The epidemiology of alopecia areata: a population-based cohort study in UK primary care*
Plain language summary available online
Summary
Background
There is a lack of population-based information on the disease burden and management of alopecia areata (AA).
Objectives
To describe the epidemiology of AA, focusing on incidence, demographics and patterns of healthcare utilization.
Methods
Population-based cohort study of 4·16 million adults and children, using UK electronic primary care records from the Oxford-Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) network database, 2009–2018. The incidence and point prevalence of AA were estimated. Variation in AA incidence by age, sex, deprivation, geographical distribution and ethnicity was examined. Patterns of healthcare utilization were evaluated in people with incident AA.
Results
The AA incidence rate was 0·26 per 1000 person-years. AA point prevalence in 2018 was 0·58% in adults. AA onset peaked at age 25–29 years for both sexes, although the peak was broader in females. People of nonwhite ethnicity were more likely to present with AA, especially those of Asian ethnicity [incidence rate ratio (IRR) 3·32 (95% confidence interval 3·11–3·55)]. Higher AA incidence was associated with social deprivation [IRR most vs. least deprived quintile 1·47 (1·37–1·59)] and urban living [IRR 1·23 (1·14–1·32)]. People of higher social deprivation were less likely to be referred for specialist dermatology review.
Conclusions
By providing the first large-scale estimates of the incidence and point prevalence of AA, our study helps to understand the burden of AA on the population. Understanding the variation in AA onset between different population groups may give insight into the pathogenesis of AA and its management.
Alopecia areata (AA) is a common, immune-mediated nonscarring alopecia and can be associated with severe psychological consequences.1, 2 Despite being a relatively common condition, robust and recent epidemiology data for AA are lacking. The only previous population-based estimate of AA incidence rate (IR) of 0·21 per 1000 person-years is from the US Rochester Epidemiology Project (REP), but this was based on only 530 people with AA.3 The mean age of AA onset has been suggested to be between 25 and 36 years,3-6 with no clear sex differences,3-9 although these estimates have largely been generated from clinic-based studies, meaning they are likely to be missing a large proportion of people with milder forms of AA who do not receive specialist management.4-9
Information on current treatment patterns and pathways to specialist care for people with AA is similarly limited. Although topical steroids, intralesional steroids and contact immunotherapy are recommended in current UK guidelines,10 the optimal treatment pathway is not clear. A 2008 Cochrane Review examined 17 randomized control trials encompassing 540 patients and found no evidence of a significant benefit compared with placebo for any treatment examined.11 For disease of limited extent topical minoxidil and dithranol may offer some benefit, while systemic immunosuppressants are sometimes used in severe alopecia areata.10 No previous studies have robustly evaluated treatment patterns in AA, or healthcare utilization after clinical diagnosis.
In the UK, general practitioners (GPs) are usually the first point of contact for new cases, with secondary care referrals reserved for those not responding to first-line treatments, those presenting with extensive or rapidly advancing disease, or where the diagnosis is uncertain.12 In this population-based setting we aimed to describe the contemporary epidemiology of AA, and common patterns of healthcare utilization and treatment.
Patients and methods
Study design
The study protocol was prespecified as part of an AA observational study series (Harries et al., paper submitted), registered with ClinicalTrials.gov (NCT04239521), and conducted following REporting of studies Conducted using Observational Routinely collected Data (RECORD) guidelines.13
Population-based primary care data were extracted from the Oxford-Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) network database. The RCGP RSC cohort is drawn from a large network of GP practices across England, representing a broadly representative sample of the UK population.14 The RCGP RSC database contains information about demographics, clinical diagnoses, clinical measurements, laboratory test results and prescriptions. Studies using RCGP RSC have been published across many common diseases, including obesity, atrial fibrillation, diabetes and rheumatoid arthritis.15-18
Study population
All adults and children registered between 1 January 2009 and 31 December 2018 were eligible for inclusion. People who opted out of record sharing were excluded (approximately 1·8% of adults).
Alopecia areata cases
We identified people diagnosed with AA (AA cases) using diagnostic Read codes specific to the condition (Harries et al., paper submitted). AA cases required an AA-specific Read code and, in the subsequent 365 days, no code for an alternative diagnosis (scarring alopecia,19 traction alopecia, congenital alopecia, androgenetic alopecia, telogen effluvium, tinea capitis, trichotillomania or secondary syphilis of the scalp).
Sociodemographic factors
Age was categorized into 5-year age groups. Ethnicity was grouped into standard major UK ethnic groups: white, black, Asian, mixed and other.20 Socioeconomic status was defined using the Index of Multiple Deprivation (IMD),21 calculated at the point of data extraction using patient postcode, and stratified by national deprivation quintile.
Statistical analyses
Incidence of alopecia areata
Incident cases were defined as people with a new-onset AA during the study period. People with a diagnosis of AA prior to the study period were excluded. To increase certainty that an AA diagnosis was incident, those diagnosed within 6 months of registering with a practice were excluded, unless under 1 year of age. We calculated IRs, stratified by age category and sex, by dividing the number of incident people by the sum of person-years of follow-up for the total eligible population. Multivariable IR ratios (IRRs) adjusted for sex, ethnicity, IMD quintile, geographical region and geographic area (urban/rural), were estimated using Poisson regression.
Point prevalence of alopecia areata
We estimated point prevalence of AA for adults, overall and age/sex stratified, on 31 December 2018, the last date of follow-up. Point prevalence represents people actively registered with their GP practice on that date, who have either active AA or who have had AA in the past. Point prevalence was calculated by dividing the total number of actively registered people with an AA diagnostic code (at any prior timepoint) by the total number of actively registered people. All people were included regardless of the date of AA diagnosis in relation to the date of registration with a practice. People with < 365 days of follow-up from date of registration were excluded from the analysis (unless under 1 year of age).
Service utilization and prescribing patterns
In people newly diagnosed with AA, we examined primary care visit rates, specialist dermatology review rates and prescribing of medications indicated for AA, in the first year after AA diagnosis.
We used a matched-cohort design to compare rates of primary care visits in people newly diagnosed with AA (AA cases) and people without AA (matched controls). Matched controls were identified by matching each AA case with four controls never diagnosed with AA at the date of diagnosis of the AA case. We used nearest-neighbour matching, by age, sex and time since practice registration, at the GP practice level.22 Matched controls required at least 1 year of follow-up when matched to minimize the risk of a nonrecorded existing diagnosis of AA. Follow-up for each control began at the start of follow-up of their corresponding AA case.
Annual rates of primary care visits were calculated by dividing the total number of visits each year by the sum of person-years of follow-up each year. The proportion of AA cases referred for specialist dermatology review and the proportion prescribed medications of interest were calculated using the Kaplan–Meier estimator, as not all people had an entire year of follow-up after diagnosis available. Visit and referral rates were stratified by age, sex, ethnicity, IMD quintile, geographical region and rural/urban geography. AA medication classes of interest were categorized as mild, moderate, potent or very potent topical corticosteroids (TCS), systemic steroids, minoxidil and dithranol.10 In sensitivity analyses, we excluded people with a diagnosis of atopic dermatitis or psoriasis prior to either their AA diagnosis or first medication prescription.
Sensitivity analysis
To check the validity of the AA case definition and identify possible changes in coding practice, annual incident trends and point prevalence of alopecia coded as ‘nonspecific’ and ‘other specified’ (either scarring, congenital, traction or traumatic, or androgenetic alopecia) (Harries et al., paper submitted) were estimated using the same approach as for the primary cohort.
Changes to the study protocol
The matched cohort was added to provide a comparator for rates of primary care visits. This provides important context as the reason underlying a visit cannot be ascertained from primary care records, meaning we could not identify whether visits related to AA itself. No other changes were made; all a priori defined objectives were reported. R v3.4.1 was used for analysis.
Ethics approval
Study approval was granted by the RCGP RSC Research Committee. Using the NHS Health Research Authority research decision tool (http://www.hra-decisiontools.org.uk/research/) showed that formal ethics board review was not required for this study.
Results
Alopecia areata incidence
Of the total study population (n = 4 163 162), 6765 people developed new-onset AA over the study period [Figure S1 (patient flow diagram); see Supporting Information]. The overall AA IR was 0·26 per 1000 person-years. AA incidence peaked at age 25–29 years in both males [IR 0·51, 95% confidence interval (CI) 0·46–0·56] and females (IR 0·43, 95% CI 0·39–0·48). The median age at diagnosis was 31 [interquartile range (IQR) 21–41] for males and 34 (IQR 22–48) for females. The incidence peak was much broader in females than males with female incidence being higher in childhood (age groups 5–14 years) and in those people aged 45+ years [Figure 1 and Table S1 (see Supporting Information)]. AA annual IRs were stable over the 10-year study period (Figure S2; see Supporting Information). Incidence of ‘nonspecific’ and ‘other specified’ alopecia were constant over the study period (Figure S3; see Supporting Information).
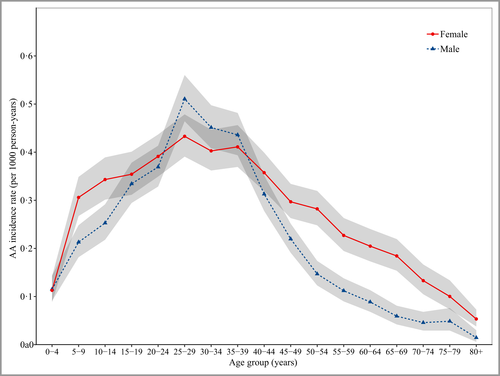
The incidence rates of AA by age at diagnosis and sex. Data are unadjusted incidence rates per 1000 person-years for 2009–2018 inclusive in a total population of 4 163 162 people. Grey shading represents 95% confidence intervals. AA, alopecia areata.
When adjusted for age and other sociodemographic characteristics, incident AA was more common in females (IRR 1·19, 95% CI 1·13–1·24), with the largest sex difference in people aged 50 years and above (Table 1). AA was substantially more common in nonwhite ethnicity groups with the highest incidence in Asians [IRR Asian vs. white ethnicity 3·32 (95% CI 3·11–3·55)]. Trends were consistent across the lifespan (Table 1). Incidence was highest in those with the highest levels of deprivation, and there was higher incidence among urban than rural dwellers (Table 1).
| Overall | 0–4 | 4–18 | 18–50 | 50+ | |
|---|---|---|---|---|---|
| Incident cases (N) | 6765 | 79 | 1200 | 4249 | 1237 |
| Person-years at risk | 25 717 205 | 1 016 305 | 4 148 944 | 11 231 654 | 9 320 302 |
| Incidence rate (per 1000 person-years) | 0·26 (0·26–0·27) | 0·08 (0·06–0·10) | 0·29 (0·27–0·31) | 0·38 (0·37–0·39) | 0·13 (0·13–0·14) |
| Sex | |||||
| Male | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) |
| Female | 1·19 (1·13–1·24)*** | 0·75 (0·48–1·17) | 1·30 (1·16–1·46)*** | 1·01 (0·95–1·08) | 2·12 (1·88–2·39)*** |
| Ethnicity | |||||
| White | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) |
| Asian | 3·32 (3·11–3·55)*** | 3·98 (2·18–7·27)*** | 3·11 (2·64–3·67)*** | 2·93 (2·70–3·17)*** | 2·62 (2·12–3·22)*** |
| Black | 1·54 (1·36–1·75)*** | 2·56 (1·04–6·31)* | 1·68 (1·28–2·20)*** | 1·36 (1·16–1·60)*** | 1·26 (0·85–1·86) |
| Mixed | 2·15 (1·82–2·54)*** | 3·83 (1·58–9·28)** | 1·59 (1·11–2·28)* | 2·09 (1·70–2·56)*** | 1·94 (1·04–3·62)* |
| Other | 2·71 (2·29–3·19)*** | 1·43 (0·19–10·55) | 1·33 (0·80–2·23) | 2·63 (2·19–3·17)*** | 2·30 (1·27–4·17)** |
| IMD quintile | |||||
| 1 (most deprived) | 1·47 (1·37–1·59)*** | 1·57 (0·79–3·13) | 1·34 (1·12–1·60)** | 1·49 (1·35–1·65)*** | 1·21 (1·01–1·46)* |
| 2 | 1·33 (1·23–1·44)*** | 1·53 (0·77–3·06) | 1·25 (1·04–1·50)* | 1·35 (1·22–1·49)*** | 1·05 (0·88–1·25) |
| 3 | 1·17 (1·08–1·26)*** | 0·64 (0·27–1·53) | 1·20 (0·99–1·44) | 1·25 (1·12–1·38)*** | 0·88 (0·74–1·04) |
| 4 | 1·07 (0·99–1·16) | 0·72 (0·31–1·66) | 1·09 (0·91–1·32) | 1·14 (1·03–1·26)* | 0·88 (0·75–1·03) |
| 5 (least deprived) | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) |
| Geographic area | |||||
| Rural | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) | 1·00 (ref) |
| Urban | 1·23 (1·14–1·32)*** | 0·95 (0·45–2·01) | 1·44 (1·20–1·72)*** | 1·16 (1·05–1·27)** | 1·01 (0·88–1·16) |
- IMD, index of multiple deprivation; ref, reference.
- Models were adjusted for sex, ethnicity, IMD and geographic area. IMD data were not available for n = 85 019; ethnicity data was not available for n = 1 039 434; geographic area data was not available for n = 81 164; patients with missing data were included as missing categories for each variable. ***P < 0·001, **P < 0·01, *P < 0·05.
The unadjusted incidence of AA was highest in the London region [overall (adults and children combined) IRR 0·42 (95% CI 0·40–0·45)] (Table S2; see Supporting Information). In adjusted analysis, there was a lower incidence of AA in the East Midlands, North West, South East and South West compared with London [Figure 2, Table S3 (see Supporting Information)]. No significant differences by region were observed in children, although numbers were smaller (Table S3).
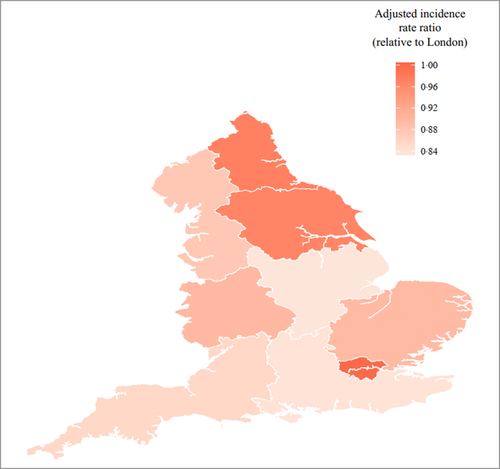
Alopecia areata point prevalence
There were 2 634 083 actively registered people on 31 December 2018 who were included in the estimation of AA point prevalence. Overall point prevalence (95% CI) of AA in adults was 0·58% (0·57–0·59), with a higher adult point prevalence in females [0·62% (0·60–0·63)] than males [0·55% (0·53–0·56)]. Point prevalence of nonspecific alopecia was 0·91% (0·90–0·93), and was markedly higher in females [1·57% (1·54–1·59)] than males [0·27% (0·26–0·28)]; point prevalence of other-specified alopecia was much lower [overall 0·082% (0·078–0·086); females 0·063% (0·058–0·068); males 0·100% (0·094–0·117)].
Primary care visits
Primary care visit rates (95% CI) for people diagnosed with AA in the year following their diagnosis were 4·32 (4·27–4·38) visits per year compared with 2·58 (2·56–2·60) in matched controls (Tables S4 and S5; see Supporting Information). Primary care visit rates for people diagnosed with AA increased over the 10-year study period and were consistently higher than visit rates for controls (Figure S4; see Supporting Information). Females made more visits than males and there was evidence of a socioeconomic gradient with more frequent visits made by those with the highest levels of deprivation. People with AA of mixed ethnicity had a lower rate of visits compared with the other ethnic groups. People with AA living in rural geographic areas made more visits than those living in urban areas (Table S4).
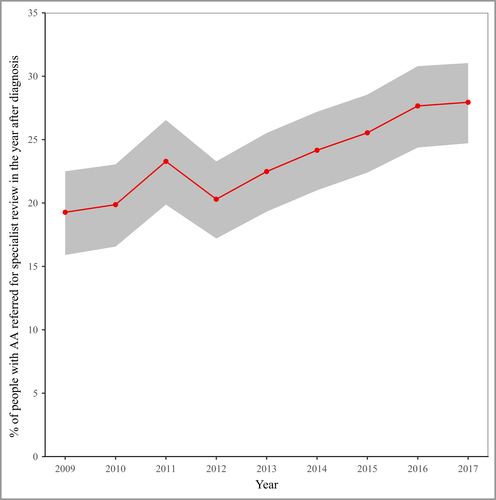
Specialist dermatology reviews
There were 1624 (24%) AA cases referred for specialist dermatology review in the year after diagnosis (Table S4). The proportion of AA cases referred increased over the study period from 19·4% (16·1–22·7) in 2009 to 27·9% (24·7–31·0) in 2017 (Figure 3). Although referrals levels were similar by ethnicity and rural/urban geography, they were higher for females (compared with males) and people living in less deprived areas (compared with those from more deprived areas) (Table S4).
Prescribing patterns
Of the total patients, 46% did not receive any prescription medication. Potent TCS were the most commonly prescribed medications in the year after AA diagnosis, and prescribing increased over the study period (23·6% in 2009, 33·1% in 2017, Figure S5; see Supporting Information). The proportion of people prescribed mild or very potent TCS remained similar across the study period ranging between 12% and 17% (Figure S5). Of note, approximately 5% of patients were prescribed systemic steroids. Minoxidil (70 prescriptions) and dithranol (16 prescriptions) were rarely prescribed. The results were similar when people with a diagnosis of atopic dermatitis or psoriasis prior to either AA diagnosis or first medication prescription were excluded (Figure S6; see Supporting Information).
Discussion
Our analysis of over 4 million people in primary care in England demonstrates that new-onset AA most commonly presents between 25 and 29 years of age. Females more commonly present with AA than males, although the overall difference was small and when age-stratified, a higher incidence in females was apparent only in children aged 4–18 years and adults aged 50+ years. Females also have a much broader peak than males in terms of age at AA onset. We show a threefold higher AA incidence in people of Asian origin, and a higher AA incidence in people from areas of higher deprivation and urban areas. One in four people with new-onset AA are referred to a dermatology specialist, with referral rates lowest in people from more deprived areas.
In comparison with other studies, to our knowledge, this is the largest population-based epidemiological study of AA to date, including 6765 people with AA compared with 530 people included in a recent US population-based study using data from the REP.3 The IRs in both studies are similar: 0·26 in our study compared with 0·21 per 1000 person-years in the REP. The mean age at diagnosis was 33 years in the REP, similar to the incidence peak we observed. As expected, the incidence estimated in our primary care-based study is lower than that reported in secondary care cohorts (ranging from 0·7% to 3·8%),5-9 where the denominator encompasses patients with skin conditions only.
Our population-based estimate of AA point prevalence (0·58%), which includes both active AA and historical AA diagnoses, is in line with the few previous epidemiological studies of AA prevalence. Historical estimates ranging from 0·2%23 to 2·1%3 have been observed in smaller US cohorts, while a more recent population-based study from France estimated an AA prevalence of 1·0%.24
We observed a higher rate of AA in those from nonwhite backgrounds and, in particular, a threefold higher rate in those of Asian origin. This is concordant with US data where a higher incidence of AA was observed in nurses of black compared with white ethnicity.25 Interestingly, AA was more common in people of black ethnicity but less common among people of Asian ethnicity in the American National Alopecia Areata Registry.26 Differences between our data and this study may relate to the lower prevalence of people from Asian backgrounds in the US cohorts, as well as reliance upon self-reporting in both US studies. While the exact pathogenesis of AA remains incompletely understood, it is generally considered to be an autoimmune phenomenon, occurring in those with a genetic predisposition in the presence of certain environmental triggers.27 Genetic variations in the HLA region known to predispose to autoimmune conditions have been observed more frequently in those of nonwhite ethnicity compared with white individuals.28, 29 Rates of other autoimmune conditions including systemic lupus erythematous have also been reported to be higher in Black and Asian, compared with white, individuals.30
Ours is the first study to identify higher deprivation and urban environments as factors associated with AA. Urbanization and air pollution have previously been postulated as environmental triggers in the development of other autoimmune conditions such as rheumatoid arthritis, potentially via the induction of systemic inflammation by particulate matter.31, 32 Social deprivation could be linked to AA development indirectly due to higher rates of life stressors, depression and anxiety, although a causal link between these factors and AA onset has not been established.33, 34
Considering the implications for primary care management of AA, 24% of patients with AA seen in primary care were referred to specialist dermatology in the first year after diagnosis, and referral rates increased from 19·4% in 2009 to 27·9% in 2017. This is comparable with secondary care referral rates for psoriasis in the UK (18·1% in 2009),35 but is significantly higher than referral rates for atopic dermatitis (4·5% in 2018).36 UK guidelines suggest dermatology referral for hair loss that does not respond to first-line treatment or if the diagnosis is uncertain.12 Given that 80% of patients with limited AA may experience spontaneous remission within 1 year,37 and hair regrowth with topical steroids has success rates of 18–60% compared with 0–27% success with placebo,38-40 a specialist dermatology review rate of 24% seems concordant with guidance. Difference in primary care visit rates for people with AA were in line with known primary care consultation patterns, with females and those from more deprived backgrounds more likely to attend their GP.41, 42
Despite higher rates of AA and primary care attendance, people from the most deprived areas were less likely to be referred to a dermatology specialist, a pattern previous reported in the UK for other conditions.42 A potential explanation is that GP consultations in deprived areas are more often complicated by complex multi-morbid presentations, compared with patient-led, single-problem consultations in more affluent areas, where patient expectations of secondary care referral are greater.43
Potent TCS were the most frequently prescribed medication after AA diagnosis, in correspondence with national guidelines which recommend potent TCS as first-line treatment for limited AA.10 The rise in the proportion of patients with AA prescribed potent TCS prescriptions over the study period may reflect a physician response to these 2012 guidelines. Low rates of systemic corticosteroid prescriptions are consistent with UK AA guidelines, which specifically state that the use of systemic steroids is not supported.10
The strengths of our study design include the use of a large, UK population-representative, primary care cohort.14 Although we lacked a validated case definition for AA, our ontological approach to detecting incident AA has higher accuracy compared with the use of AA-specific codes alone.44 Exclusions of recurrent AA episodes and other, potentially confounding, conditions (i.e. other causes of hair loss) are likely to have improved the accuracy of our estimates.
There are several study limitations. We are likely to have underestimated the true incidence and point prevalence of AA as some cases will have been coded using other terms including nonspecific alopecia. This study also does not capture mild cases of AA not presenting to primary care. We assume that all diagnoses of AA in primary care are correct and that AA is a simple diagnosis to make: diagnosis is usually easier in patch-type AA but it is possible that we underestimate true incidence based on the difficulty in diagnosing diffuse types of AA, although this will be a much smaller percentage of the overall incidence. Neither the reason for visits or specialist dermatology reviews, nor the indication of prescriptions, was available, which means we were unable to identify AA-specific events; our service utilization estimates therefore provide a measure of overall primary care use in people with AA. We were unable to assess secondary care prescribing in AA, as at present there is no mechanism in the UK for capturing secondary care prescriptions. As with all single-country epidemiological studies, our results should not be extrapolated to dissimilar populations.
In conclusion, by providing the first large-scale population-based estimates of the incidence and point prevalence of AA, our study helps to understand the burden of AA on the population across the lifespan. The marked variation in AA onset, especially across different ethnic groups, may give insight into the pathogenesis of AA and its management.
Acknowledgments
Patients and practices who are members of the Oxford-Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) network, who allow their data to be shared for surveillance, research, quality improvement and education. The collaboration of primary care computerized medical record system providers EMIS, TPP, InPractice Systems, Microtest and Wellbeing in facilitating the RCGP RSC data. Data access support from the RCGP RSC team of the University of Oxford and University of Surrey. Medical writing and statistical input from Anita Lynam and Louise Jordan of Momentum Data and project management support from Filipa Ferreira of the University of Oxford and University of Surrey.
Author Contribution
Matthew Harries: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing-review & editing (equal). Abby Macbeth: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing-review & editing (equal). Susan Holmes: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing-review & editing (equal). Wing Sin Chiu: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing-review & editing (equal). William Romero Gallardo: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing-review & editing (equal). Monica Nijher: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing-review & editing (equal). Simon de Lusignan: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing-review & editing (equal). Christos Tziotzios: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing-review & editing (equal). Andrew G Messenger: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing-review & editing (equal).




