Macrophage and chondrocyte phenotypes in inflammation
Abstract
Inflammation is a complex biological process protecting the body from diverse external threats. Effectively performing this task requires an intricate, well-regulated interplay of different cells and tissues. Furthermore, several cells participating in inflammation can assume diverse phenotypes.
A classic and relatively well-studied example of phenotypic diversity in inflammation is macrophage polarization. Based on the TH1/TH2 phenotypes of T helper cells, this scheme has proinflammatory “classical/M1” activation contrasted with the anti-inflammatory and healing-promoting “alternative/M2” phenotype. Some authors have extended the concept into an M17 phenotype induced by the classic TH17 cytokine IL-17. Phenotypic changes in chondrocytes have also been studied especially in the context of osteoarthritis (OA), and there are indications that these cells can also assume polarized phenotypes at least partly analogous to those of TH cells and macrophages. The therapeutic success of biological agents targeting TH1/TH2/TH17 inductor and/or effector cytokines displays the utility of the concept of polarization. The aim of this focused review is to survey the internal and external factors affecting macrophage and chondrocyte phenotypes (such as inflammatory cytokines, widely used medications and natural products) and to explore the possibility of ameliorating pathological states by modulating these phenotypes.
Plain English Summary
Inflammation is a mechanism that protects the body from both internal and external threats, requiring a complex interplay of various cell types. To effectively perform their functions, these cells can assume different phenotypes (patterns of gene expression). Macrophages, central immune cells, can adopt inflammation-promoting “classical/M1” and inflammation-attenuating “alternative/M2” phenotypes, as well a “M17” phenotype induced by IL-17. Chondrocytes, cells of the cartilage, can also assume distinct analogous phenotypes. Modulating the phenotypes of these cells holds a promise for treating diseases such as osteoarthritis, a highly prevalent and debilitating disease with no disease-modifying drugs currently available.
1 INTRODUCTION
Inflammation is a complex biological process protecting the body from diverse internal and external threats. Effectively performing this task requires an intricate, well-regulated interplay of different cells and tissues. If the regulation of inflammation fails, development of illnesses such as autoimmune or allergic diseases may result. A central example of a disease associated with chronic inflammation is osteoarthritis (OA). OA is the most common joint disorder worldwide, causing considerable suffering and significant costs to healthcare systems. OA most commonly affects hip and knee joints, as well as the joints in the hand. The disease is characterized by constant low-grade inflammation in the joints, narrowing of joint spaces, transient exacerbations of synovial inflammation and formation of osteophytes (bony overgrowths) along joint margins; the disease may eventually lead to the complete degradation of cartilage in the affected joint. These changes cause pain, stiffness and loss of mobility.1 Several cells participating in OA-related inflammatory processes can assume diverse phenotypes depending on environmental signals and the phase of the inflammatory response.2
A classic and relatively well-studied example of phenotypic diversity in inflammation is macrophage polarization, in which proinflammatory “classical/M1” activation is contrasted with anti-inflammatory and healing-promoting “alternative/M2” activation.2
The phenotypes of chondrocytes, cells of the cartilage, have also been studied especially in the context of OA, an extremely common joint disease for which no effective disease-modifying medications are currently available.3 Chondrocyte phenotype has been shown to change during different phases of OA, and more recently, studies utilizing single-cell RNA sequencing have revealed distinct subpopulations of chondrocytes within an OA joint.4, 5 However, these phenotypes are less well characterized than those of TH cells or macrophages. Chondrocyte phenotypes induced by the classic TH1/TH2/TH17 cytokines, glucocorticoids and nonsteroidal anti-inflammatory drugs (NSAIDs) were recently characterized by the authors.6 Understanding these phenotypic changes promotes understanding of OA pathogenesis and may help with the development of disease-modifying OA drugs in the future.
This focused review is based on Antti Pemmari's PhD thesis, examined on May 2022 and awarded the 2023 PhD thesis award of the Finnish Pharmacological Society.
2 MACROPHAGE PHENOTYPES
Macrophages are versatile, multifunctional cells whose physiological functions include cleaning tissues of debris via phagocytosis, recycling of nutrients (e.g., iron) and tissue remodelling. In infections, macrophages phagocytose microbes and present their antigens to T cells. They also direct inflammatory response by producing cytokines and promote the resolution of inflammation after its cause has been eliminated. Performing these diverse functions requires macrophages to adopt different phenotypes2 (Figure 1).
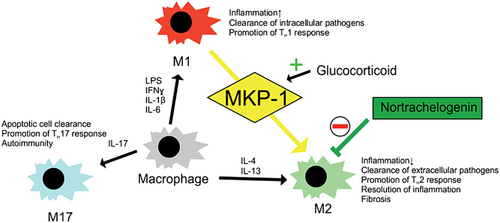
The concept of T helper (TH) cell polarization, developed in the 1980s, was relatively soon extended to macrophages. The “traditional” version of this scheme has proinflammatory “classically activated” or “M1” macrophages contrasted with anti-inflammatory and healing-promoting “alternatively activated” or “M2” macrophages. Nowadays, this scheme is considered somewhat simplistic, and it is increasingly recognized that macrophage phenotypes form a “spectrum” rather than a strict dichotomy (or a strict division into more distinct phenotypes). However, macrophage polarization is still thought to be a useful concept for understanding the various functions of these cells in the context of inflammation and tissue healing.7
2.1 Classical macrophage activation
Classically activated (M1) macrophages promote inflammation and effectively destroy intracellular pathogens. These macrophages play a crucial role in the early stages of inflammation, promoting the eradication of pathogens and the initiation of adaptive immune responses. The polarization of macrophages towards the M1 phenotype is orchestrated by various factors, including microbial products, such as lipopolysaccharide (LPS), and proinflammatory cytokines like interferon gamma (IFN-γ). Activation of Toll-like receptors (TLRs) on macrophages by pathogen-associated molecular patterns (PAMPs) triggers the M1 phenotype, leading to enhanced phagocytosis and antigen presentation.7
Inducible nitric oxide synthase (iNOS) is traditionally regarded as a central marker of M1 activation. In inflammation, iNOS converts the amino acid L-arginine into nitric oxide (NO) and L-citrulline; the direct cytotoxic effects of NO are then employed to fight pathogens, but they can also have deleterious effects on host tissues.8 Murine macrophages readily express iNOS and produce NO, but the role of these factors in human cells is less well established; iNOS expression has not been successfully demonstrated in many “resting state” human macrophage populations. However, in inflammatory states (such as tuberculosis, atherosclerosis and loosened prosthetic joints),9 human macrophages have been shown to robustly express iNOS, demonstrating the proinflammatory role of this factor also in humans.10
M1 macrophages produce a variety of proinflammatory cytokines such as interleukins (IL) 1 and 6, tumour necrosis factor alpha (TNFα) and monocyte chemotactic protein 1 (MCP-1, also known as chemokine C-C motif ligand 2 or CCL2). Compared to the short-lived NO, these mediators can act systemically, directing body-wide inflammatory responses. IL-1 promotes the proliferation and/or activation of various leukocytes such as neutrophils, lymphocytes (both B and T cells) and macrophages. IL-6 activates neutrophils and lymphocytes while suppressing anti-inflammatory regulatory T cells. It also increases the production of acute-phase proteins in the liver. IL-1 also has some anti-inflammatory effects via induction of IL-1 receptor antagonist (IL-1RA) and IL-10, probably as a kind of endogenous negative feedback loop.2 TNFα induces neutrophil proliferation and migration into tissues, activates the synthesis of acute-phase proteins and supports classical macrophage activation. MCP-1 is a chemokine (factor promoting cell movement, i.e., chemotaxis) that attracts monocytes, T cells and dendritic cells to the site of inflammation. Proinflammatory cytokines, especially IL-1 and TNFα, also act as endogenous pyrogens; they raise body temperature via increased prostaglandin E2 (PGE2) synthesis in the hypothalamus.2
2.2 Alternative macrophage activation
Macrophages whose effects are anti-inflammatory rather than proinflammatory are collectively called alternatively activated or M2 macrophages. They protect the body from harms caused by excessive M1/TH1 responses and contribute to resolution of inflammation and tissue repair.7 M2 macrophages produce anti-inflammatory cytokines and are involved in scavenging debris and promoting tissue remodelling. Owing to the variety of inducing factors and effector cytokines observed in M2 macrophages, different schemes subdividing the M2 phenotype have been proposed, but there is little agreement about their exact details.11, 12
A relatively well-established subset/subpopulation of alternatively activated macrophages are the so-called “wound-healing” cells, which are induced by the canonical TH2 cytokines IL-4 and IL-13. These cytokines signal through a shared pathway, a heterodimer receptor complex consisting of alpha IL-4 receptor (IL-4Rα) and alpha IL-13 receptor (IL-13Rα1). The activation of the receptor complex activates insulin receptor substrates (IRS) 1 and 2 as well as signal transducer and activator of transcription 6 (STAT6), the “classic” transcription factor promoting M2 activation. These cells use arginase to produce extracellular matrix (ECM) precursors from arginine via ornithine. They also direct the actions of other healing-promoting cells such as fibroblasts and myofibroblasts by producing profibrotic growth factors and other mediators.13 In addition to arginase, the wound-healing M2 phenotype is classically associated with the expression of mannose receptor (MRC1/CD206) and chitinase-like proteins. Tissue healing promoted by M2 macrophages is mediated by various growth factors that stimulate ECM production and other healing-promoting processes. These include platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β) and fibroblast growth factor 2 (FGF2).14
While human macrophages display an alternative phenotype induced by IL-4 and IL-13, finding a robust M2 marker in human macrophages has proven relatively difficult. Human macrophages do not express widely used murine M2 markers such as Ym1 (also known as chitinase-like 3 or Chil3) or Fizz1 (also known as resistin like alpha or Retnla),15 and they meaningfully express arginase 1 only under special conditions such as trauma or treatment with certain immunostimulatory compounds.16 One relatively well-established candidate is chemokine (C-C motif) ligand 13 (CCL13), which has been shown to be markedly upregulated in IL-4-stimulated human macrophages.17 In vivo, CCL13 expression by M2 macrophages is especially linked with fibrosis, allergic reactions and asthma18; it promotes the chemotaxis of macrophages, T lymphocytes and eosinophils.19 Other chemokines identified as human alternative activation markers in transcriptomic studies include CCL18 and CCL23.17 The growth factor PDGF20 and MRC1/CD20621 have also been showed to be upregulated in human M2 cells.
2.3 M17 macrophage activation
Some authors have extended macrophage polarization to a phenotype analogous to TH17 cells. The “M17” phenotype is less well defined and established than M1 and M2 phenotypes; however, these cells have been reported to promote inflammation in the early phases of inflammatory response and to later facilitate the clearance of apoptotic cells and resolution of inflammation. They may also promote further TH17 responses and be involved in autoimmunity.22
2.4 More complex factors affecting macrophage phenotype
The model of macrophage polarization presented above has attracted some criticism. According to several authors, the nomenclature has become increasingly confusing, and terms for the phenotypes (especially the “alternative” ones) are used somewhat inconsistently. Also, a rough M1/M2/M17 division is not straightforwardly supported by the evidence obtained from modern transcriptomics.12 The role of factors such as cellular metabolism (controlled by, e.g., mTor signalling) behind the phenotypic changes is also being increasingly appreciated.23 One central issue is the difference in established (or postulated) alternative activation markers between human and murine cells (see above). However, macrophage polarization seems to capture at least several central features of macrophage phenotypes observed in different physiological and pathological states.7 Also, the somewhat more specific phenotypes of LPS-induced M1 and IL-4 + IL-13-induced “wound-healing” M2 activation can be delineated with modern sequencing technologies and thus can be considered (patho)physiologically important.12 This gives credence to the notion that macrophage polarization can be a useful concept for understanding the functions of macrophages in health and disease.
Macrophage phenotypes are also not fixed but exhibit remarkable plasticity. Dynamic switching between classical and alternative phenotypes occurs often in a tissue in response to changing microenvironmental signals, ensuring effective control of inflammatory response and a balance between pro- and anti-inflammatory processes.24
2.5 Macrophage phenotypes in diseases
The dysregulation of macrophage phenotypes is implicated in various diseases, including chronic inflammatory conditions and fibrotic diseases. Understanding the factors that influence macrophage polarization provides potential therapeutic targets for modulating the immune response, and targeting specific macrophage phenotypes may offer novel approaches for the treatment of inflammatory and fibrosing disorders.13, 25
Excessive classical (M1) macrophage activation has been linked to various disease states involving chronic inflammation. An example is rheumatoid arthritis, in which radiological joint damage has been shown to correlate with the degree of macrophage infiltration into the joint.26 Furthermore, these macrophages have been shown to be heavily skewed towards the M1 phenotype as determined by comparing the expression of central M1 and M2 markers. They can also present autoantigens to T cells, perpetuating the autoimmune process.27 The therapeutic success of biological agents specifically targeting TH1/M1-associated cytokines (such as TNFα blockers) in autoimmune diseases provides further evidence for the central role of macrophages in these disorders.28 In addition to the manifest joint inflammation observed in rheumatoid arthritis, macrophages also contribute to the more subtle chronic synovial inflammation present in OA, and significant amounts of macrophages have been detected in up to 90% of end-stage knee OA synovial samples.29
In addition to “overt” autoimmune diseases, macrophages are also implicated in pathological states involving more subtle low-grade inflammation. These include many “diseases of affluence” such as atherosclerosis,30 obesity, nonalcoholic fatty liver disease31 and insulin resistance32 in addition to OA. In obesity-linked diseases, the predominant phenotype of adipose tissue macrophages changes to M1, and the proinflammatory c-Jun N-terminal kinase (JNK) and NFκB pathways display constant activation.33 Some of these effects may be caused by direct TLR4-mediated M1 activation by saturated fatty acids.34
Alternatively activated (M2) macrophages protect the body from harmful excessive inflammation and support tissue healing. However, alternative activation has also been linked to pathologic conditions. Most of these are fibrosing diseases that involve excessive ECM deposition and thus can be thought of as dysregulated tissue repair processes. In these diseases, M2 macrophages may directly produce ECM precursors and affect the actions of other cells involved in the fibrosing process, such as fibroblasts and myofibroblasts.13 Diseases linked with dysregulated M2 activation include pulmonary fibrosis,35 chronic pancreatitis36 and systemic sclerosis (SSc).37 The relationship between macrophage polarization and fibrosis is not, however, completely straightforward. Studies on dermal38 and lung39 fibrosis show that at least some of the macrophages express also proinflammatory mediators such as IL-1β and TNFα, as well as matrix metalloproteinase (MMP) enzymes traditionally linked to M1 activation. Thus, fibrotic lesions may include both classically and alternatively activated macrophages with their relative proportions depending on the age of the lesion and the state of the disease.
A display of the utility of the concept of immune cell polarization is the success of several immunomodulatory medications specifically targeting the inductor and/or effector cytokines of the different phenotypes. Relative abundances of different TH cell and macrophage phenotypes, as well as central inductor cytokines thereof, can be thought to determine the “working mode” of immune system. Showing the benefits of this approach, biological agents such as IL-6 and TNFα inhibitors are used as effective treatments of inflammatory diseases.40
Some compounds and medications have been proposed to have beneficial effects in OA via modulation of macrophage phenotype. For example, high molecular weight hyaluronic acid has been shown to inhibit M1 activation and to promote M2 activation,41 and the plant flavonoid quercetin has been implicated to alleviate experimental OA via increased M2 activation of synovial macrophages.42 Metformin, a widely used antidiabetic that has been studied in various age-related diseases, inhibits M1 activation43 and has been shown to be attenuate cartilage degradation in experimental OA.44
2.6 Effects of MKP-1 and glucocorticoids on macrophage phenotype
Mitogen-activated protein kinase phosphatase 1 (MKP-1) is a phosphatase that regulates the activity of MAP kinases, and it is known as a central endogenous regulator of inflammation. Animals deficient in this gene are more prone to hyperinflammatory septic disease and mortality compared to wild-type animals, and their macrophages display an enhanced propensity to M1 activation.45 In addition, MKP-1 has been shown by the authors not to merely suppress proinflammatory macrophage activation but to shift macrophage phenotype towards the anti-inflammatory alternative activation; MKP-1 deficient macrophages display reduced expression of M2 markers.46 Glucocorticoids, widely used medications in the treatment of inflammatory diseases, were shown to attenuate M1 and promote M2 activation via MKP-1.46
In contrast to many widely used anti-inflammatory treatments attenuating M1 activation, inhibition of M2 activation has generally attracted less interest. However, owing to the role of alternatively activated macrophages on many fibrosing diseases (see above), compounds inhibiting this phenotype could have therapeutic potential. Compounds that inhibit M2 activation include peroxisome proliferator activated alpha (PPARα) agonists, medications also used in the treatment of dyslipidemia,47 as well as nortrachelogenin, the major polyphenol component of knot extract from Scots pine (Pinus sylvestris).48 In a murine model mimicking scleroderma, nortrachelogenin has been shown by the authors to prevent the development of skin fibrosis and to reduce the expression of arginase 1 (along with several collagens) suggesting that it can ameliorate fibrosis via the inhibition of M2 activation.48 Inhibiting alternative macrophage activation with the phosphodiesterase 4 (PDE4) inhibitor rolipram37 and the tyrosine kinase inhibitor nintedanib49 has also been shown to ameliorate experimental dermal fibrosis in mice.
3 CHONDROCYTE PHENOTYPES
Chondrocytes are derived from mesenchymal stem cells (MSCs), and their differentiation is regulated by the growth factors FGF2, TGFβ and bone morphogenetic protein 4 (BMP4), as well as by Wnt signalling. As chondrocytes reside in an avascular, hypoxic environment, they express large amounts of hypoxia-inducible factor 1 alpha (HIF1α). It facilitates chondrocyte metabolism in hypoxic conditions, increasing the expression of aggrecan and type II collagen via Sox9. Chondrocytes play a central role in endochondral ossification (formation of bone from hyaline cartilage during fetal development); during this process, they adopt a so-called hypertrophic phenotype that is marked by increased cell volume and synthesis of ECM components. In mature cartilage, chondrocytes maintain cartilage structure via a delicate balance of anabolic and catabolic processes: They secrete constituents of the ECM and simultaneously promote its breakdown, which results in a steady turnover of cartilage constituents. Mature chondrocytes generally do not proliferate3 (Figure 2).
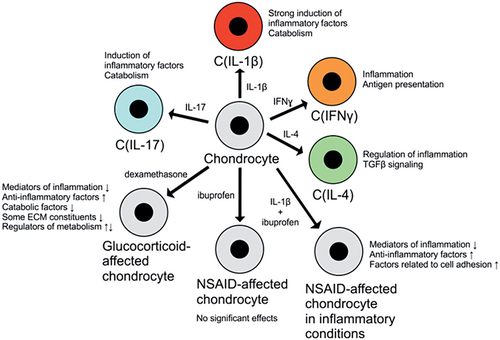
While chondrocytes synthesize ECM and support its integrity, ECM components also affect chondrocyte phenotype and function via surface receptors such as integrins, discoidin domain receptor 2 (DDR 2) and CD44. When cartilage is damaged by trauma or degenerative changes (such as in OA), chondrocytes upregulate the production of ECM components to partially repair the damage. The cartilage produced in these situations is, however, partially fibrous and mechanically inferior to normal, healthy hyaline cartilage.50
3.1 Chondrocyte phenotype in OA
Changes in chondrocyte phenotype have been most thoroughly documented in the setting of OA. The disease is associated with loss of chondrocytes, and gene expression in the surviving cells has been shown to be markedly altered. Some of these changes are thought to be anabolic and beneficial to cartilage (such as enhanced production of some ECM components), and others catabolic and deleterious (e.g., production of proteolytic enzymes and inflammatory mediators as well as altered expression of enzymes participating in the posttranslational modification of glycosaminoglycans).51 Several changes in chondrocyte metabolism have also been observed in OA. These include impaired mitochondrial function, increased production of reactive oxygen and nitrogen species and defective AMP activated protein kinase (AMPK) signalling. Also, the main energy production mode in the cells appears to shift from mitochondrial respiration towards much less effective anaerobic glycolysis.52
In OA, chondrocytes also acquire some characteristics of premature senescence, and their (already small) capability to proliferate is further reduced. However, the potential causality (and direction thereof) between these changes and OA is still largely ambiguous.53 Genome-wide expression analyses comparing chondrocyte gene expression in degraded and preserved OA cartilage have identified thousands of genes whose expression is significantly altered by OA-induced damage. These include genes involved in cartilage development (such as FRZB), inflammation (such as microsomal prostaglandin E synthase-1, PTGES and IL11) and pain (such as NGF).54 One of the best-studied changes observed in OA chondrocytes is abnormal hypertrophy (increase in cell size) associated with upregulation of type X collagen.55 Chondrocytes undergo hypertrophy also during endochondral ossification (see above), and other features of this process, such as vascularization and calcification, can also be observed in OA cartilage. Thus, the disease has been hypothesized to involve “recapitulation” of embryonic ossification. For example, osteophyte formation, a central feature of OA, is thought to proceed first by MSCs differentiating into chondrocytes, followed by transformation of cartilage into bone.50 Synovial fluid from OA joints contains increased amounts of inflammatory cytokines such as IL-1β, IL-17 and TNFα, and chondrocytes are driven to produce further proinflammatory factors (such as IL-8, IL-6, NO and PGE2) in OA. NO produced by chondrocytes via iNOS is another central inflammatory and catabolic factor.56 Acting in both autocrine and paracrine manner, it exerts widespread catabolism-promoting effects on chondrocyte phenotype. These include inhibition of ECM synthesis, increased production of matrix-degrading enzymes (such as MMPs), increased susceptibility to injury from oxidants (e.g., H2O2) and apoptosis.53
A central intracellular mediator of cartilage damage caused by proinflammatory cytokines is hypoxia-inducible factor 2 alpha (HIF2α). Like HIF1α (see above), it is induced by low oxygen levels, but while HIF1α has benign or beneficial effects on cartilage, HIF2α induces many catabolic enzymes such as MMPs 1, 3, 9 and 13 as well as a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) 4 and 5. HIF2α also inhibits protective autophagy and promotes chondrocyte apoptosis.53 Interestingly, chondrocytes in OA have been observed to resemble classically activated macrophages in that, for example, both cell types produce proinflammatory cytokines such as IL-1β and TNFα in a manner driven by the transcription factor NF-κB.57
In addition to the aforementioned proinflammatory and degradative changes in chondrocyte phenotype, several counteracting anti-inflammatory and reparative changes are also observed in OA. Insulin-like growth factor (IGF-1) promotes chondrocyte differentiation and survival, and it also simultaneously increases ECM component production while opposing procatabolic signals. Even though the levels of this important anabolic cytokine are normal or even increased in OA, the responsiveness of chondrocytes to it seems to be diminished. This has been hypothesized to be due to increasing presence of reactive oxygen species, which alters IGF-1- triggered signalling cascades.58
The role of FGF family, especially FGF2, has also been extensively studied in OA. FGF2 is stored in the cartilage matrix from which it is released by mechanical injury or loading. It strongly promotes proliferation in adult chondrocytes, but its effects on ECM production are less clear: Both positive and negative effects have been reported. This may be due to different FGF receptors (FGFRs) utilized by FGF2 having different effects in chondrocytes: FGFR3 activation appears to be chondroprotective and FGFR1 activation deleterious. Interestingly, FGFR3/FGFR1 ratio has been shown to be reduced in OA, potentially shifting the balance between the anabolic and catabolic effects of FGF2 into the catabolic direction. FGF18 is another member of the FGF family whose anabolic effects on chondrocytes have attracted interest regarding OA pathophysiology and treatment.3 Recombinant FGF18, known as sprifermin, is currently under investigation as a potential disease-modifying drug in OA.59
Another important anabolic pathway in the cartilage is TGFβ/BMP signalling. TGF-β isoforms have several anti-inflammatory and anticatabolic effects in chondrocytes: TGF-β1 downregulates MMP-1 and MMP13 as well as IL-1β and TNF receptors, while TGF-β2 reduces the degradation of type II collagen. BMPs also stimulate chondrocyte differentiation and ECM production.3 A member of the BMP family that has recently gained increased attention is growth differentiation factor 5 (GDF5, also known as BMP-14). GDF5 regulates skeletal development and chondrocyte differentiation, and polymorphisms in this gene have been linked to OA. Recombinant GDF5 and compounds that increase GDF5 signalling are studied as potential therapeutic strategies in OA.60
In recent years, Single-cell RNA sequencing has allowed the study of chondrocyte phenotypes in unprecedented detail. Studies investigating chondrocytes in OA cartilage have identified distinct subpopulations of these cells. These subpopulations include preinflammatory and inflammatory chondrocytes as well as prehypertrophic and hypertrophic chondrocytes, which are thought to play a central role in OA-related changes in cartilage composition.5, 61 Cytokines produced by synovial cells differentially affect carious chondrocyte phenotypes, modulating cartilage physiology and pathological processes.62 An interesting recent finding is the identification of the “prefibrocartilage chondrocyte” subpopulation. This subpopulation might allow the stratification of OA patients into (inflammatory and noninflammatory) subtypes, potentially facilitating more targeted therapies in the future.4
3.2 Effects of the central TH1/TH2/TH17 cytokines on chondrocyte phenotype
The framework of macrophage polarization was modelled after that of TH cells, and it has proven to be a useful concept for understanding the roles of macrophages in various physiological and pathological states. As chondrocytes are also characterized by great phenotypic heterogeneity, and there are similarities between classically activated macrophages and chondrocytes from OA joints, an intriguing question is whether chondrocytes can also adopt “polarized” phenotypes analogous to those of TH cells and macrophages.
In a recent study by the authors, chondrocytes were found to assume distinct phenotypes when treated with the central TH1/TH2/TH17 cytokines.63 The chondrocyte phenotype induced by IL-1β [C(IL-1β) phenotype] was found to be characterized by a widespread, marked upregulation of proinflammatory and catabolic genes. A smaller set of proinflammatory and chemotactic factors was induced by IL-17 [C(IL-17) phenotype]. The C(IFNγ) phenotype induced by IFNγ (a mainly TH1-type cytokine) was quite distinct from the C(IL-1β) and C(IL-17) phenotypes; IFNγ upregulated many genes involved in antigen processing and presentation.63 Chondrocytes are not “classic” antigen-presenting cells, but there is some evidence that they can present cartilage proteoglycans as antigens to CD8+ T cells, which potentially contributes to joint inflammation in arthritis.64 Effects of the TH2 cytokine IL-4 on chondrocyte phenotype [C(IL-4) phenotype] were much more limited but included the upregulation of some inflammation-regulating genes, while several downregulated genes were linked to cell proliferation and migration as well as to TGFβ signalling.63
3.3 Effects of medications on chondrocyte phenotype
Intra-articular glucocorticoid injections are widely used to treat inflammatory OA exacerbations and other types of arthritis, and a recent study by the authors65 and few others66, 67 have investigated the effects of glucocorticoids on the transcriptome of chondrocytes from OA patients. In these studies, glucocorticoids were found to affect (mostly downregulate) several genes associated with inflammation such as COX-2, IL-6 and CCL2/MCP-1. Many ECM-degrading enzymes (MMPs 1, 13 and 16), but conversely also various collagens and anabolic factors, are also downregulated by glucocorticoids.65 There are hints that glucocorticoids increase oxidative stress in chondrocytes,66 but they also seem to induce antioxidative and cytoprotective genes such as FOXO3 and SOD2.65 Another effect of dexamethasone that may be very relevant in OA is the downregulation of NGF,65 a central mediator of OA pain.68 Monoclonal antibodies targeting NGF have been shown to be effective in treating OA pain, but they may also accelerate cartilage degradation in a subset of patients.69 Whether glucocorticoid-induced NGF downregulation might have such harmful effects remains to be studied. In addition to NGF, dexamethasone also reduces the expression of cyclo-oxygenase 2 (COX-2) and vascular endothelial growth factor alpha (VEGFA).65 The prostaglandin products of COX-2 (particularly PGE2) and VEGFA mediate OA pain.70 Thus, their downregulation by glucocorticoids is likely to contribute to the analgesic effects of these medications in OA. In a study by the authors, dexamethasone was found to partially normalize gene expression in OA chondrocytes, shifting it from that observed in degraded OA cartilage towards preserved cartilage.65
Like glucocorticoids, NSAIDs are widely used in arthritis. They inhibit pain and inflammation by reducing the synthesis of prostanoids (especially PGE2) by COX enzymes. COX-2 activity and PGE2 production has been shown to be increased in chondrocytes from OA joints, and PGE2 may modulate the degradation of cartilage proteoglycans.71 These findings suggest that COX-2 inhibition might “normalize” chondrocyte phenotype in OA. This is supported by the finding that the NSAID ibuprofen decreases markers of joint tissue turnover in knee OA.72 In contrast to these potential benefits, some studies have implicated NSAIDs in accelerating radiological OA progression. These studies have, however, been criticized for, for example, not controlling for the severity of joint pain as a potential confounder.73
In a study by the authors,74 ibuprofen alone (in the absence of added OA-mediating cytokines) had no statistically significant effects (after appropriate multiple testing correction) on gene expression in chondrocytes from OA patients. This supports the belief that ibuprofen has no overt harmful effects on chondrocyte phenotype. When the cells were stimulated with IL-1β to mimic the low-grade inflammation present in OA joints, ibuprofen displayed nuanced effects on chondrocyte gene expression: Several anti-inflammatory genes, but also some proinflammatory factors, were among the upregulated genes. Several proinflammatory factors (such as IL-6 and IL-23) were among the most strongly downregulated genes. Interestingly, ibuprofen upregulated peroxisome proliferator activated receptor gamma (PPARγ) and its coactivator 1 beta (PPARGC1B).74 The expression of PPARγ has been shown to be reduced in OA cartilage, and its presence may influence OA pathogenesis by decreasing joint inflammation, downregulating catabolic enzymes and inhibiting chondrocyte apoptosis.75 In functional analysis, integrin signalling was found to be activated by ibuprofen.74 This is interesting, as dysregulated integrin signalling may play a role in OA pathogenesis.76 Integrins upregulated by ibuprofen include ITGA5, ITGB2 and ITGB3,74 which have been found to be linked to OA. ITGA5 (as a part of the α5β1 fibronectin receptor) promotes chondrocyte survival.77 On the other hand, ITGB2 expression in the synovium has been shown to correlate with OA severity,78 and ITGB3 (as part of the αVβ3 dimer) has been reported to promote inflammation and cartilage degradation.79 Thus, integrin-related effects of ibuprofen on cartilage and OA pathophysiology are likely to be complex.
Ibuprofen also activated MAPK/ERK signalling,74 and some of the potentially beneficial effects of ibuprofen on chondrocytes (reduction of apoptosis and prevention of dedifferentiation) have been linked to ERK signalling instead of COX-2/PGE2.80 cAMP signalling was also upregulated by ibuprofen,74 and this pathway has been shown to have a central role in chondrocyte differentiation.81 On the other hand, phosphatase and tensin homologue (PTEN) signalling was inhibited by ibuprofen.74 PTEN has been shown to be upregulated in chondrocytes from OA patients, and it reduces ECM production. Blocking PTEN signalling may retard the development of OA-associated changes in cartilage,82 which suggests another potentially beneficial action of ibuprofen on OA pathogenesis. Interestingly, genes downregulated by ibuprofen included genes such as hyaluronan synthase 1 (HAS1) and stanniocalcin 1 (STC1),74 whose expression has been shown to be increased in inflamed OA synovium compared to noninflamed.83 This suggests that NSAIDs might, to some extent, “normalize” the phenotype of OA joint tissue under inflammatory conditions.
In addition to the aforementioned NSAIDs and glucocorticoids, the effects of some other medications on chondrocyte phenotype have been studied for potential therapeutic benefits in OA. For example, the immunosuppressive drug rapamycin has been shown to promote chondrogenic phenotype and to reduce proinflammatory gene expression in chondrocytes.84 Intriguingly, some observational studies show that the use metformin, which affects both macrophage (see above) and chondrocyte85 phenotypes, is linked with reduced development of OA both alone86 and in combination with COX-2 inhibitors.87 Direct targeting of cytokines affecting chondrocyte (or macrophage) phenotype via biological agents would be a logical avenue for OA treatment, and medications such as IL-1 and TNFα inhibitors have been widely studied in this context; however, the results have generally been disappointing. This has been hypothesized to be due to the clinical/phenotypic heterogeneity of OA, and some subpopulations have shown more encouraging results.88 Thus, better understanding of OA pathophysiology, including the roles of different macrophage and chondrocyte phenotypes, can be expected to increase the changes of success for developing new therapies.
4 CONCLUSIONS
Macrophages, as “master regulators” of inflammation, play a central role in both development and amelioration of various inflammatory and fibrotic states. They can assume different pro- and anti-inflammatory phenotypes depending on the signals received from the environment, and these phenotypes are affected by various endogenous and exogenous factors. Recent studies indicate that like macrophages, chondrocytes also undergo phenotypic changes in the setting of OA and other arthritides, and they can adopt pro- and anti-inflammatory phenotypes induced by the central TH1/TH2/TH17 cytokines. Furthermore, medications commonly used in the treatment of OA have widespread effects on chondrocyte phenotype (Figure 2). Elucidating the mechanisms governing macrophage and chondrocyte phenotypes, and the effects of medications on them, offers promising avenues for therapeutic interventions in various inflammatory and fibrotic diseases.
ACKNOWLEDGEMENTS
This study was supported by grants from The Academy of Finland, Finnish Society of Rheumatology, Tampere Rheumatism Foundation, the competitive research funding (VTR funding) of Tampere University Hospital and the Scandinavian Rheumatology Research Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




