Mechanisms of resistance to daratumumab in patients with multiple myeloma
Funding information: None.
Abstract
Multiple myeloma (MM) is an incurable cancer in the bone marrow. The treatment of MM has developed significantly during the last 20 years, which has resulted in increased survival. Daratumumab is the first CD38 antibody approved for the treatment of MM. It has improved the treatment of MM even further. This is an evaluation of the modes of action of daratumumab and a description of the development of resistance with a focus on inhibitory checkpoint receptors on CD8+ T-cells, complement activation and extracellular vesicles.
Plain English Summary
Multiple myeloma (MM) is an incurable cancer in the bone marrow. The treatment of MM has developed significantly during the last 20 years, which has resulted in increased survival. Chemotherapy dominated the treatment landscape two decades ago, but it has been outpaced by immunotherapy. Daratumumab is the first CD38 antibody approved for treatment of MM. It has improved the treatment of MM even further. This is an evaluation of the modes of action of daratumumab and a description of the development of resistance with focus on how daratumumab interacts with different parts of the immune system.
1 MULTIPLE MYELOMA
Multiple myeloma (MM) is an incurable malignancy of the B-cell lineage, characterized by neoplastic, monoclonal expansion of plasma cells (PCs) in the bone marrow (BM), which may cause anaemia, osteolytic lesions of the bones, hypercalcemia and renal failure also known as CRAB criteria.1 MM accounts for 10% of all hematologic cancers, which makes it the second most prevalent hematologic malignant disorder after non-Hodgkin's lymphomas. The mean age at diagnosis is 69 years, and the median overall survival (OS) is just above 4 years.2
1.1 TREATMENT OF MULTIPLE MYELOMA
Since MM is an incurable disease, the main purpose of treatment is to give the patients deep and durable responses with as few side effects as possible. The typical disease course consists of remissions and relapses. Generally, with increasing lines of therapy, the more superficial is the response, and the shorter is the duration of the remission.2, 3 The following types of drugs constitute the backbone of MM therapy: immunomodulatory drugs (IMIDs): thalidomide, lenalidomide and pomalidomide; proteasome inhibitors (PIs): bortezomib, carfilzomib and ixazomib; alkylating drugs: melphalan and cyclophosphamide; corticosteroids: dexamethasone and prednisone; and monoclonal antibodies targeting CD38: daratumumab (DARA) and isatuximab or SLAM-F7: elotuzumab. Very recently, approved therapies encompass B-cell maturation antigen (BCMA)-targeting approaches such as Belantamab Mafodotin, bispecific antibodies, chimeric antigen receptor (CAR) T-cells and a whole new range of active therapies are in the pipeline.
The initial treatment is dependent on the age and co-morbidities of the patient. For young (≤70 years) patients without severe co-morbidities, an induction regimen followed by high-dose treatment (HDT) and autologous stem cell treatment (ASCT) is the standard first line. A typical induction regimen consists of 4 cycles of lenalidomide, bortezomib and dexamethasone.4 For older (>70 years) patients, the initial treatment has recently been changed to DARA, lenalidomide and dexamethasone due to convincing results from the MAIA study.5 Most patients with MM will eventually relapse and be in need of a second line of therapy.2 Treatment for relapse is less standardized and more tailored to the individual patient taking into account the former treatment, response to treatment, duration of response and co-morbidities. In Denmark, most patients are treated with DARA in combination with dexamethasone and lenalidomide or bortezomib in their second line of treatment.6
2 DARATUMUMAB
2.1 Modes of action
DARA is a human immunoglobulin G1 (IgG1) kappa monoclonal antibody (mAb) that targets CD38. CD38 is a 45-kDa type II transmembrane glycoprotein acting as both a receptor and an ectoenzyme. It is highly expressed on PCs—and especially on malignant PCs (MM cells), and to a lesser extent on red blood cells, platelets, natural killer (NK) cells, a subset of B and T cells, and numerous non-haematological tissues such as airway epithelium, smooth muscle cells, striated-muscle cells including heart tissue, central and peripheral nerve tissue, glial cells, osteoclasts and endocrine cells of the pancreas.7
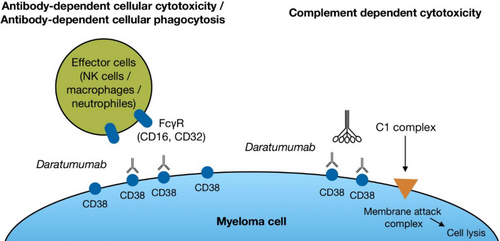
Three different effector mechanisms seem to be essential for the direct killing of malignant PCs by DARA: antibody-dependent cellular phagocytosis (ADCP), where the Fc region of DARA bound to CD38 (the “tumour antigen”) on MM cells, reacts with the Fc receptor on effector cells, for example, macrophages (Figure 1). This induces phagocytosis of the MM cell. If the antigen-bound DARA reacts with the Fc receptor on an NK-cell or T-/NK-cell, they will release perforin and GrB, which will result in cytolysis of the MM cell termed antibody-dependent cellular cytotoxicity (ADCC). In the preclinical studies, it seems that especially complement-dependent cytotoxicity (CDC) is important for MM cell lysis.8 During complement activation, the complement protein C1q binds to the Fc region of DARA attached to CD38. Each Fc region has a single binding site for C1q, and each C1q molecule must bind to at least two DARA Fc regions to become activated. Since each DARA molecule has only one Fc region, multiple DARA molecules must be brought together to initiate the process of complement activation.8, 9 An in vitro comparison of DARA and three other CD38 mAbs indicates that DARA is the most efficient activator of CDC.10
Besides these abilities to directly kill tumour cells, DARA affects the immune system through several different modes of action. Studies of patient material collected during clinical trials where DARA was given as monotherapy showed that DARA may eliminate CD38+ populations of regulatory T-cells, B-cells and monocytes/macrophages that impose a break on the cytotoxic T-cells.11 Consequently, cytotoxic T-cells proliferate and become activated following treatment with DARA. This immune response correlates with the clinical response to the treatment. Furthermore, DARA inhibits MM cell adhesion to the BM stromal cells (BMSCs) via CD38 internalization through the endocytic machinery rendering the myeloma cells more sensitive to concomitant therapy.12 Recently, another function of CD38 was identified. Marlein et al. showed that CD38-dependent tumour-derived tunnelling nanotubes (TNTs) could be established between BMSCs and MM cells. Mitochondrial transfer via these TNT is a method for the MM cell to provide energy for further tumour growth. This transfer was dependent on CD38 and was significantly decreased when using a CD38 blocking antibody or “knock down” of CD38 in vitro.13
3 MECHANISM OF RESISTANCE TO DARATUMUMAB
DARA has improved the treatment of MM significantly and improved both progression free survival (PFS), overall response rate (ORR), minimal residual disease (MRD) negativity rates and OS.14, 15 Nevertheless, even though the clinical benefits of DARA are well documented, a recent evaluation of all Danish MM patients, who have received DARA before 2019, showed that the majority of patients eventually relapse on DARA and that the median OS after progression is only approximately 12 months.16 These findings highlight a need to examine the reasons for the failure of DARA therapy in order to improve the outcome. In this article, resistance is defined as the progression of disease during treatment with DARA according to standard guidelines.17 Resistance can be either primary, that is, a formal response has never been obtained, or acquired, that is, a response has been obtained and later during the treatment a progression has evolved.
3.1 The complement system
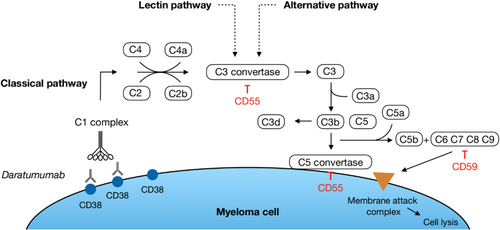
The complement system is a part of the humoral immunity. Activation of the complement system results in the formation of the membrane attack complex (MAC) on the surface of the target cell (Figure 2). The MAC forms pores in the plasma membrane, which results in osmotic swelling and rupture of the target cell. The plasma level of the complement degradation and activation products, for example, C3a, C3d, C5a and C5b-9, reflects the extent of complement activation.18-20 Normal cells have several complement inhibitory proteins (CIPs) on the cell surface to protect them from the effects of complement. CD59 is an important CIP, which blocks the final formation of the MAC and thereby inhibits the function of this element of the complement system. CD55 accelerates the decay of C3- and C5-convertases (Figure 2). Analysis of samples collected during monotherapy with DARA has shown an increased level of the CIPs CD55 and CD59 on the myeloma cells at the time of loss of response and disease progression.21 Thus, inhibition of CDC may be a cause of the development of resistance to DARA.
Preclinical studies have revealed that both CDC and ADCC are more effective when the expression of CD38 is high, but immediately following the first infusion with DARA, there is a pronounced reduction of the expression of CD38 on the remaining MM cells.9, 21, 22 In vitro studies showed that all-trans retinoic acid (ATRA) improves the DARA-mediated CDC and ADCC by upregulation of CD38 on MM cells including MM cells, which were resistant to DARA monotherapy.22 This points towards a potential clinical advantage of a high expression of CD38 on myeloma cells. Indeed, a high expression of CD38 before initiation of DARA is a predictor of obtaining a partial response or better.21 Based on these observations, a phase 1/2 study combining ATRA with DARA in DARA-refractory patients was initiated.23 Despite the promising in vitro experiences, the results of the clinical study were disappointing. The ORR was only 5% and the mean PFS was 2.8 months. Panobinostat can also upregulate the expression of CD38 by myeloma cells and enhance the anti-myeloma effect of DARA in vitro but given the disappointing clinical results with ATRA it is unlikely that this avenue will be pursued further.24
The fact that CD38 expression by MM cells is downregulated immediately after initiation of treatment with DARA but a response may be maintained over many months or even years suggests that other modes of action of DARA than CDC, ADCC and ADCP are important to obtain a long term response.21 The long-term effects of DARA may be more dependent on its immunomodulatory effects (reduced production of immunosuppressive adenosine, elimination of CD38 positive regulatory cells) or the inhibition of formation of nanotubes and transfer of mitochondria from stromal cells to the MM cells. Thus, low CD38 expression by myeloma cells may in fact be beneficial to maintain control of the disease and one may indeed envision CD38 as a growth and survival factor for MM cells. A clinical aspect of this discussion is whether the treatment with DARA should continue with a change of partner drug despite progression on a DARA-containing regimen, or whether treatment with DARA should be paused, so the MM cells regain the CD38 expression, which takes about 6 months.21 Recently, Szabo and colleagues did a retrospective, nationwide evaluation of all MM patients in Denmark receiving DARA before 2019. The course of therapy for 474 patients was evaluated, and the OS from the time point where each patient progressed on DARA for the first time was determined. They found that the median OS for patients continuing to receive DARA but combined with another anti-myeloma therapy than before the relapse was 23.6 months, and the OS for patients not receiving DARA after progressing on DARA was 11.3 months. The difference was clinically significant in a multivariate analysis counting for age, cytogenetic risk and time since diagnoses among others.25 This points towards the benefit of continuing treatment with DARA without interruption and suggests that the low CD38 expression by MM cells imposed by DARA is compatible with and may in fact support a continued clinical response.
In the clinical setting complement activation caused by DARA has only been measured during the first 8 weeks of treatment and only by measuring native complement factors, among these C2.21 The results show that there is a decrease of the native complement factors after initiating DARA therapy, but the signal disappears after a few weeks of treatment, indicating that the complement system is mainly active during these first weeks or that an increased production of complement factors compensate for the consumption. In an attempt to make CDC even more potent, a new CD38 mAb is under development.26 This CD38 mAb called GEN3014 has a hexamerization-enhancing mutation, which increases the binding of C1q and thereby enhances the activation of the complement cascade.27 The data from the first human trials of DARA and GEN3014 confirms this assumption. There was a much more profound decrease in C2 in patients treated with GEN3014 than in patients treated with DARA.21, 26 To explore the impact of complement activation during long-term treatment with DARA, the complement split product C3d was measured in the PB of patients who had received DARA for a median of a minimum of 300 days. The level of C3d in patients receiving DARA that had obtained a partial response or better was compared to patients progressing on DARA. There were no significant differences between the two groups. This supports the hypothesis that CDC is of less importance for the long-term response to DARA.
3.2 T CELLS
The MM BM microenvironment seems to be in an immunosuppressive state and both B- and T-cells show significant impairment of their functions, possibly due to an increased concentration of adenosine in the BM.28 Such impairment is reflected by higher expression of markers of T-cell exhaustion such as programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte associated protein-4 (CTLA-4).29 Several checkpoint inhibitors that block PD-1, programmed cell death ligand 1 (PD-L1) or CTLA-4 have been approved for solid cancers but, so far, checkpoint inhibitors have not shown convincing efficacy in MM.30 T-cell immunoglobulin and ITIM domains (TIGIT) is an inhibitory checkpoint molecule highly expressed by CD8+ T-cell from MM patients and may play a more important role in the inhibition of the T-cell response against MM.31
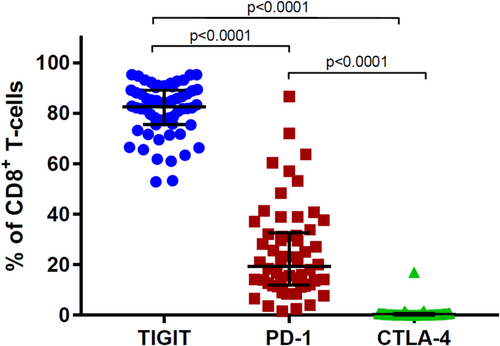
When initiating treatment with DARA, the number of CD4+CD25+CD127dim Tregs declines immediately. These Tregs are also CD38+ and therefore sensitive to DARA. This decline is followed by an expansion of CD8+ T cells and an increase in the ratios of CD8+:CD4+ and CD8+:Tregs.11 These immunomodulatory changes are more pronounced in patients with a good, clinical response to DARA. Whether exhaustion of T-cells plays a role in the development of resistance to DARA is not fully clarified, but adding durvalumab, a PD-L1 inhibitor, to DARA in DARA-refractory patients did not induce a clinical response.32 Iversen et al. examined the expression of checkpoint molecules on CD8+ T-cells isolated from the BM of newly diagnosed MM patients (NDMM) and patients relapsing on DARA.33 They found no difference in the expression of PD-1 on CD8+ T-cells in DARA-refractory patients compared to NDMM patients. Furthermore, PD-1 was only expressed in around 20% of CD8+ T-cells from MM patients, whereas TIGIT was present in around 80% (Figure 3). Paiva et al. confirmed the observation on the percentage of PD-1 expressing CD8+ T-cells.34 Two studies confirm that TIGIT is more frequently expressed by CD8+ T-cells in myeloma patients than PD-1.31, 35 Altogether, it seems that PD-1 is not a key immune regulatory checkpoint in MM. Iversen et al. did not find a difference in the expression of TIGIT on CD8+ T-cells when comparing NDMM to DARA-refractory patients, which was supported by the findings by Guillerey.31 Neri et al. found a higher expression of TIGIT on T-cells from DARA-resistant patients compared to responders.36 Therefore, it seems that there is a difference in the expression of TIGIT on T-cells when comparing DARA-refractory patients to patients currently in clinical response, but not when comparing DARA-refractory patients to NDMM. Anti-TIGIT mAbs are currently being tested in clinical trials of patients with relapsed MM.
3.3 NK CELLS
Prior studies have shown that the number of NK cells decreases after initiation of DARA and that this decrease is more pronounced in the CD56dim subset.37, 38 Furthermore, it has been shown that the expression of CD16, the receptor necessary for initiating ADCC, by the remaining NK cells is low.39 After the decrease in CD38+ NK cells, there is an expansion of CD38− NK cells. This expansion is more pronounced in responders compared to progressors.40 Patients with disease primary refractory to DARA had NK cells with an exhausted phenotype even prior to the first treatment.41 This NK cell depletion and dysfunction could be involved in both primary and acquired resistance to DARA.
We confirm that the number of circulating NK cells decreases in patients treated with DARA and that this decrease is more pronounced in the CD56dim subset (data not yet published). Furthermore, a higher percentage of these CD56dim NK cells lacks the activation marker CD16 necessary for ADCC. Notably, NK cells from patients relapsing on DARA appeared to be in an exhausted state with a lower expression of the stimulatory checkpoint receptor DNAM-1 and a higher expression of the inhibitory receptor TIGIT compared to NK cells from DARA-naïve MM patients. This difference was even more pronounced in the CD56dim subset of the NK cells.
3.4 EXTRACELLULAR VESICLES
Extracellular vesicles (EVs) are membrane-bound organelles that are released from all cells to the extracellular space and biological fluids, for example, blood. They can contain molecular cargo (e.g., RNA, protein and metabolites) and carry cell-specific markers on their surface, which unveils their cell of origin. In recent years, the focus on EVs and their role in oncogenesis, metastatic disease and resistance to cancer therapy has expanded.42 Caivano et al. showed that many patients with haematological malignancies, including MM, had a significantly higher amount of EVs in peripheral blood (PB) compared to healthy individuals. Most EVs from the haematological patients expressed a cancer antigen specific for the individual disease on their surface. EVs from MM patients expressed CD38.43 The MM BM microenvironment is a complex network of several different cell types, and EVs may contribute to the cross talk between malignant and non-malignant cells. Multiple studies in different types of cancer suggest that EVs may play a role in drug resistance by several different mechanisms. For example by suppression of immune cells, by transferring drug-efflux pumps from drug-resistant cells to drug-sensitive cells, or by binding therapeutic mAbs in the circulation and thereby preventing them from reaching their target.44 In vivo studies have revealed that lenalidomide resistance can be transmitted from resistant to non-resistant MM cells via EVs.45 Breast carcinoma cells, which express human epidermal growth factor receptor 2 (HER2), can be treated with trastuzumab, a mAb like DARA. These carcinoma cells secrete EVs expressing HER2, which then binds trastuzumab off target and inhibits the interaction of trastuzumab with the tumour cells.46 The same process of capturing and neutralizing a therapeutic antibody has been observed with rituximab in lymphoma.47 In line with the findings of HER2-expressing cells in breast cancer and CD20-expressing cells in lymphomas, an in vitro study has revealed that DARA-treated MM cell lines secrete EVs expressing a CD38/DARA complex.48 Likewise, EVs isolated from MM patients receiving DARA express CD38.49 Brennan et al. performed mass spectrometry on EVs from PB of 10 DARA treated patients (5 responding to DARA and 5 progressing on DARA) and 10 untreated healthy control EV samples. The majority of peptides identified in both the DARA-treated MM EVs and healthy control EVs matched the DARA sequence. This is due to the fact that DARA is a fully human IgG. However, in 9 out of 10 patients treated with DARA, they found EVs containing a peptide sequence that was not detected in the 10 control samples, with several MM patients having multiple DARA-specific peptides. This finding supports the hypothesis that DARA is present on EVs from patients treated with DARA and thus bound off target. Whether the binding of DARA off target contributes to the development of resistance is not known. In addition, the expression of the CIPs CD55 and CD59 was higher on EVs isolated from DARA-treated MM patients compared to healthy controls.49 In combination with the high amounts of CD55 and CD59 on the plasma membrane of the myeloma cells this may inhibit CDC mediated by DARA.21
4 CONCLUSION
DARA has improved the treatment of myeloma patients significantly, but patients relapsing on DARA is still a clinical challenge. It seems that a high expression of CD38 on malignant plasma is essential for the initial response, but might be a disadvantage for the long-term response. The immune checkpoint receptor TIGIT is highly expressed in CD8+ T-cells from myeloma patients but has not proven its importance in the clinical setting yet. The number of circulating CD38+ NK cells decreases after initiation of DARA, and the remaining NK cells seem to be in an exhausted state, at least in progressors. Whether exhaustion of T-cells and/or NK cells is important for the development of resistance to DARA is still not known. Furthermore, the formation of circulating EVs binding DARA off target could potentially contribute to this development. Further research is needed.
ACKNOWLEDGEMENTS
This focused review is based on the PhD thesis on resistance to daratumumab in patients with multiple myeloma.50
CONFLICT OF INTEREST STATEMENT
The author declares no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.