Regulation of mitochondrial dysfunction by estrogens and estrogen receptors in Alzheimer's disease: A focused review
[Correction added on 7 June 2024, after first online publication: ORCID ID of author Albert Gjedde has been added.]
Abstract
Alzheimer's disease (AD) is a neurodegenerative disorder that primarily manifests itself by progressive memory loss and cognitive decline, thus significantly affecting memory functions and quality of life. In this review, we proceed from the understanding that the canonical amyloid-β hypothesis, while significant, has faced setbacks, highlighting the need to adopt a broader perspective considering the intricate interplay of diverse pathological pathways for effective AD treatments. Sex differences in AD offer valuable insights into a better understanding of its pathophysiology. Fluctuation of the levels of ovarian sex hormones during perimenopause is associated with changes in glucose metabolism, as a possible window of opportunity to further understand the roles of sex steroid hormones and their associated receptors in the pathophysiology of AD. We review these dimensions, emphasizing the potential of estrogen receptors (ERs) to reveal mitochondrial functions in the search for further research and therapeutic strategies for AD pharmacotherapy. Understanding and addressing the intricate interactions of mitochondrial dysfunction and ERs potentially pave the way for more effective approaches to AD therapy.
1 INTRODUCTION
Alzheimer's disease (AD), the most common cause of dementia, is a neurodegenerative disorder mainly characterized by progressive memory loss and functional impairment in at least one additional cognitive domain. The main risk factor of AD is age; therefore, as the population ages, the prevalence of AD is expected drastically to rise.1 AD manifests the impairment in different cognitive domains and, as a result, not only affects the individual's quality of life but also imposes considerable burdens and emotional toll on families, caregivers and healthcare systems. Lost productivity and the need for long-term care also introduce additional economic burdens.
Besides the loss of neurons and synapses, the pathological hallmark of AD is the presence of extracellular amyloid-β (Aβ) plaques and intracellular tau-neurofibrillary tangles (NFTs) that provided the foci of therapeutic interventions for the past few decades. However, many therapeutic approaches targeting this system have faced setbacks, causing scepticism to arise about the Aβ hypothesis.2 The inconsistent outcomes observed with the recently developed anti-amyloid antibody aducanumab, with modest clinical effects even at the highest dose (the clinical efficacy of lecanemab is yet to be established), along with the realization that AD is a multifaceted disease with a complex interplay of a multitude of pathophysiological processes underscore the shortcomings of the canonical Aβ hypothesis that appears to be of limited therapeutic usefulness. A broader perspective of multiple pathological pathways is needed to advance the testing of more effective treatments for AD.
Sex differences in AD offer increased insights into the pathophysiology of dementia.3 The prevalence of AD is two-to-three times higher in women than in men after the age of 65.4 Moreover, sex-related differences are evident in the symptomatology, laboratory data and clinical manifestations of AD. For instance, women exhibit higher rates of cognitive decline with a higher rate of brain atrophy after diagnosis of mild cognitive impairment (MCI).5 The difference is attributable to a wide range of factors that include but are not limited to sex hormones and genetic profiles. In women, perimenopause is a window of time that evinces some neurological alterations, with the potential to predispose individuals to neurodegenerative disease.6 In this window, the circulating estrogen levels vary and coincide with a decline in the brain's glucose metabolic rate.6
Glucose metabolism is closely linked to mitochondrial function to maintain cellular energy required to support neural and glial cell activity. Mounting evidence supported the rise of a mitochondrial cascade hypothesis, according to which the mitochondrial dysfunction drives the pathogenesis of AD.7 Mitochondria play a crucial role in maintaining optimal neuronal activity, and their dysfunction is implicated in the progression of AD. As mentioned, women exhibit a higher likelihood of developing the disease after the age of 65. Hormonal influences, particularly related to estrogen, are considered pivotal in this context. Estrogen and its receptors are associated with neuroprotective effects, and fluctuations in estrogen levels during the perimenopausal period coincide with increased susceptibility to AD in women. The relevance of studying estrogen receptors (ERs) could also lie in their potential influence on mitochondrial function. Despite existing studies demonstrating this impact, the need for further exploration into the intricate interplay between ERs and mitochondria in AD is emphasized, especially considering that most current studies are outdated. In this focused review, we touch upon the current hypotheses of the pathophysiology of AD, with a particular emphasis on mitochondrial dysfunction. We then delve into the role of ERs in modulating mitochondrial dysfunction, and we further elaborate on the potential for targeting this system to explore novel avenues for AD research.
2 PATHOPHYSIOLOGY OF AD IN A NUTSHELL
2.1 The original amyloid hypothesis of AD
The original amyloid hypothesis of AD originated from the discovery of Aβ plaques in the cerebral cortex of patients with AD in 1984.8 Aβ is a peptide produced by cleavage of the amyloid-β precursor protein (APP).8 This cleavage proceeds in two ways: a non-amyloidogenic pathway initiated by α-secretase or an amyloidogenic pathway initiated by β-secretase.8 The amyloidogenic pathway leads to the production of senile plaques composed of extracellular oligomers of Aβ. In the course of the splicing, β-secretase cleaves the extracellular domain of APP, producing the membrane-bound carboxyl-terminal fragment CTF99 that subsequently undergoes cleavage of its transmembrane domain by γ-secretase, producing the 40-42 residue Aβ peptide.8 The Aβ peptides then aggregate to form oligomers and extracellular senile plaques through a process involving apolipoproteins and proteoglycans.8
The possible neurotoxicity of Aβ is commonly held to be due to multiple subsequent processes. For instance, extracellular Aβ oligomers may activate cell surface “death” receptors that trigger caspase-mediated apoptotic pathways.1 Intracellular accumulation of Aβ may stress functions of endoplasmic reticulum and mitochondria, leading to caspase activation.8 According to Shi et al., senile plaques also activate glial cells, causing neuroinflammation and neuronal degeneration.1 Furthermore, it is possible that Aβ can initiate the formation of intracellular NFTs of hyperphosphorylated τ-protein, which are themselves neurotoxic.8 Further consequences of Aβ production will appear in the sections below.
2.2 The cholinergic hypothesis of AD
The proper functioning of the cholinergic system is crucial to the integrity of many processes known to be impaired in AD, including memory, learning, attention, sleep and plasticity.8, 9 This observation led to the cholinergic hypothesis of AD.2
Numerous findings suggest that deficits in cholinergic neurotransmission are central to the pathophysiology of AD. Inhibition of muscarinic receptor function impairs memory.9 Acetylcholine (ACh) production is reduced in AD, and various drugs such as donepezil that increase the synaptic concentration of ACh by inhibiting acetylcholinesterase, the enzyme responsible for degrading synaptic ACh, are one of the main treatments used in AD to delay the decline in memory and cognition.9 Key elements of the cholinergic system show atrophic changes occurring before the manifestation of AD.9 Interestingly, an allosteric muscarinic and σ-1 agonist appears to lower the development of Aβ pathology, suggesting a potential role of the cholinergic system in the pathogenesis of AD.9
2.3 The τ hypothesis of AD
The τ-protein is a microtubule-associated protein (MAP)1 that exists in both axons and dendrites of neurons and regulates the stability and dynamics of microtubules.8 The τ-protein is phosphorylated by a diverse range of kinases, including protein kinase A (PKA). Phosphorylation of τ lowers its ability to interact with and stabilize microtubules but raises the protein's tendency to form aggregates.8, 10 We know hyperphosphorylated τ-aggregates as threads when they form in the axons or dendrites of neurons but as NFT when they form in the soma.8 Decreased interaction between τ and microtubules caused by hyperphosphorylation leads to microtubule disintegration, and together with threads and NFT, the disintegration disturbs cell signalling, dysregulates dendritic smooth endoplasmic reticulum (SER) Ca2+ dynamics and eventually leads to cell death.8, 10
2.4 The neuroinflammation hypothesis of AD
In addition to being characterized by Aβ, NFT and cholinergic deficits, the brains of AD patients also show signs of chronic inflammation11 that led to the neuroinflammation hypothesis of AD. A substantial body of evidence demonstrates complex bidirectional interactions between neuroinflammation and Aβ- and τ-protein pathologies.11 Specifically, neuroinflammation exacerbates Aβ- and τ-pathologies, while Aβ- and τ-pathologies promote neuroinflammation, thus establishing a positive feedback loop.11 Long-term use of anti-inflammatory treatments such as non-steroidal anti-inflammatory drugs (NSAIDs) in one study reduced the risk of developing AD by as much as 50%.5
Microglia play a crucial role in neuroinflammatory processes, and the presence of Aβ in AD prompts microglia to undergo conversion from a homeostatic surveillance phenotype to an activated pro-inflammatory state.11 Microglial activation, in turn, has a biphasic impact on AD. In the first phase, activated microglia phagocytose and degrade Aβ, counteracting disease progression. In the second phase, prolonged neuroinflammation is accompanied by a gradual loss of the ability of microglia to clear Aβ while the production of pro-inflammatory cytokines remains intact.11 The pro-inflammatory environment leads to the production of reactive oxygen species (ROS), nitric oxide (NO) and other substances associated with neuronal and glial degeneration.11 An R47H loss-of-function mutation in the innate immune receptor labelled Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) increases the risk of developing late onset AD by 2 to 4.5 times, making it the second strongest risk gene next to apolipoprotein E-ε4 (ApoE4).11 TREM2 dysfunction lowers the ability of microglia and macrophages to clear Aβ and other cellular components, leading to less dense and more neurotoxic fibrillary plaques and oligomeric Aβ proteins that may exacerbate τ-pathology.11
Concerning immune receptors, several immune-related cytokines play a central part in the pathophysiology of AD.11 For instance, increased levels of tumour necrosis factor alpha (TNF-α) receptors occur in the cerebrospinal fluid of MCI patients that reach the diagnosis of AD.11 Deletion of tumour necrosis factor receptor 1 (Tnfr1) reduces plaque load and improves the cognitive performance of transgenic mice.11 Interestingly, TNF-α signalling can lead to increased production of β-secretase and increased γ-secretase activity, which, in turn, aggravates Aβ pathology.11 IL-1β promotes Aβ pathology by increasing the production and secretion of APP by glial cells and directing APP processing towards the amyloidogenic pathway.11 IL-6 is elevated in AD, increases the production of APP and hyperphosphorylation of τ and predicts cognitive decline.11 Nuclear factor-κB (NFκB) increases the transcription of β-secretase, thus promoting Aβ production.12 Since NFκB also constitutes one of the pathways by which Aβ increases cytokine production, a positive feedback loop exists between NFκB, cytokine production and Aβ pathology.11 IL-10 expression is upregulated in AD, and IL-10 levels correlate with AD progression. IL-10 may inhibit the beneficial functions of microglia in AD.11 IL-10 knockout mice show a reduction in Aβ deposition and reduced synaptic loss.11 The cytokine transforming growth factor beta 1 (TGF-β1) has neuroprotective actions in AD, including decreased production and deposition of Aβ, protection of neurons from Aβ-induced damage and a general reduction of neuroinflammation.11 Furthermore, TGF-β1 inhibits the phosphorylation of τ and increases the expression of anti-apoptotic proteins.11 TGF-β1 mRNA expression is negatively correlated with the concentration of NFT, suggesting protective properties of TGF-β1 against τ-pathology. In support of this possibility, TGF-β1 addition rescued memory impairment in relation to an intracerebroventricular Aβ challenge.11
2.5 The calcium hypothesis of AD
The calcium hypothesis postulates that neurons of brains with AD are subject to a dysregulation of Ca2+ in- and efflux with a net increase of cytoplasmic [Ca2+].13 The increase, in turn, affects the processing of APP and promotes the production of Aβ, the deposition of senile plaques and the tendency of τ to become hyperphosphorylated and to form NFTs. Conversely, we now know that Aβ oligomers, at least in part, are responsible for the increase in cytoplasmic [Ca2+] by modulation of Ca2+ transport at the plasma membrane and Ca2+ release from the endoplasmic reticulum.13 The modulation establishes a positive feedback loop between Aβ pathology and Ca2+ overload that aggravates disease progression.13 The disturbances of Ca2+ homeostasis occur before the onset of AD symptoms, suggesting that the disruptions may be involved in the early steps of AD progression.13 In fact, we now know that some of the genetic variations associated with increased risk of AD affect Ca2+ homeostasis.13
Aβ oligomers raise cytoplasmic [Ca2+] by upregulating and stimulating voltage-gated calcium channels (VGCCs), as well as forming calcium-permeable Aβ-pores in the membrane and changing the dielectric properties of the membrane, leading to increased permeability.13 The ability of glial cells to remove glutamate from the extracellular space is compromised in AD and raises the activation of AMPA- and NMDA-receptors, with an increased influx of calcium across neuronal membranes.13
The Aβ-induced increase of [Ca2+] in the cytoplasm may initiate a self-perpetuating process, as elevated levels of cytoplasmic Ca2+ appear to cause dysfunction of proteins involved in Ca2+ homeostasis, both at the plasma membrane and intracellular organelles.13 For instance, high [Ca2+] in cytoplasm leads to overexpression of the L-type calcium channel Cav 1.2, increasing Ca2+ influx.13 The resulting calcium overload is associated with a series of harmful effects. For example, high [Ca2+] levels in cytoplasm activate β-secretase with increased production of Aβ.13 High cytoplasmic Ca2+ levels are associated with increased ROS production and activation of calcineurin (CaN), a phosphatase involved in long-term depression (LTD), leading to loss of dendritic spines and synapses, resulting in cognitive impairment.13 Calcium-mediated activation of a diverse range of kinases may also raise τ-phosphorylation.13
Mitochondria are key facilitators of Ca2+ homeostasis.13 A major accomplishment of this function is the absorption of Ca2+ ions from the cytoplasm in situations of Ca2+ overload.13 However, if the calcium concentration increases too much at the mitochondrial matrix due to high cytoplasmic [Ca2+], it compromises mitochondrial function.13 The functional decline may cause problems regarding energy production and oxidative stress, in addition to more specifically harmful effects such as permeabilization of the outer mitochondrial membrane with subsequent release of pro-apoptotic factors, including cytochrome C and an apoptosis-inducing factor.13 This process ultimately leads to neuronal damage and cell death.13
As seen from the descriptions of the five hypotheses of the pathophysiology of AD given above, it is important to note that the different hypotheses by no means are mutually exclusive. Instead, Aβ- and τ-pathology, cholinergic dysfunction, neuroinflammation and Ca2+ dyshomeostasis are closely linked and mutually perpetuating. One of the key tasks is to identify the processes that are part of the initial pathogenetic events of AD and those that are mere consequences of the underlying pathology. This knowledge will be important in developing a potential cure that addresses the fundamental pathological process of AD.
3 MITOCHONDRIAL DYSFUNCTION IN THE PATHOPHYSIOLOGY OF AD
Mitochondria are dynamic cellular organelles essential for energy turnover and the resulting plethora of biosynthetic activities within the body. It is well established that mitochondria play a pivotal role in cellular bioenergetics, indispensable to energy conversion. The primary cellular role executed by mitochondria is the synthesis of adenosine triphosphate (ATP) through the operation of the electron transport chain.14 Mitochondria also play a crucial role in regulating Ca2+ homeostasis, participating in apoptosis and generating and eliminating ROS.15-17
The structural configuration of mitochondria is remarkably dynamic, and substantial variability exists in both the structural attributes and the proteomic composition across different cell types. The distinctions enable mitochondria to function in exceptionally adaptable manners, catering to the specific demands of each cell type. In alignment with the multifaceted roles, disruptions of mitochondrial function occur under the pathological conditions of disorders of metabolism,18 cardiovascular diseases,19 cancers20 and disorders intricately linked to neurodegenerative processes and aberrant cell death.21
Accumulating evidence suggests that deficient mitochondrial functions play pivotal roles in the onset and progression of AD.22, 23 Indeed, an alternative hypothesis of AD pathology, known as the “mitochondrial cascade,” posits that individuals who inherit mitochondrial genes associated with reduced rates of mitochondrial activity or increased rates of mitochondrial decline have an elevated risk of developing the disease.24 Considering that mitochondria are engaged in a diverse array of processes, encompassing apoptosis, neuronal cell death, oxidative stress and calcium homeostasis, all of which are observed in AD, it is not surprising that mitochondrial dysfunction may play a central role in the pathogenesis of AD. The next section provides a brief overview of the mitochondrial dysfunction observed in AD (Figure 1).
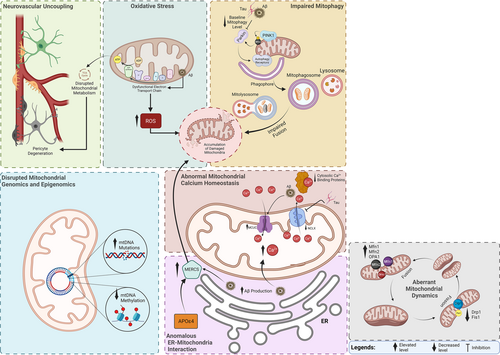
3.1 Aberrant mitochondrial structure and dynamics in AD
Studies consistently reveal changes in mitochondrial structure and dynamics in individuals affected by AD. Notably, variable mitochondrial morphology and distributions are evident in patients with AD. Generally, mitochondrial distribution in the cells affected by AD pathology is perinuclear, with few metabolic organelles located in the distal processes, where they usually reside in healthy cells and act in the service of exocytosis, ion channel pumping, synaptic function and other functions.25 Electron microscopy reveals that neurons within the mammillary bodies of humans with AD exhibit abnormally small mitochondria, with a substantial proportion displaying disrupted cristae.26 Morphological abnormalities similarly appear in the hypothalamic neurons of individuals with AD compared to normal individuals.27, 28 Alterations in mitochondrial morphology and distribution in fibroblasts from AD patients also present with elongated mitochondria in perinuclear areas in marked contrast to normal human fibroblasts.29
Apart from morphological alterations of mitochondria, aberrant mitochondrial dynamics also prevail in AD. Generally, mitochondria undergo dynamic cycles of fusion and fission, forming and deforming interconnected networks.30 Accordingly, mitochondrial fission generates two daughter mitochondria from the division of a single mitochondrion, whereas mitochondrial fusion is the merging of two mitochondria to create a single, larger mitochondrion. The processes are regulated by proteins belonging to the dynamin GTPase superfamily. Under physiological conditions, the fusion of mitochondrial outer and inner membranes relies on dynamin-related proteins mitofusins 1 and 2 (Mfn1 and Mfn2), situated in the outer mitochondrial membrane, and optic atrophy protein 1 (Opa1), located within the inner mitochondrial membrane. On the other hand, mitochondrial fission is regulated by the dynamin-like protein 1 (Drp1) and the small protein fission 1 (Fis1).31 The equilibrium between fusion and fission is pivotal for mitochondrial function.
In post-mortem brain samples from individuals with AD, a significant decrease in the levels of mitochondrial fusion proteins MFN1, MFN2 and OPA1 has been observed in the frontal cortex. Conversely, substantial increases in the levels of DRP1 and Fis132 suggest a shift towards enhanced fission, leading to mitochondrial fragmentation. Increased Drp1 also appears in both streptozotocin-induced33 and transgenic34 AD models, highlighting the excessive contribution of mitochondrial fission to AD-related neuropathology. Therefore, targeting the proteins that regulate fusion and fission of mitochondria, such as by inhibition of Drp1, has emerged as an AD treatment strategy where evidence shows that the inhibition seen in AD models may ameliorate synaptic depression, Aβ deposition and cognitive impairment.35
3.2 Disrupted mitochondrial genomics and epigenomics in AD
Mitochondria possess multiple copies of a semi-autonomous genome known as mitochondrial DNA (mtDNA) that encodes a total of 13 subunits for the complexes involved in the electron transport chain, in addition to 2 ribosomal RNAs (rRNAs) and 22 transfer RNAs (tRNAs).36 The remaining subunits of the electron transport chain, as well as other mitochondrial proteins, are encoded by nuclear DNA. The absence of protective histones, limited recombination capability reduced DNA repair capacity and the proximity to the electron transport chain, collectively render mtDNA particularly vulnerable to the detrimental effects of oxidative stress.37 As a result, the mutation rate of mtDNA is significantly higher than that of nuclear DNA.38
Several studies show that mtDNA damage may be associated with AD pathology. For instance, reports from a particular study indicated that the extent of mtDNA damage is elevated in individuals with AD compared to control subjects. Intriguingly, the mtDNA of AD patients exhibited approximately a 10 times higher level of oxidized bases compared to nuclear DNA.39 Likewise, in some studies, an elevated incidence of mtDNA mutations in the brains of individuals with AD surpassed the anticipated levels associated with normal ageing.40 Furthermore, an analysis of the coding region of mtDNA in brain specimens revealed a higher occurrence of tRNA mutations in approximately 17% of individuals with AD.41
In addition to genomic disruptions, other modifications to mtDNA potentially affect its transcription and overall functionality. Mitochondrial epigenetics, often referred to as “mitoepigenetics,” is a relatively nascent area of investigation with a limited body of research on mtDNA methylation. A few studies show that altered mtDNA epigenetics may be involved in AD pathogenesis, including evidence of mtDNA methylation patterns in AD-related pathology,42 such as analyses of D-loop methylation levels in blood samples that show significant reduction of mtDNA methylation in AD patients compared to healthy individuals.43 The findings suggest that dysregulation of both genomic and epigenomic aspects of mitochondria may potentially contribute to the pathology of AD.
3.3 Disrupted haemostasis of mitochondrial calcium signalling in AD
Ca2+ serve as crucial regulators of essential physiological processes of neurons that include synaptic plasticity, secretion, proliferation, apoptosis and metabolic regulation, and mitochondria play a pivotal role in maintaining cellular Ca2+ homeostasis.44 Considering that the hypothesis of dysregulated Ca2+ homeostasis as a critical pathology underlying AD is widely recognized,45 it is not surprising that mitochondrial calcium signalling may play a role in the pathogenesis of AD.
Samples from individuals with AD or models of AD generally reveal elevated levels of mitochondrial Ca2+ related to multiple altered regulatory processes, including decreased expression of cytosolic Ca2+ binding proteins and changes in mitochondrial Ca2+ influx and efflux capacities.46 In a study of an animal model of AD, the authors suggested that Aβ elevates mitochondrial Ca2+ levels by means of effects on the mitochondrial calcium uniporter, an essential transporter responsible for regulating the influx of calcium ions into mitochondria.47 Findings show that excessive mitochondrial Ca2+ levels contribute to the progression of AD by raising the generation of superoxide that induces metabolic dysfunction and triggers neuronal cell death.48 Other results demonstrate that the presence of misfolded tau protein blocks the efflux of calcium from mitochondria.49 A more recent study also showed that inhibition of mitochondrial calcium uptake protects neurons and astrocytes against the effects of Aβ, including dementia.50 Taken together, claims of altered mitochondrial Ca2+ signalling as potential causative factors now explain the origin of neurodegeneration. However, insight into the precise consequences of the underlying molecular mechanisms and the longitudinal progression of the abnormalities needs further research.
3.4 Compromised mitophagy in AD
Mitophagy is the cellular process that selectively removes damaged or dysfunctional mitochondria from the cell by means of autophagy. Mitophagy plays a crucial role in maintaining cellular health by promoting the turnover of mitochondria and preventing the accumulation of dysfunctional mitochondria, which could contribute to cellular degeneration. The most extensively studied mitophagy pathway involves the stabilization and activation of PTEN-induced kinase 1 (PINK1) at the outer mitochondrial membrane of dysfunctional mitochondria. PINK1 phosphorylates MFN2 and ubiquitin and further recruits the E3-ubiquitin ligase, Parkin, to mitochondria. Parkin ubiquitylates proteins that are then recognized by the ubiquitin-binding proteins, which facilitate the recruitment of these mitochondria into the autophagy pathway.51
Mitochondria are principal targets of autophagic degradation in AD models, and there is compelling evidence indicating the presence of autophagy or lysosome dysfunction in AD.52, 53 Studies have demonstrated a reduction of approximately 30–50% in the baseline levels of mitophagy in post-mortem hippocampal brain samples from AD patients compared to normal individuals.54 These findings are substantiated by identifying impaired fusion between mitophagosomes and lysosomes, leading to the accumulation of damaged mitochondria within autophagic vesicles in the brains of individuals with AD.54, 55 Furthermore, alterations in PINK1, Parkin and ubiquitination of mitochondrial proteins have been found in the accumulated mitochondria in neurons of various AD models, implicating a disrupted mitophagy process.23 Overall, inadequate mitophagy capacity in eliminating the increased number of damaged mitochondria and impairment in the later steps in mitophagy involving lysosomal degradation results in the accumulation of damaged mitochondria and disturbance in mitochondrial homeostasis. Hence, targeting deficient mitochondrial mitophagy by overexpressing proteins implicated at various stages of this process has been considered a potential therapeutic strategy in AD.56
3.5 Anomalous interaction between endoplasmic reticulum and mitochondria in AD
The endoplasmic reticulum is a continuous network of tubular membranes within the cytoplasm that shares several common functions with mitochondria. Mitochondria-associated endoplasmic reticulum membranes (MAMs) are lipid domains in the endoplasmic reticulum that interact with the outer mitochondrial membrane. Mitochondria–endoplasmic reticulum contact sites (MERCS) are established when approximately 20% of the mitochondrial surface closely interfaces with MAMs.57, 58 Accumulating evidence suggests that MERCS are critical regulators of a range of physiological functions, including the calcium exchange between endoplasmic reticulum and mitochondria, phospholipid metabolism, mitochondrial dynamics, autophagy, apoptosis and inflammasome activation.59, 60
Given the vital cellular functions attributed to MERCS, emerging evidence suggests that part of the cellular pathways governing mitochondrial function and neuropathological processes related to AD converge at these sites. Thus, changes in the structure and function of MERCS may potentially represent a key event driving the pathology associated with AD.58 For instance, studies have shown significantly enhanced Aβ production in the MERCS of the mouse brain, suggesting that these sites could serve as focal points for toxic effects associated with Aβ.61 Furthermore, findings from another study have revealed the presence of up-regulated proteins associated with MAMs in the AD brain.62 Intriguingly, this study also exhibited that Aβ exposure could increase the number of MERCS and mitochondrial calcium concentrations.62 A more recent study also demonstrated that Aβ increased MERCS, leading to further alterations of mitochondrial function and autophagosome formation in AD-related models.63 Other studies have also highlighted the interaction of ApoE4 and MAMs. ApoE4 represents the primary genetic or causative risk factor for AD and is recognized to target mitochondria and several MAM-associated processes.64 Accordingly, it has been shown that endoplasmic reticulum–mitochondrial interaction and MAM function were significantly increased in cells treated with ApoE4,65 implying that mitochondria-endoplasmic reticulum interplay plays a fundamental mediating role in the effects of ApoE4 on AD. These emerging findings suggest that dysfunction in the interaction between endoplasmic reticulum and mitochondria is a significant underlying mechanism in AD pathology.
3.6 Oxidative stress and mitochondrial dysfunction in AD
Glucose metabolism in the brain primarily relies on oxidative processes in the mitochondria by TCA and electron transport chains. This dependence makes the brain susceptible to oxidative damage due to its high rate of oxygen consumption.66 Also, brain parenchyma contains a high level of polyunsaturated fatty acids (easily targeted by free radicals) and a low level of antioxidants.67 Ions of the ROS are accountable for cellular injury in ageing and neurodegenerative disorders like AD. While ROS ions are essential signalling molecules at regular levels, they become harmful when produced in excessive amounts.68 In AD, neuronal death can result from the accumulation of Aβ protein induced by ROS, eventually causing lysosome membrane degradation.67, 69 A prominent defect in the mitochondrial electron transport chain is cytochrome c oxidase deficiency, leading to increased ROS production, reduced energy stores and disruptions of energy metabolism. This mitochondrial dysfunction exacerbates oxidative stress and contributes to neuronal degeneration in AD.66 Conversely, it has been found that the insertion of Aβ as oligomers into the mitochondrial bilayer can lead to ROS development, thereby initiating lipid peroxidation of membranes, followed by intracellular protein and nucleic acid oxidation.69
The interplay between oxidative stress and mitochondrial dysfunction in AD is complex. On one hand, oxidative stress damages mitochondrial components, diminishing their function and energy production. On the other hand, dysfunctional mitochondria generate more ROS, intensifying oxidative stress. This self-vicious cycle accelerates the progression of AD.68
Therapeutically, understanding this intricate relationship offers potential therapeutic interventions. Strategies aimed at reducing oxidative stress and restoring mitochondrial function hold promise in slowing or halting AD progression. Further research must investigate the complexities and translate findings into effective therapeutic strategies.
3.7 Roles of mitochondrial dysfunction in neurovascular uncoupling in AD
Neurovascular coupling responsible for functional hyperemia is a function of the neurovascular unit (NVU) consisting of neurons, astrocytes, the endothelial cells of the blood–brain barrier, myocytes, pericytes and the extracellular matrix. The NVU regulates the hemodynamic changes of cerebral blood flow in response to neural activity. One of the primary regulatory mechanisms is vasodilation, which occurs through the release of NO via nearby neurons.70, 71 Cerebral endothelial cells in the brain rely heavily on mitochondrial respiration for energy and survival, possessing a significantly larger mitochondrial volume compared to peripheral endothelial cells. Their vulnerability to apoptosis and disruption of tight junctions under hypoxic or ischemic conditions further underscores their reliance on mitochondrial metabolism.72
Growing evidence indicates a potential impairment of neurovascular coupling in AD, leading to disrupted blood flow responses to neural activity. This disruption may result in a mismatch between metabolic demands and the supply of oxygen and glucose, ultimately leading to reduced neuronal activity and clinically evident cognitive decline.71, 73, 74 Recent findings suggest that individuals with AD who carry the ApoEε4 allele exhibit blood–brain barrier impairment due to pericyte degeneration, resulting in significantly reduced pericyte coverage and total capillary length compared to those without the allele.75 Furthermore, pericyte degeneration has been linked to extensive white matter pathology characterized by hypoxia and myelination loss, contributing to the disruption of structural and functional brain connections typically observed in some cases of AD.76
4 ERs IN AD
A growing body of evidence suggests that both estrogen and ERs play significant roles in the pathophysiology of specific neurological and psychiatric disorders such as AD.77-79 As female rats age, levels of both ER alpha (ERα) and ER beta (ERβ) at the CA1 synapses in hippocampus decline.77, 80-82 Hu et al. demonstrated that women with AD have reduced non-nuclear ERα in the CA1 and CA2 areas of the hippocampus, compared to healthy matched controls.83 Conversely, increases of ERα and ERβ, particularly nuclear ERα, in specific brain regions such as the nucleus basalis of Meynert, the vertical limb of the diagonal band of Broca, the infundibular nucleus of the hypothalamus and the medial mammillary nucleus (only for ERα) have been associated with the pathophysiology of AD compared to age- and sex-matched controls.84-87 Although some conflicting findings concern the role of ERα in AD pathophysiology, ERα has been demonstrated to be involved in AD risk and progression.77 On the other hand, research on ERβ had more consistent results. For instance, overexpression of ERβ has been shown to reduce Aβ deposition in animal models of AD.88 Moreover, study of the brains of women with AD has also revealed reduced neuronal mitochondrial ERβ (mtERβ) in the frontal cortex.89
4.1 Regulation of mitochondrial dysfunction through estrogens and ERs
Mitochondrial ERs initially were found in MCF7 breast cancer cells, with subsequent confirmation of their presence in the CNS.90 Intra-mitochondrial ERα was shown to be expressed in brain endothelial cells and in rat hippocampus,91 while ERβ was found in hippocampal neurons of rats and mice as well as in mouse forebrain.90, 92, 93
Estrogen can influence mitochondrial functions both directly and indirectly.94, 95 As members of the family of nuclear receptors, ERα and ERβ regulate the mtDNA transcription. Activation of genomic ERα and ERβ raises transcription of the nuclear respiratory factor-1 (NRF-1) that in turn stimulates transcription of mitochondrial transcription factor A (TFAM), along with transcription factors AB types 1 and 2 (TFB1 and TFB2), and mitochondrial respiratory chain (MRC) genes that affect the regulation of mtDNA-encoded genes and mitochondrial biogenesis.96
mtERβ is also situated at the mitochondrial interface, serving as a central point of communication with the MAMs.93 Positioned intracellularly, mtERβ crucially regulates mtDNA and mitochondrial genes,97 influencing their function and expression that serve to adjust the cellular energy homeostasis.
4.2 Regulation of mitochondrial bioenergetics by estradiol (E2)
Lack of ovarian sex hormones has been shown to inhibit mitochondrial functions significantly, inducing oxidative stress and reducing brain bioenergetics. Accumulation of mitochondrial Aβ and Aβ-binding alcohol dehydrogenase (ABAD) is evident in the hippocampal region of the brains of ovariectomised mice, with exacerbation in the triple-transgenic AD model and preventable by treatment with E2, one of three estrogens98 (for an overview of this model, please refer to Oddo et al.99). The findings imply that loss of ovarian sex hormones incurs the same phenotype and pathophysiology that characterize triple-transgenic AD mice model.
The estrogen E2-enhanced mitochondrial respiratory reserve capacity follows activation and expression of electron transport chain complex IV (cytochrome c oxidase), accompanied by a subsequent reduction in the rate of reactive oxygen leak and lipid peroxidation that points to improved efficiency of brain mitochondrial function.97, 100 However, when progestin is added, the result is different. A progesterone derivative, medroxyprogesterone, reduced the E2-induced potentiation of mitochondrial respiratory reserve capacity in primary hippocampal neuronal and glial cells.101 Studies of the effect of E2 on mitochondrial bioenergetics in rat embryonic hippocampal neurons revealed an increase of maximal respiratory capacity.93, 98
Selective activations of both ERα and ERβ stimulate baseline oxygen consumption (OCR) and extracellular acidification rates (ECARs) that serve as surrogate measures of lactate production and glycolysis,102 and the greater increase of basal OCR followed E2 treatment.93, 103 Yao et al. pointed out that E2 differentially regulates glial and neuronal mitochondrial bioenergetics in primary hippocampal cultures of female Sprague–Dawley rats obtained from E18 embryos. Maximal reserved respiratory capacity and OCR were enhanced in mixed glia after a 24-h treatment with E2, while basal respiration remained unaltered in neurons. While the ECAR did not significantly change in neurons after treatment, it was substantially higher in mixed glia, indicating enhanced glycolysis.98
4.3 Estrogen regulation of glucose metabolism and insulin resistance
E2 exerts its modulatory effect on glucose metabolism by tuning the rate of glucose metabolism through modulation of enzymes involved in glycolysis, as well as by promoting the availability of glucose through increased glucose uptake (Figure 2).105 In the brain, hexokinase, phosphofructokinase and pyruvate kinase have long been known to be under the influence of E2,106 facilitating ATP production and supply. E2 also regulates other key metabolic enzymes, such as pyruvate dehydrogenase, aconitase and ATP-synthase.97
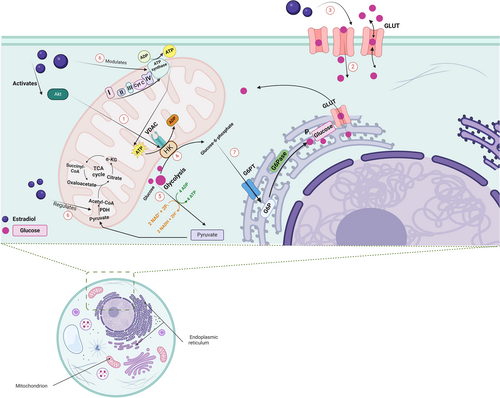
E2 promotes glucose uptake by regulating the expression and activation of Glut1 (mainly found in glial cells and capillaries), as well as Glut3 and Glut4 found primarily on cortical neurons.107 Upon activation of ERs, downstream signalling of PI3K-Akt and MAPK-ERK facilitates the insertion of the Glut3 and Glut4 transporters, compensating for the compromised glucose metabolism pathway. In an APP/PS1 double-transgenic postmenopausal mouse model of AD, a consistent decline of the levels of Glut1 and Glut3 in the middle and late stages of menopause followed a transient rise during the early stage of menopause, suggesting impaired glucose metabolism.108 Additional alterations of mtERβ morphology led the authors to postulate that estrogen deficiency may be associated with an aberrant mtERβ-mediated IGF-1 energy metabolism signalling pathway.108 The translocation of hexokinase to the voltage-dependent anion channel (VDAC) at the mitochondrial membrane, which directs intramitochondrial ATP synthesis towards glucose metabolism,104 was shown to be driven by the activation of Akt induced by estrogen (Figure 2).109, 110
There is a well-established interplay between ERs and insulin-like growth factor (IGF-1) in the brain.107, 111 E2 raises the expression of IGF-1 in the brain,107 and IGF-1 can regulate the recruitment of Glut transporters in the brain,112, 113 suggesting that the modulation of glucose metabolism by means of ERs may be influenced by IGF-1 levels as well. Moreover, E2 consistently demonstrates an ability to increase insulin sensitivity, a factor that potentially goes awry in AD.114-117 Studies pointing to a negative correlation between brain levels of IGF-1 and the accumulation of Aβ118, 119 provide additional support for the potential role of E2 and its cognate receptors in managing AD symptoms by increasing IGF-1 levels. In islet β cells, E2 improved glucose uptake through the up-regulation of GPER and Glut 2 and increased Akt/mTOR signalling, thus highlighting a pivotal role for GPER.120 Apart from its role in the regulation of Glut transporters, E2 has been found to stimulate 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3, leading to elevation of fructose-2,6-bisphosphate that affects glycolytic flux and boosts glucose uptake in ERα-positive breast cancer cells.105
4.4 Estrogen regulation of mitochondrial dynamics and mitophagy
Female rodents that undergo ovariectomy consistently increase raise mitochondrial fission and lower mitochondrial fusion in transgenic AD animal models and non-transgenic rodents.98, 121 The changes are reflected in elevated levels of Drp1 and reduced levels of optic atrophy 1 GTPase (OPA1) in the brain, indicative of enhanced mitochondrial fragmentation due to the absence of ovarian sex hormones. Notably, administration of E2 reverses these effects and restores mitochondrial dynamics.121 Several additional studies shed light on the role of ERα in the up-regulation of Mfn1, Mfn2 and OPA1, as well as the downregulation of Fis1, through the observation that these regulatory changes only occur in ERα-positive MCF7 breast cancer cells and not in triple-negative breast cancer cells, MDA-MB-231 or T47D. The regulation of Drp1, however, appears to be ERα-independent.122
Looking at the reverse side, selective activation of ERβ triggers PKA signalling at the mitochondrial membrane through ERβ. This activation leads to the phosphorylation of Drp1, effectively inhibiting mitochondrial fission induced by the Aβ-oligomer.123 The inhibition of the ERβ overexpression has been shown to mimic the response of MCF7 cells to E2, resulting in decline of fission and increase of fusion. Finally, aberrant mitochondrial morphology, impaired mitochondrial fission, calcium handling and ATP production have been reported in the muscle specific ERα knockout mice.124 However, in this context, the extent of the abnormalities and the possibility of tissue-specific effects of estrogen signalling125 are caveats that should be further explored, especially in the brain.
Enhanced expression of the G-protein-coupled estrogen receptor (GPER) was associated with higher expression levels of Mfn1, Mfn2 and Parkin, as well as lower levels of Fis1, confirming the regulatory role that GPER plays in mitochondrial dynamics and its involvement in increased mitophagy.126 Selective activation of GPER has also been implicated in increased mitochondrial function and mitochondrial membrane potential in the hippocampus of aged female rats.127
4.5 Estrogen regulation of calcium signalling
There is a bidirectional link between ERs and calcium signalling.128 For instance, activities of ERα and GPER, but not activity of ERβ, are subject to tight regulation via calcium-dependent interaction with calcium-calmodulin.129-132 In the brain, membrane ERs rapidly mediate calcium signalling, and activation of these membrane-bound ERs leads to rapid influx of calcium through close interaction with L-type calcium channels, mediating the Src/ERK/CREB/Bcl2 downstream pathway.133, 134 Astrocytic membrane-associated ERs have been shown to control calcium flux via the PKC pathway.135 Multiple ligands of ERs rapidly regulate the activity of the mitochondrial calcium uniporter that serves as the primary means by which calcium enters the mitochondria.136 Activating ERs elevated mitochondrial calcium uptake, while antagonizing the receptors with tamoxifen inhibited its uptake within minutes.136 This rapid activation alludes to the involvement of the non-genomic pathway, and it appeared that this effect should reflect ERs placed either within mitochondria or closely associated with it, when the effect was tested in permeabilized cells.136
In rat synaptosomes of the hippocampus and nucleus caudate, E2 exhibits a U-shaped dose-dependent effect on mitochondrial calcium efflux, where calcium efflux is inhibited at a concentration of 1-nM E2, followed by an exponential increase in calcium efflux with doses exceeding 10 nM.137, 138 The study concludes that E2 suppresses sodium-dependent calcium efflux from mitochondria and affects calcium retention within mitochondria. The authors, however, could not show any E2-induced changes to the mitochondrial calcium influx.137, 138 However, Nilsen and colleagues reported increased mitochondrial calcium uptake and further claimed that elevated levels of mitochondrial calcium induced by E2 can protect neuronal cells from the excitotoxic effect of glutamate by sequestering calcium in mitochondria, thereby decreasing available cytoplasmic calcium.139 The authors took one step further and showed that mitochondria preserve their respiratory capacity after treatment with E2, despite calcium accumulation.140 In a complementary study, E2 treatment sustained the calcium homeostasis of neurons derived from aged brains, compared to middle-aged brains, exhibiting a protective role for E2 in the prevention of age-related neuronal dysregulation.140 Finally, in a preclinical study, Burstein and colleagues demonstrated sex-specific differences of the function of the brain mitochondrial permeability transition pore, exhibiting a lower threshold for calcium in females than in males.141 Additionally, the authors highlighted the importance of ERβ, when antagonizing or knocking out this receptor inhibited the mitochondrial permeability transition pore. ERβ functionally protected against neurotoxicity in primary neurons and against oxygen–glucose deprivation in hippocampal slice cultures.141
4.6 Estrogen regulation of neurovascular coupling and ROS
Diminished endothelial nitric oxide synthase (eNOS) expression in brain blood vessels and nearly absent eNOS expression in cerebral cortical and white matter vessels were reported in AD brains.142 Preclinically, eNOS deficiency has also been shown to contribute to altered neurovascular coupling, linking cerebral vasculopathy and neurodegeneration.143 Membrane ERs are implicated in the rapid induction of NO via stimulation of eNOS,144-147 contributing to neurovascular coupling and responding to oxygen demand of the brain.143, 148 NO is then converted into reactive nitrogen species (RNS) that initiates the process of reversible S-nitrosylation, with an outcome of nitrothiol production that changes the function of mitochondrial proteins involved in energy (redox) metabolism.94 In addition to protecting against vasculopathy, estrogen has been shown to lower mitochondrial superoxide production by acting on ERs in vitro while also decreasing mitochondrial ROS production in vivo in both male and female rats.149 The study illuminates the role of endogenous estrogen as modulator of mitochondrial ROS production in the brain by enhancing the activity of manganese superoxide dismutase responsible for catalyzing the breakdown of superoxide radicals.149 In a subsequent in vitro study on human brain microvascular endothelial cells, researchers revealed that ERα and not ERβ mediates this antioxidant effect of estrogen, and they further suggested that the concomitant increase in the mitochondrial cytochrome c levels could be the underlying reason for this protective effect.91
5 CONCLUSION
Mitochondria are crucial to preserving optimal neuronal activity and function that are progressively impaired in AD. It is a claim that mitochondrial dysfunction may drive the onset and progression of AD, initiating a cascade of the downstream effects of tauopathy and amyloidopathy. AD is also known to exhibit notable sex disparities, with women being 2–3 times more likely to develop the disease after the age of 65. Women show different cognitive trajectories and susceptibility to AD compared to men as well. These differences in AD prevalence, trajectory and progression suggest that sex-specific factors, including hormonal influences, may play a crucial role.
Estrogen and its receptors share an association with multiple neuroprotective effects, and fluctuations of estrogen levels during the perimenopausal period coincide with a phase when women become more predisposed to contract AD. These hormonal fluctuations can be crucial in AD pathophysiology, making the study of ERs and their influence on mitochondrial function more pertinent. Estrogen and its associated receptors have been widely shown to impact mitochondrial function; however, most studies are quite old, and the intricate interplay between ERs and mitochondria in the context of AD underscores the need to delve deeper into these receptors' modulation of mitochondrial function.
Mitochondrial dynamics are disrupted in AD, characterized by altered mitochondrial morphology, decreased fusion and increased fission. Reduced mitochondrial calcium efflux, enhanced calcium uptake, diminished mitophagy and decreased glucose consumption are prominent in AD. Estrogen and various ERs were revealed to increase fusion, decrease fission and enhance mitophagy by regulating critical proteins involved in these processes. Estrogen also influences oxidative phosphorylation and glycolysis, which are altered during the course of AD. Estrogenic modulation as well can also revert the increase of ROS levels and related reductions of cytochrome c oxidase activity that typically occur in AD, leading to increased expression and activity of cytochrome c oxidase, ultimately improving the function of the MRC. Estrogen and ERs also play pivotal roles in restoring neurovascular coupling, regulating calcium signalling, promoting glucose metabolism and insulin sensitivity, all of which are compromised in AD.
Although it is evident that estrogen and ER can have beneficial therapeutic modes of action in the management of AD, there are caveats that need to be addressed in future research. One important issue is that Aβ can influence the metabolism and, therefore, levels of estrogen E2 by fine tuning the enzymatic activity of mitochondrial ABAD. Apart from this, the effects of E2 and ER can be tissue-specific, and we may draw incorrect conclusions from data obtained on tissues other than the brain, particularly as the number of studies focusing on the brain is scarce. Differences among the impacts of different subtypes of ER (ERα, ERβ and GPER) on multiple mitochondrial functions require further exploration. Also, genomic and non-genomic contributions of ER to the regulation of mitochondrial functions and AD pathophysiology merit further in-depth investigations. By elucidating the mechanisms by means of which ERs influence mitochondrial health, we may, in the best of cases, unlock insights that lead to targeted therapies, not only shedding light on AD's complex pathophysiology but also offering new hope for more effective treatments, particularly with sex-specific considerations in mind.
ACKNOWLEDGEMENTS
Shokouh Arjmand thanks the Brain & Behavior Research Foundation and the Molly Lou Foundation for the award of the BBRF Young Investigator Grant, Grant Number 31803, during the tenure of which the manuscript was drafted.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.




