Understanding colorectal cancer risk for symptomatic patients in primary care: A cohort study utilising faecal immunochemical tests and blood results in England
The Handling Editor for this article was Professor Colin Howden, and it was accepted for publication after full peer-review.
Summary
Background
A faecal immunochemical tests (FIT) cut-off of ≥10 μg Hb/g faeces is now recommended in the UK as a gateway to urgent (suspected cancer) investigation for colorectal cancer (CRC), based on an expected CRC risk threshold of 3%.
Aims
To quantify the risk of CRC at FIT cut-offs by age, haemoglobin and platelet strata.
Methods
A cohort study of a symptomatic CRC pathway based on primary care FIT tests in Nottingham, UK (November 2017–2021) with 1-year follow-up. Heat maps showed the cumulative 1-year CRC risk using Kaplan–Meier estimates.
Results
In total, 514 (1.5%) CRCs were diagnosed following 33,694 index FIT requests. Individuals with a FIT ≥ 10 μg Hb/g faeces had a >3% risk of CRC, except patients under the age of 40 years (CRC risk 1.45% [95% CI: 0.03%–2.86%]). Non-anaemic patients with a FIT < 100 μg Hb/g faeces had a CRC risk of <3%, except those between the age of 70 and 85 years (5.26% 95% CI: 2.72%–7.73%). Using a ≥3% CRC threshold in patients <55 years calculated using FIT, age and anaemia might allow 160–220 colonoscopies per 10,000 FITs to be re-purposed, at a cost of missing 1–2 CRCs.
Conclusions
FIT alone with a single cut-off is unlikely to be a panacea for optimising CRC diagnosis, as risk varies by FIT, age and anaemia when faecal haemoglobin levels are below 100 μg Hb/g. Tailored FIT cut-offs for investigation on a CRC pathway could reduce the number of investigations needed at a 3% CRC risk threshold.
1 INTRODUCTION
In 2015, National Institute for Health and Care Excellence (NICE) guidelines ‘Suspected Cancer: recognition and referral (NG12)’ the stated ‘risk threshold’ for urgent referral or investigation was a 3% positive predictive value of cancer, that is those referred by primary care on a cancer diagnostic pathway should have a risk of the specific cancer of 3% or more.1, 2 This threshold was chosen to improve the diagnosis of cancer over a previously used threshold of 5% by targeting those at greatest risk for the most appropriate investigation1 but was a pragmatic compromise as patient preferences are for lower cut-offs.3 These guidelines were still mostly focussed on signs, symptoms and age, despite the fact that patients with early stage colorectal cancer (CRC) often have no or only vague symptoms.
The NHS Cancer Plan (2019) defines diagnosis at an earlier stage as a key strategy for improving outcomes and identifies the faecal immunochemical test (FIT) as a key enabler to the aspiration of diagnosing 75% of CRC at Stage I or II,4 and over the past few years, evidence for the use of FIT has accrued.1, 2, 5 The COVID-19 pandemic and its constraints (face-to-face patient contact, staffing resource and concerns around aerosol-generating procedures) accelerated the use of FIT in symptomatic patients across the United Kingdom, yet the implementation has been piecemeal.6-8 One uncertainty is the actual CRC risk at specific cut-offs of FIT when used freely in Primary Care, and what impact these cut-offs might have on diagnostic demand, as highlighted in the recent Association of Coloproctology of Great Britain & Ireland and the British Society of Gastroenterology guidelines endorsing the use of FIT in symptomatic patients.1, 2, 5-9 These guidelines recommend the use of FIT to guide clinicians in choosing the most appropriate referral pathway for symptomatic patients (without a palpable rectal mass)—specifically that patients with a faecal haemoglobin (fHb) ≥10 μg Hb/g faeces should be referred on an urgent cancer diagnostic pathway. The cut-off of ≥10 μg Hb/g faeces (FIT10) was originally suggested for patients with a low risk of CRC who were being seen in primary care because of concern to avoid ‘missed cancer’ in ‘high-risk’ symptoms. Further research was recommended for those considered at higher risk and the optimum cut-off to use in different groups.1, 10 It is also likely that many people (i.e. those under 50 years of age and those with normal blood parameters) are investigated for CRC and exposed to the risks and other negative consequences of investigation, despite having a very small chance of having the disease. This will contribute to diagnostic services being overwhelmed with the associated reduction in capacity and risk of delays in diagnosis for others. However, there is an absence of population-based studies with enough people to assess the impact of FIT cut-offs9 or to balance the risk of CRC against the ability of the health service to investigate and diagnose cancer in an appropriate and timely manner.
In Nottingham, we introduced FIT with mandatory blood tests in Primary Care prior to all urgent cancer referrals for all symptoms other than rectal bleeding or rectal mass in November 2017. We have used all available electronic health data associated with FIT referrals over a 4-year period spanning before and during the COVID-19 pandemic to assess the risk of CRC in people who had a FIT. Our aim was to determine the empirical thresholds of CRC risk in a representative population at different FIT cut-offs to assess the optimal use of FIT in patients with symptoms of CRC.
2 METHODS
2.1 Nottingham rapid colorectal cancer diagnosis pathway (NRCCD)
In Nottingham, a locally commissioned 12-month service evaluation of FIT in urgent CRC pathways allowed ‘local agreement’ of a new pathway designed by all stakeholders [General Practitioners (GPs), secondary care, Clinical Care Commissioning Groups (CCGs) and the Bowel Cancer Screening Hub]. This incorporated FIT as a triage tool for symptomatic suspected CRC referrals, except rectal bleeding and palpable rectal mass, as described elsewhere.11-13 The data from this service evaluation led to a re-design of the local pathways. Following approval and rollout of this pathway in November 2017,8 GPs were able to request FIT (and blood tests) independently for the investigation of CRC. FIT and FBC were mandated for all CRC referrals, other than rectal bleeding or rectal mass, irrespective of symptoms or age. FIT and blood results were used to prioritise access to urgent investigations based on multiple thresholds and published evidence, and continuous local data evaluation and national context were used to guide iterative changes to the pathway as described in Figure S1.
2.2 Study setting
- Adults (>=18 years of age)
- Patients within Nottinghamshire registered at a General Practice that would refer to Nottingham University Hospitals (Nottingham City and South Nottingham Integrated Care Partnerships)
- From November 01, 2017 until November 31, 2021
FIT requests and results reporting was electronic. FIT dispatch and return were entirely postal and kits were analysed according to manufacturer's protocols by our accredited BCSP Hub laboratory. All samples were analysed using an OC-Sensor™ platform (Eiken Chemical Co.) as previously described.13
2.3 Exclusion criteria
FIT results in patients younger than 18 years old or those who were not registered with a GP in Nottingham City and South Nottingham ICPs or FIT results conducted outside the study time period.
2.4 Data management
The variables of interest were extracted and linked by patients' unique identification numbers using Microsoft SQL Server. The data were then anonymised prior to being accessed by the researchers, so the researchers did not have access to identifiable patient-level data and no patient-level data left NUH NHS Trust. The anonymous data for analysis were transferred to a separate secure server within NUH that only the investigative team could access for analysis (CC, JW), so no patient data left NUH NHS Trust.
2.5 Outcomes
CRC was defined from linked Infoflex (Civica) data where all cancers diagnosed at NUH NHS Trust are recorded. Fact and date of death were obtained from the NHS personal demographics service and the underlying cause of death (coded with ICD-10) from https://www.hed.nhs.uk/Info/. Patients were followed up for up to 1 year for CRC diagnosis or death.
2.6 Exposures
Each individual had their first recorded FIT per year (index FIT) identified and was subsequently linked to all the required data sets within NUH NHS Trust's Enterprise Data Warehouse. This included age (at the date of FIT) using the year of birth, sex (defined as male/female) and blood tests that were extracted for all patients and included haemoglobin/platelets using any result up to 1 year prior and 14 days following the index FIT. The closest of these tests to the index FIT in time were used. Ferritin was not measured frequently enough to be included in this study. Cut-offs from the Nottingham pathway and published literature were used to define strata within this study fHb < 4 μg Hb/g faeces, 4–9.9 μg Hb/g faeces, 10–19.9 μg Hb/g faeces, 20–39.9 μg Hb/g faeces, 40–99.9 μg Hb/g faeces and ≥100 μg Hb/g faeces, along with anaemia (≤130 g/L in men, ≤120 g/L in women) and abnormal platelet count (≥400 × 109/L).13, 15 We identified all relevant investigations for CRC (colonoscopy, flexible sigmoidoscopy, Computed Tomography colonography) that occurred within 6 months of the index FIT.
2.7 Statistical analysis
The analyses were carried out using R16 within R Studio on NUH NHS Trust devices. We calculated numbers and percentages of baseline age group, sex and subsequent investigations and outcomes in the study population. Baseline FIT and blood test results were described by median and interquartile ranges (IQR). We then stratified these baseline measures by FIT category using the cut-offs defined above.
We displayed the time from index FIT to CRC diagnosis and/or death using histograms. We described the completeness of the data and classified missing data as missing for this descriptive study.
One-year cumulative CRC risks were calculated as one minus the 1-year Kaplan–Meier survival estimate. These 1-year cumulative CRC risks were calculated with 95% confidence intervals within each stratum of FIT category, age, anaemia and thrombocytosis. We also undertook an analysis stratified by an FIT level of ≥10 μg Hb/g faeces as per the current national guidance.17 These 1-year cumulative CRC risks were then presented as heat maps.
For each of the strata presented in the heat maps, we identified the corresponding FIT threshold as the FIT value within that stratum with a 1-year cumulative CRC risk >3%. For a more conservative FIT threshold estimate, we identified the FIT value within that stratum whose lower 1-year cumulative CRC risk 95% CI was >3%.
Finally, we estimated the number of investigations that could potentially be re-purposed and the number of CRCs that would be missed if investigation was restricted to only those groups with a 3% or greater 1-year CRC risk.
3 RESULTS
3.1 Demographics
In total 34,435 patients returned 39,774 FIT kits, with 37,216 tests with 1 year follow-up, after excluding 2558 (6.4%) returning more than one test within a year of the initial test. Only 6% of the population was under the age of 40 years and the number of FIT requests was greatest in those aged 55–85 years (Table 1).
| Number of patients with measurement | Number with repeated FIT | Number of tests within 14 days or year prior to FIT | % missing with results carried within 14 days or prior year | N (%) or median value (IQR) | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 15,061 | 1120 | 16,274 (44%) | ||
| Female | 19,374 | 1438 | |||
| Age (years) | |||||
| Age 18–40 | 2247 | 74 | 2278 (6%) | ||
| Age 40–55 | 7210 | 370 | 7516 (20%) | ||
| Age 55–70 | 10,991 | 761 | 11,738 (32%) | ||
| Age 70–85 | 11,803 | 1126 | 12,927 (35%) | ||
| Age > 85 | 2598 | 227 | 2757 (7%) | ||
| Test results | |||||
| FIT | 34,435 | 2558 | 37,216 | 0 | 4 (4–8) |
| Blood tests | |||||
| Hb (g/dL) | 30,999 | 33,694 | 9.5 | 131 (118–143) | |
| Platelet count | 30,901 | 33,586 | 9.8 | 268 (223–322) | |
| Ferritin | 28,182 | 30,725 | 17.4 | 65 (23–139) | |
| Investigations | |||||
| Colonoscopy, CT colonography within 6 months | |||||
| Colonoscopy | 6540 | ||||
| CT colonography | 3103 | ||||
| CRC diagnoses and deaths within 1 year | |||||
| Colorectal cancer | 533 | 1.5 | |||
| Colorectal cancer deaths | 79 | 0.2 | |||
| Non-colorectal cancer deaths | 1469 | 4.3 | |||
3.2 Colorectal cancer diagnoses
During the study period, a total of 533 (1.5%) CRCs were diagnosed following the index FIT. In the year following the index FIT, there were 79 deaths from CRC and 1469 (4.3%) deaths from other causes. The largest proportion of CRC was diagnosed in those patients with an FIT of >100 μg Hb/g faeces (Table 2 only shows patients with blood tests available). Median time to diagnosis was 35.8 days (33.7–39.8 days) and for non-CRC death was 165.6 days (157.6–176.1 days), and the distribution of diagnoses and deaths relative to the index FIT are shown in Appendix S1.
| N (% within FIT strata) | fHb < 4 μg Hb/g faeces | fHb 4–9.9 μg Hb/g faeces | fHb 10–19.9 μg Hb/g faeces | fHb 20–39.9 μg Hb/g faeces | fHb 40–99.9 μg Hb/g faeces | fHb ≥ 100 μg Hb/g faeces |
|---|---|---|---|---|---|---|
| Colorectal cancer diagnosis | 26 (0.1%) | 27 (0.5%) | 24 (1%) | 41 (2.3%) | 67 (4.3%) | 329 (16.8%) |
| Colorectal cancer death | 10 (0%) | 3 (0.1%) | 3 (0.1%) | 6 (0.3%) | 10 (0.6%) | 37 (1.9%) |
| Non-colorectal cancer deaths | 565 (2.8%) | 233 (4.2%) | 129 (5.4%) | 120 (6.9%) | 133 (8.5%) | 207 (10.6%) |
| Gender | ||||||
| Male | 8925 (44%) | 2233 (40%) | 1038 (44%) | 785 (45%) | 748 (48%) | 1011 (52%) |
| Female | 11,562 (56%) | 3337 (60%) | 1335 (56%) | 964 (55%) | 810 (52%) | 946 (48%) |
| Age (years) | ||||||
| Age 18–40 | 1296 (6%) | 247 (4%) | 79 (3%) | 125 (7%) | 42 (3%) | 49 (3%) |
| Age 40–55 | 4682 (23%) | 888 (16%) | 325 (14%) | 183 (10%) | 162 (10%) | 230 (12%) |
| Age 55–70 | 6834 (33%) | 1763 (32%) | 703 (30%) | 427 (24%) | 412 (26%) | 468 (24%) |
| Age 70–85 | 6567 (32%) | 2205 (40%) | 968 (41%) | 786 (45%) | 725 (47%) | 920 (47%) |
| Age > 85 | 1108 (5%) | 467 (8%) | 298 (13%) | 228 (13%) | 217 (14%) | 290 (15%) |
| Anaemia – yes | 6197 (30%) | 2013 (36%) | 1019 (43%) | 817 (47%) | 796 (51%) | 1046 (53%) |
| Abnormal platelets | 1413 (7%) | 492 (9%) | 233 (10%) | 188 (11%) | 160 (10%) | 295 (15%) |
3.3 Missing data
Approximately, 9.7% of patients had no recorded valid haemoglobin measurement, and 9.8% had no recorded valid platelet count within 1 year prior to and 14 days post-FIT request. The remainder of the results is presented in those with complete data, consisting of 33,694 unique FIT results from 30,999 patients with 514 (1.5%) CRC diagnoses.
3.4 One-year cumulative CRC risks by age and FIT level
Only 26 (5.1%) cancers were diagnosed in those patients with an fHb of <4 μg Hb/g faeces. Most cancers were diagnosed in those patients with an fHb of ≥100 μg Hb/g faeces 329 (64%). At a reported fHb of <10 μg Hb/g faeces, 53 cancers were diagnosed compared to 461 with an FIT of ≥10 μg Hb/g faeces. Stratifying FIT level by age demonstrated that all patients with an fHb of <10 μg Hb/g faeces had a 1-year cumulative CRC risk of <3% (Figure 1 shows the stratified risks, and Figure S7 also includes histograms of the number of patients in each stratum). All patients with an fHb of ≥10 μg Hb/g faeces had a >3% risk of CRC except those under the age of 40 years who had a cumulative CRC risk of 1.45% (95% CI: 0.03%–2.86%). Stratifying by all our fHb cut-offs (Figure 2 shows the stratified risks, and Figure S8 also includes histograms of the number of patients in each stratum) shows that the 3% threshold for FIT is ≥100 μg Hb/g faeces for patients under 70 years and ≥40 μg Hb/g faeces for those over 70 years. A lower 95% CI bound of the Kaplan–Meier estimate would require an FIT cut-off of ≥20 μg Hb/g faeces for all patients over 40 years.
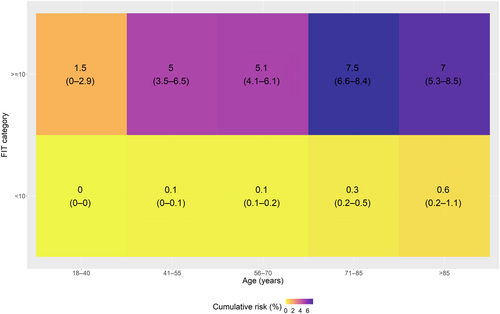
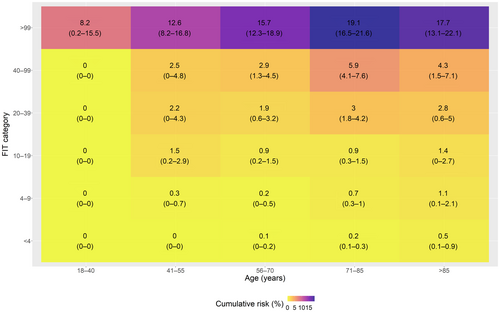
3.5 One-year cumulative CRC risks by age, anaemia and FIT levels
Following stratification of CRC diagnosis by age, FIT level and anaemia, non-anaemic patients with an fHb of <100 μg Hb/g faeces had a cumulative CRC risk of <3% (Figure 3 shows the stratified risks, and Figure S9 also includes histograms of the number of patients in each stratum) except those between the age of 70 and 85 years (5.26%, 95% CI: 2.72%–7.73%). A lower 95% confidence interval bound of the Kaplan–Meier estimate would require a cut-off of ≥40 μg Hb/g faeces for all non-anaemic patients under 70 years and ≥20 μg Hb/g faeces for patients over 70 years. In contrast, in patients who had anaemia, the 3% threshold was met over 40 years in the FIT 20–40 μg Hb/g faeces category. In anaemic patients under 40 years of age, no further cancers were detected in those with an FIT of <100 μg Hb/g faeces. Using a lower 95% CI bound of the Kaplan–Meier estimate in anaemic patients would require an FIT cut-off of ≥100 μg Hb/g faeces for all patients under 40 years and ≥20 μg Hb/g faeces for patients over 40 years.
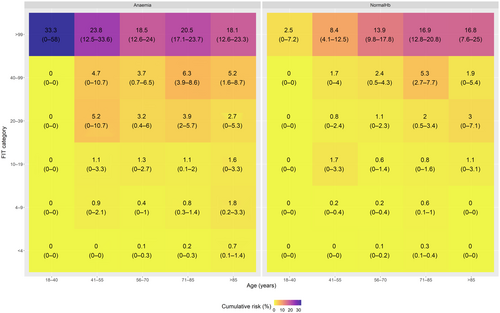
3.6 One-year cumulative CRC risks by age, anaemia, thrombocytosis and FIT levels
In patients who are not anaemic and have a normal platelet count with an FIT between 40 and 100 μg Hb/g faeces, only those over the age of 70 years met the 3% threshold for investigation. All patients over the age of 40 years with an fHb of ≥100 μg Hb/g faeces met the threshold for investigation (Figure S10 shows the stratified risks, and Figure S11 also includes histograms of the number of patients in each stratum). A lower 95% CI bound of the Kaplan–Meier estimate would require an FIT cut-off of ≥40 μg Hb/g faeces for all patients with normal blood tests under 70 years and ≥20 μg Hb/g faeces for patients over 85 years. In patients with abnormal platelets and anaemia, the threshold for investigation at 3% was met at a younger age and lower FIT category, for example an FIT between 10 and 20 μg Hb/g faeces and age of 40–55 years.
3.7 Estimates of investigations that could be re-purposed and potential cancers missed
A total of 7637 patients had an FIT of ≥10 μg Hb/g faeces and so would have required a luminal investigation under a ≥10 μg Hb/g faeces cut-off (as currently recommended nationally), with 53 CRCs missed in patients with fHb < 10 μg Hb/g faeces. Table 3 shows the effect of selecting the FIT threshold that strictly meets the 3% threshold from the above heat maps, by showing the number of patients in those strata with an FIT value below the 3% 1-year risk of CRC threshold, but above 10 μg Hb/g faeces (i.e. would have had a colonoscopy/CT colonography under a uniform FIT ≥10 μg Hb/g faeces cut-off). This showed that almost 1000 colonoscopies could be avoided by selecting an FIT threshold of 100 μg Hb/g faeces for patients under 55, but an additional 13 cancers would be missed. Adding in anaemia almost halves the number of missed cancers in these strata but requires delivery of 200 more urgent colonoscopies or equivalent. Abnormal platelets had a minimal additional change in the numbers after anaemia had been included. Using a 3% CRC threshold in low-risk patients, <55 years for investigation including FIT, age and anaemia strata approximately 160–220 colonoscopies per 10,000 FITs could be avoided at a cost of missing 1–2 CRCs (range indicating mean and lower 95% CI excluding 3% threshold).
| Threshold for low-risk strata | Investigations freed at 3% threshold compared to test all FIT ≥ 10 (i.e number in strata with FIT below CRC risk threshold and FIT ≥ 10 or above) | Potential cancers missed at a 3% threshold (i.e number in strata with FIT below CRC risk threshold and FIT ≥ 10 or above) | Investigations freed at 95% CI of 3% threshold compared to test all FIT ≥ 10 (i.e. number in strata with FIT below 95% CI of CRC risk threshold and FIT ≥ 10 or above) | Potential cancers missed at 95% CI of 3% threshold (i.e. number in strata with FIT below 95% CI of CRC risk threshold and FIT ≥ 10 or above) |
|---|---|---|---|---|
| Age (years) | ||||
| Age 18–40 | 246 (FIT ≥ 100) | 0 | 246 (FIT ≥ 100) | 0 |
| Age 40–55 | 670 (FIT ≥ 100) | 13 | 325 (FIT ≥ 20) | 5 |
| Not anaemic and | ||||
| Age 18–40 | 246 (any FIT) | 1 | 206 (FIT ≥ 100) | 0 |
| Age 40–55 | 480 (FIT ≥ 100) | 7 | 361 (FIT ≥ 40) | 5 |
| Not anaemic and no thrombocytosis | ||||
| Age 18–40 | 228 (any FIT) | 1 | 190 (FIT ≥ 100) | 0 |
| Age 40–55 | 447 (FIT ≥ 100) | 6 | 337 (FIT ≥ 40) | 4 |
4 DISCUSSION
We show that the introduction of FIT in primary care as a gateway test to urgent pathways for CRC diagnosis, identified a population that GPs chose to test with an overall 1-year cumulative risk of CRC of 1.5%. We show that the CRC risk in FIT strata varies hugely by age and whether a patient is either anaemic or has thrombocytosis, and due to the large, representative nature of our study, we can give estimates of these differences. For example, non-anaemic patients do not meet the 3% threshold set by NICE for investigation until they have an FIT of ≥40 μg Hb/g faeces. In contrast, those patients with anaemia meet the 3% threshold at an FIT of ≥20 μg Hb/g faeces. Patients under 40 years of age only meet the 3% threshold for investigation in those who have an FIT of ≥100 μg Hb/g faeces and are anaemic. Estimating the risk in our study just above the current guideline-recommended approach of a single cut-off at ≥10 μg Hb/g faeces showed that patients with an FIT of 10–20 μg Hb/g faeces had an overall uncensored risk of 25/2266 = 1.1%, well below the 3% threshold.
In our population, we conservatively estimate that by using a stratified approach to FIT cut-offs in low-risk patients (<55 years and not anaemic) up to 600 urgent investigations (of which the majority are colonoscopy) for CRC can be forgone over 4 years within our catchment of over 37,000 FIT tests at a cost of missing approximately four CRCs. When extrapolated to the whole country this could represent a re-purposing of 160–220 colonoscopies per 10,000 FITs carried out at a cost of 1–2 cancers missed (using lower to mid-point estimates) for other purposes such as screening, surveillance or routine investigation. This compares to the current nationally recommended approach (FIT > 10 μg Hb/g) which leads to over 2000 investigations per 10,000 FITs. Our stratified results show that using more information from blood tests, varying the FIT cut-off can change the balance between the number of tests performed and the number of cancers missed in the investigation of symptomatic patients for CRC. The balance of investigations required, cancers diagnosed and missed is crucial to consider together when attempting to optimise diagnostic accuracy and health service provision in the real world. The consensus among all stakeholders needs to be reached on the threshold (risk of CRC) at which urgent investigation should be triggered, taking all these factors into account to optimally define this balance. This approach might allow the released diagnostic capacity to be used to support the lowering of the screening age in CRC screening in line with the NHS Long Term Plan.4
4.1 Strength and limitations
The size of the cohort and number of cancers diagnosed means there is sufficient power to stratify our results to understand the additional benefits of using age, sex and blood tests to identify those patients most at risk of CRC and potentially in which groups investigation can be safely avoided. It is important to note that these data reflect FIT in clinical use but still mirror the findings of research studies on FIT in selected populations. We used the OC-Sensor™ platform (Eiken Chemical Co.) to determine FIT levels. Other analysers are utilised in symptomatic pathways with the other most frequently used being the HM-Jackarc analyser. There is a possibility that different analysers may be inconsistent in certain demographics or parts of the fHb spectrum. The analysis presented focuses on the diagnosis of CRC. Other diagnoses such as inflammatory bowel disease (IBD) and polyps need to be considered FIT may present opportunities to diagnose IBD earlier or treat potentially premalignant polyps. However, for the context of this study, we have focused on the diagnosis of CRC as this is the primary purpose of the urgent diagnostic cancer pathway and freeing up diagnostic capacity could allow these patients timelier access to diagnosis. Finally, it is important to note the relatively high risk of death from other causes in our cohort which means that previously reported risks of CRC from studies that did not account for loss to follow up, or the competing risk of death will be overestimates of the 1-year cumulative risk of CRC. The high non-CRC death risk will potentially have impacted studies of diagnostic accuracy also where the follow-up was at least 1 year.
Our study assessed existing empirical categorisations of FIT, age and anaemia. Ideally, further optimisation and validation of pathways could be achieved by deriving and externally validating cut-offs and strata using continuous modelling of FIT, age and blood test results in this and other population-based data sets.
4.2 Context of what is already known
A recent review of 28,832 patients,18 selected for urgent referral and fully investigated, found a pooled sensitivity of 88.7% and specificity of 80.5% using a cut-off of 10 μg Hb/g faeces for CRC. In a further systematic review (16 studies, n = 35,945), the summary estimates of sensitivity and specificity were 91.0% (95% CI: 88.9–92.7) and 75.2% (95% CI 69.6–80.1) for all patients with a cut-off 10 μg Hb/g faeces for CRC. Furthermore, a systematic review of FIT in primary care reported that the at a cut-off of 20 μg Hb/g faeces only one additional CRC would be missed per 1000 patients investigated at a CRC prevalence of 2%.19 A number of other research studies have observed optimal FIT cut-offs of ≥20 μg Hb/g faeces or higher.15, 17 NICE recommended a threshold for investigation of patients suspected of having CRC of 3% yet current studies often have a detection risk of just 1%–2% suggesting further optimisation of the pathway could be achieved to identify patients above a 3% threshold of CRC.8, 20 A 3% threshold for investigation would result in fewer investigations such as colonoscopy which currently have limited capacity due to COVID-related backlogs and increasing demands for investigation.6 However, this need to reduce investigations has to be set against the need for a safety net and minimise the likelihood of missed cancer diagnoses to ensure the successful implementation of symptomatic FIT pathways.
Higher cut-offs for FIT above the currently recommended ≥10 μg Hb/g faeces cut-off have been previously suggested. For example, an optimal FIT cut-off of 19 μg Hb/g faeces was found in 5040 patients giving a sensitivity of 85.4% in a population with a risk of cancer of 3.0% (151/5040).15 The authors suggest a tailored approach to the use of FIT and produced estimates for optimal cut-offs based on age and referring symptoms but focused on diagnostic accuracy rather than, as we do, the balance of risks and benefits to the health service as a whole. In an analysis of a population from Oxford, UK of 16,604 patients with low-risk symptoms for CRC and a CRC risk of 0.8% the addition of blood tests to FIT results while improving specificity decreased sensitivity for the diagnosis of CRC.20 The authors concluded FIT plus blood tests did not improve discrimination for CRC. However, this was a low-risk population, and the authors did not consider the threshold at which the investigation should be performed. Our data on the additional value of blood tests at the lower end of the fHb spectrum are consistent with other studies, including two from Scotland that have recently reported.7, 21 Data from Tayside, the first to describe FIT for symptomatic patients in the UK, have also shown the potential value of haemoglobin and microcytosis in optimising triage.7 Our results suggest that FIT, blood tests and age could be used to refine protocols to implement FIT into 2WW pathways maintaining a balance between detection of CRC and the need to undertake endoscopic investigations.
Previous attempts to optimise FIT with other markers have shown limited benefit (FAST, COLONPREDICT).22, 23 However, these tools have used different combinations across the full range of fHb results—it is unlikely that any marker will add to the value of high fHb (≥100 μg Hb/g faeces or similar). Reduction of missed CRC below any threshold for urgent referral, based on an FIT result alone or in combination with other markers, may be improved by repeat testing.24, 25 We have not included the repeat-tested group in our analysis. Although further work is required to validate this approach the ‘cost’—financial and otherwise of a missed CRC is much higher than that of a repeat FIT. As such, the two approaches may be complementary in improving the use of FIT. Optimisation of FIT is likely to be of greatest value when the fHb result is ‘intermediate’, although a consensus definition of such terms is required.26
4.3 Clinical significance
The value of FIT results and the ‘added value’ of other factors to an intermediate or low FIT result depends on the context. The decisions in primary care are often based on heuristic judgements such as ‘Does this patient have cancer?’ or ‘Does this patient need to be referred, and if so on which pathway?’ As such, GPs may use FIT in settings where their pre-test clinical judgement of CRC risk is low and FIT is used for reassurance as well as situations where pre-test clinical suspicion of cancer is high, and FIT is used to confirm the suspicion of CRC specifically, and everything in between. For example, a ‘negative’ FIT result might lead GPs to consider (and investigate or refer for) other types of cancer that can cause gastrointestinal symptoms. Alternatively, intermediate fHb results in pre-menopausal women with anaemia may lead to needless anxiety, urgent referral and invasive examinations of little value. We have experienced all these scenarios in Nottingham since the inception of our pathway (Figure S1), and this current study provides the evidence to support a more nuanced understanding of how a combination of blood tests and demographics may be helpful to GPs when counselling patients in Primary Care before and after an FIT test. Our current pathway in Nottingham includes a threshold of 20 μg Hb/g with normal blood tests and the use of a cut-off of 4–20 μg Hb/g with abnormal blood tests (Figure S1).
Healthcare policy-makers and future guidelines should note that FIT alone with a single cut-off is unlikely to be a panacea for optimising colorectal cancer diagnosis. We show that the 1-year cumulative risk of CRC among symptomatic people undergoing an FIT in primary care varies hugely in intermediate FIT ranges, depending on their age and whether they have anaemia (the value of thrombocytosis was less). Our results are likely to be generalisable to the whole of England and most of the UK where FIT is being widely used to triage patients for investigation. Furthermore, our work demonstrates that a cut-off of FIT10 identifies sub-populations that have a 1-year cumulative risk of CRC much lower than anticipated. This inevitably contributes to the overwhelming backlog for relevant investigations, in particular colonoscopy, which competes with the resource required for a more effective CRC screening programme. If a nationally agreed cancer threshold of 3% is to be applied then future guidance may need to reconsider what FIT cut-offs should be recommended, and how age and anaemia should be included in these pathways. Operational delivery of such pathways across primary and secondary care is possible as evidenced by the uptake of FIT usage in our population.
AUTHOR CONTRIBUTIONS
Colin J Crooks: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); writing – original draft (equal). Ayan Banerjea: Data curation (equal); investigation (equal); project administration (equal); writing – review and editing (equal). James Jones: Data curation (equal); formal analysis (equal); visualization (equal); writing – review and editing (equal). Caroline Chapman: Data curation (equal); methodology (equal); writing – review and editing (equal). Simon Oliver: Data curation (equal); project administration (equal); writing – review and editing (equal). Joe West: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); writing – original draft (equal). David James Humes: Conceptualization (equal); data curation (equal); methodology (equal); writing – original draft (equal).
CONFLICT OF INTEREST STATEMENT
All authors declare no competing interests.
ACKNOWLEDGEMENTS
We would like to acknowledge all members of the Lower GI steering group in Nottingham who have helped implement this pathway.
Declaration of personal and funding interests: None.
AUTHORSHIP
Guarantor of the article: David Humes acts as guarantor for the article. All authors have approved the final version of the manuscript.




