Disease modification in chronic spontaneous urticaria
Pavel Kolkhir, Manuel P. Pereira, and Frank Siebenhaar contributed equally to the preparation of this manuscript.
Abstract
Chronic spontaneous urticaria (CSU) is a debilitating, inflammatory skin condition characterized by infiltrating immune cells. Available treatments are limited to improving the signs and symptoms. There is an unmet need to develop therapies that target disease-driving pathways upstream of mast cell activation to inhibit or delay the progression of CSU and associated comorbidities. Here, we aim to define disease modification due to a treatment intervention and criteria that disease-modifying treatments (DMTs) must meet in CSU. We have defined disease modification in CSU as a favorable treatment-induced change in the underlying pathophysiology and, therefore, the disease course, which is clinically beneficial and enduring. A DMT must fulfil the following criteria: (1) prevents or delays the progression of CSU, (2) induces long-term, therapy-free clinical remission, which is the sustained absence of CSU signs and symptoms without the need for treatment, and (3) affects the underlying mechanism of CSU, as demonstrated by an effect on disease-driving signals and/or a biomarker. DMTs in CSU should slow disease progression, achieve long-lasting disease remission, target disease-driving mechanisms, reduce mast cell-activating IgE autoantibodies, target cytokine profile polarization, and normalize the gut microbiome and barrier. Treating CSU at the immune system level could provide valuable alternatives to pharmacotherapy in CSU management. Specific DMTs in CSU are yet to be developed, but some show potential benefits, such as inhibitors of Bruton's Tyrosine Kinase, IL-4 and IL-13. Future therapies could prevent CSU signs and symptoms, achieve long-term clinical benefits after discontinuing treatment, and prevent associated concomitant disorders.
Abbreviations
-
- Ab
-
- antibody; aaCSU, autoallergic; CSU
-
- aiCSU
-
- autoimmune CSU
-
- AIT
-
- allergen immunotherapy
-
- APC
-
- antigen-presenting cell
-
- ASST
-
- autologous serum skin test
-
- ASU
-
- acute spontaneous urticaria
-
- BHRA
-
- basophil histamine release assay
-
- BTK
-
- Bruton's tyrosine kinase
-
- CSU
-
- Chronic Spontaneous Urticaria
-
- CU
-
- chronic urticaria
-
- CSA
-
- cyclosporine
-
- DMARDs
-
- disease-modifying antirheumatic drugs
-
- DMMDs
-
- disease-modifying-migraine-drugs
-
- DMT
-
- disease-modifying treatment
-
- FDA
-
- Food and Drug Administration
-
- IBD
-
- Inflammatory bowel disease
-
- Ig
-
- immunoglobulin
-
- IL
-
- interleukin
-
- IVIG
-
- intravenous immunoglobulin
-
- JAK/STAT
-
- Janus kinase/signal transducer and activator of transcription
-
- LPS
-
- lipopolysaccharide
-
- S1PR
-
- sphingosine-1-phosphate receptor
-
- MC
-
- mast cell
-
- MS
-
- multiple sclerosis
-
- QoL
-
- quality of life
-
- RA
-
- rheumatoid arthritis
-
- SCF
-
- stem cell factor
-
- SCFAs
-
- short-chain fatty acids
-
- TNF
-
- tumor necrosis factor; TPO, thyroid peroxidase
-
- TSLP
-
- thymic stromal lymphopoietin
-
- UAS
-
- Urticaria Activity Score
-
- UAS7
-
- weekly Urticaria Activity Score
1 INTRODUCTION
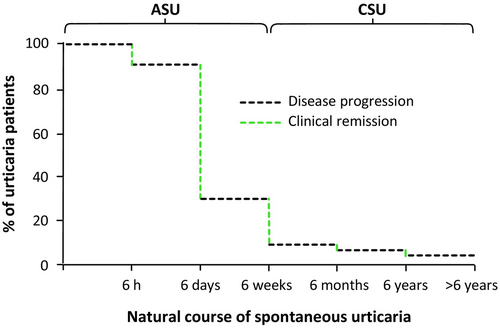
Chronic spontaneous urticaria (CSU) is a serious, debilitating skin condition of long duration with a relapsing–remitting course and an overall lifetime prevalence of about 1.4% in the global population.1, 2 The clinical signature of CSU is the recurrent sudden appearance of intensively itchy wheals and/or angioedema.1 Besides these highly disfiguring visible skin changes, which alone make CSU tremendously burdensome, in many cases, periods of years or even decades elapse before a patient's CSU is fully controlled by responding to treatment or achieving spontaneous remission, meaning they endure a long ordeal (Figure 1). Moreover, the cutaneous signs and symptoms markedly impact patients' quality of life (QoL)3 and have an enormous socioeconomic impact.4
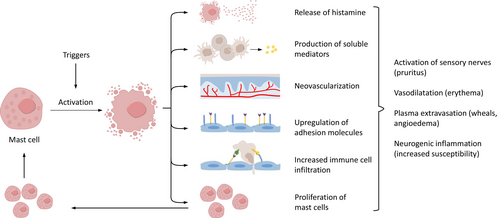
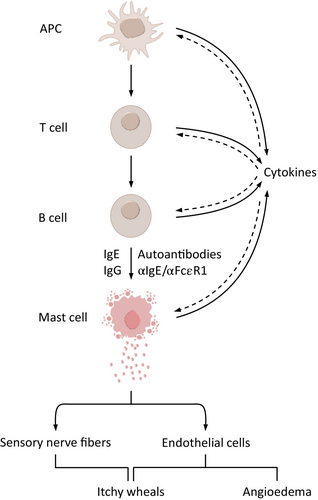
Recent interest in CSU pathogenesis has led to major insights into the complexity of the underlying disease mechanisms. Today, it is clear that CSU is a mast cell (MC)-dependent disease and that the activation and degranulation of skin MCs are responsible for the signs and symptoms. It is also clear that CSU is a chronic inflammatory disease. Structural changes of affected tissue or organs occur in many other chronic inflammatory diseases. In asthma, for example, lung changes often lead to alterations in the airway tissue composition and structure, such as increased thickness in airway smooth muscle, known as airway remodeling.5 CSU does not involve skin remodeling; nevertheless, the skin of some patients with CSU shows features of chronic inflammation, including increased numbers of MCs, eosinophils, basophils, and other infiltrating cells, upregulated cytokines, neovascularization, and increased expression of vascular adhesion molecules (Figure 2).6, 7 Together, these changes are linked to clinical consequences and may explain why the skin of CSU patients is more susceptible to MC-degranulating signals and wheal formation.6 For example, raised MC numbers in the skin correlate with disease activity; those with active CSU have more skin MCs than those in remission or healthy controls.8 Patients with CSU also have an increased cellular infiltrate of CD4+ T cells primarily of the Th2 subtype; however, Th1 cells, Th17 cell-derived cytokines, neutrophils, and monocytes are also present.9
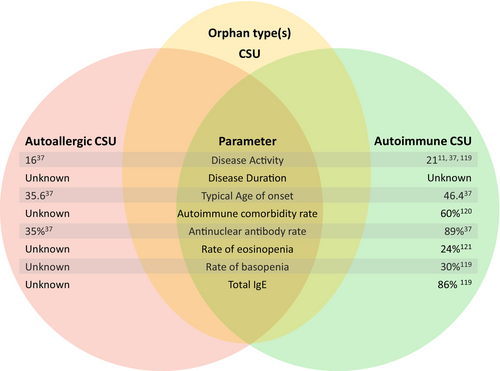
Importantly, major downstream drivers of skin MC activation and degranulation have been identified, and two CSU endotypes have been described, which differ in their pathogenic mechanisms: type I autoimmune or autoallergic CSU (aaCSU) and type IIb autoimmune CSU (aiCSU, Figure 3).10 In aaCSU, autoantigens crosslink IgE autoantibodies bound to their high-affinity receptor, FcεRI, on skin MCs, causing their degranulation.11 In aiCSU, skin MCs are activated and degranulated by IgG autoantibodies that target FcεRI or FcεRI-bound IgE. Most patients with CSU have aaCSU, with MC-activating IgE autoantibodies, or aiCSU, with MC-activating IgG autoantibodies. Some patients have both, and others have neither, pointing to the need to identify and characterize additional relevant mechanisms of MC activation in CSU.1, 10-13 More than half of CSU patients have aaCSU, aiCSU is less common, and about 7% have both.14
Further research is needed to understand what causes the production of MC-activating autoantibodies that leads to CSU and how autoantibody profiles change during the disease.
Immune cells such as basophils, eosinophils and T cells are known to migrate from the blood into the skin of patients with CSU, further contributing to urticaria symptoms. About 14% and 10% of patients show decreased basophil and eosinophil counts, which are associated with aiCSU, high CSU activity, and poor response to treatment.15
Acute spontaneous urticaria (ASU) is frequent, often associated with upper airway infections, and, in most cases, is self-limited.16 According to most studies, ASU progresses to CSU in less than 10% of cases, although some studies report higher rates.1 Based on cumulative average estimates, only 17% of patients experience CSU remission in the first year; in most patients, it persists much longer.17 Currently, it is unclear what factors drive this. CSU, on average, lasts for several years and can then show spontaneous remission. In most cases, CSU is self-limiting, with patients experiencing spontaneous remission after 2–5 years.18 However, up to 50% of patients have CSU for over 5 years. Cumulative weighted averages estimate that the proportion of patients remitting at 5 and 20 years is 45% and 73%, respectively.17 Markers linked to longer disease duration and severity include anti-thyroid antibodies, concomitant chronic inducible urticaria, hypertension, and poor control with antihistamine updosing or second-line treatments.1 However, what causes spontaneous remission has not been fully defined. CSU relapses in about one-fifth of patients, and those with increased serum levels of IgE and anti-thyroid peroxidase antibodies are at higher risk of CSU recurrence.19 Many patients with CSU express IgE antibodies against thyroid peroxidase (TPO), which can cause autoallergic MC activation. IgE-anti-TPO may activate MCs in addition to TPO, which is not in the skin, cross-reacting autoantigens such as eosinophil peroxidase, which is present in CSU skin, can crosslink IgE-anti-TPO.20 Wheals can present in IgE-mediated diseases such as allergies and anaphylaxis (usually acute), which is a rare differential diagnosis of CSU.
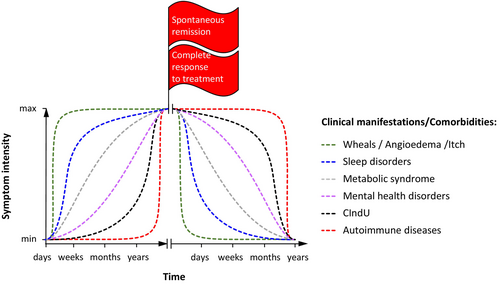
Patients with CSU develop certain comorbidities more often than the general population, and this can increase in frequency and intensity over time, indicating progression of the disease and increasing patient burden. In around 80% of patients with CSU who have autoimmune diseases, these diseases appeared within 10 years of the CSU diagnosis.21 In addition to autoimmune diseases, CSU comorbidities include sleep disorders,3, 22 sexual dysfunction,23 and mental health issues.24 One, 2, and 3 years after disease onset, 7.5%, 9.6%, and 10.9% of patients with CSU have depression and/or anxiety, respectively (Figure 4).25
Currently, available CSU treatments are limited to improving the signs and symptoms.26 Licensed treatments recommended by the international guideline for urticaria, that is, antihistamines and omalizumab, inhibit MC mediator effects and activation, respectively.26 There is a high medical need to develop and introduce novel therapies into clinical practice that modulate CSU pathology with the potential for disease modification. Such treatments must target disease-driving pathways upstream of MC activation so that inhibition of these pathways prevents or delays the progression of CSU, including the development of comorbidities. Upstream drivers of skin MC activation include autoantibodies, cytokines, and possibly gut microbiome changes with subsequent barrier dysfunction (Figure 5).12, 27, 28 The concept of disease modification in CSU has not yet been defined. To address this, we developed a definition of disease modification due to a treatment intervention and criteria that must be met by disease-modifying treatments (DMTs) for CSU. We also provide guidance on how to differentiate DMTs from symptom-preventing therapies. Additionally, we outline current limitations and potential DMTs under development for CSU.
2 THE CONCEPT OF DISEASE MODIFICATION
The concept of disease modification was first introduced in the field of rheumatology with the term disease-modifying antirheumatic drugs (DMARDs) in 1976.29 The idea of DMARDs then gained momentum in the 1980s, with several publications describing their use.30 Traditionally, disease-modifying potential was used to describe drugs that slow the progression of rheumatoid arthritis (RA),31 “reducing, suppressing, or eliminating disease activity, and thereby preventing the extended and perhaps permanent effects of disease activity.”32 In disease modification, drug treatments target the underlying pathophysiology, with the ultimate long-term aim of achieving an enduring clinical benefit after discontinuation. DMTs are now available for many diseases; Table 1 provides an overview of some selected chronic diseases.
| Disease modification definition | Disease-modifying treatments |
|---|---|
| Food allergies | |
| To induce (1) short-term efficacy, (2) a sustained clinical effect, (3) long-term efficacy and (4) disease-modifying effects, defined as sustained, significant, and clinically relevant efficacy in the post-treatment years. The goal is defined as (5) the cure of allergy with a sustained absence of allergic symptoms in post-treatment years.114 | Allergen immunotherapy (AIT) is used in the disease-modifying treatment of allergies, such as food allergies.115 |
| Allergic rhinitis | |
| Long-term remission of allergic symptoms and clinical benefits that can persist for years even after discontinuing treatment.43 | AIT is used in the disease-modifying treatment of allergic rhinitis to prevent rhinitis from progressing to asthma and lower medication use.115 |
| Asthma | |
| Prevention of asthma and new sensitizations and clinical benefits that can persist for years after discontinuing treatment.43, 44 | A treatment that can potentially achieve asthma remission, defined as the sustained absence of asthma symptoms and exacerbations, stable lung function, and no need for systemic corticosteroids to treat asthma. Some biologics, such as omalizumab and dupilumab, have shown potential for disease modification.116 AIT has demonstrated sustained, long-term reductions across clinical outcomes in asthma, such as exacerbations, pneumonia, and hospitalisations.117 |
- Abbreviations: AIT, allergen immunotherapy.
Interestingly, the Food and Drug Administration (FDA) abandoned the “disease-modifying effect” terminology in the recently updated 2018 draft guidance, which now refers to a “persistent effect on disease course”. Whereas the guidelines from the FDA and European Medicines Agency previously provided recommendations on obtaining a specific label claim for disease modification, the guidelines from 2018 provide recommendations relating to treatment goals only, i.e., without guidance on getting specific label claims.33
3 DIFFERENTIATING DISEASE-MODIFYING TREATMENTS FROM SYMPTOM-PREVENTING TREATMENTS
There are distinct differences between symptom-preventing and disease-modifying drug treatments (Figure 6). The former relieve or prevent the signs and symptoms of a disease without changing its course. For example, first-line CSU treatment with a second-generation H1-antihistamine stabilizes the histamine H1 receptor and inhibits its activation by histamine.16 Histamine plays an important role in CSU downstream of the activation and degranulation of skin MCs. However, histamine does not drive MC activation and degranulation, and antihistamines do not modify CSU. Furthermore, histamine is not the only mediator released when MCs degranulate. Effects of other inflammatory mediators released by MCs may activate tissue-resident immune cells and/or drive the recruitment of other immune cells into the skin.
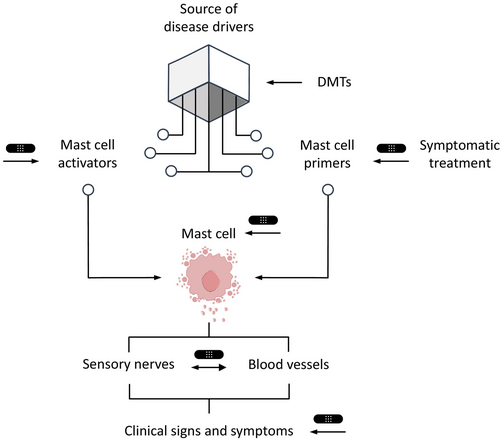
In contrast, DMTs mediate their effect by targeting a disease's underlying pathophysiology, resulting in a long-term, enduring, therapy-free and clinically beneficial change in the disease course. This could be slowing or preventing the recurrence of further signs and symptoms or the development of comorbidities. Thus, in CSU, a DMT must target pathogenic drivers upstream of skin MC activation and degranulation, for example, by reducing the production of IgE antibodies against autoantigens in aaCSU or MC-activating IgG autoantibodies in aiCSU. DMTs can be curative therapies, such as using antibiotics to treat an infection, which achieves complete disease eradication and restores health.34 “Cure” results from a treatment that heals a health problem, meaning it is unlikely to return. A curative treatment is the ultimate DMT, and for this to apply to CSU, the treatment would need to do two things: achieve clinical remission and reduce the risk of relapse to the level of people who have never experienced CSU.
4 THE DEFINITION OF DISEASE MODIFICATION IN CSU
To evaluate the potential disease-modifying properties of a CSU treatment, it is important to understand the criteria that define disease modification developed for other chronic inflammatory diseases; this can help determine what disease modification for CSU must include, as described below.35
4.1 Preventing or delaying disease progression
A DMT should prevent or delay the progression of CSU by targeting its underlying molecular pathomechanisms and pathophysiology, as is the case with lecanemab in Alzheimer's disease. This monoclonal antibody removes β-amyloid plaques from the brain and may slow cognitive and functional decline.36
In multiple sclerosis (MS), sphingosine-1-phosphate receptor (S1PR) modulators have shown disease-modifying abilities. Widely expressed in the body, S1PRs regulate pathological immune responses and neurological functions. Subtype 1 of the S1PR (S1PR1) is expressed on lymphocytes and is involved in MS pathogenesis; thus, S1PR1-directed pharmacological interventions modulate immune cell trafficking of autoreactive lymphocytes and reduce infiltration into the central nervous system.37
From a clinical viewpoint, disease progression in CSU is characterized by changes in the pathogenesis that lead to worsening clinical manifestations and the occurrence of comorbidities, the “urticarial march”. However, unlike MS or RA, there is no organ damage in urticaria. Patients with CSU are more likely to develop at least one comorbidity than the general population.38 Comorbidities that occur and worsen during the disease include, but are not limited to, sleep disorders and mental health conditions such as depression and anxiety. Studies have also shown a significant association between chronic urticaria (CU) and metabolic syndrome and its components of obesity, diabetes, hyperlipidemia and hypertension.39 Additionally, in almost two-thirds of patients, the onset of chronic inducible urticaria coincides with the onset of CSU.
From clinical experience, some comorbidities, such as mental health and sleep disorders, are likely driven by having CSU rather than being due to the mechanisms that cause CSU. Thus, symptom-preventing non-disease modifying therapies that induce a complete response may prevent some comorbidities or delay their development, but not others. This alone is insufficient to regard a therapy as a DMT. From an immunological viewpoint, the progression of disease in CSU remains ill-defined. There is some evidence that patients with aaCSU may develop aiCSU over time, but further studies are needed to confirm this and characterize the underlying mechanisms.14 If so, treatments that prevent or delay this process should be regarded as DMTs, as CSU is not a disease where the intensity of symptoms increases over time.
4.2 Inducing long-term disease modification and therapy-free clinical remission
A recent consensus report concludes that “remission” is the best term to describe the total absence of signs or symptoms of CSU in the absence of treatment.40 However, there is no consensus on defining “long-term” remission, i.e., the minimum duration of absence of signs and symptoms after cessation of therapy.41 Six months without recurrence of CSU signs and symptoms has been proposed to constitute long-term remission.42 Defining “long-term remission” requires further discussion and consensus.
Allergen immunotherapy (AIT) is considered disease-modifying or curative in allergic diseases; it can induce a long-lasting immunological and clinical tolerance toward the causal allergen, with clinical remission that persists after the treatment is stopped. IgE-mediated allergy may be causative in a small number of patients with CU, and specific immunotherapy with these allergens may benefit those patients.41 AIT is the only currently available treatment for allergic rhinitis with disease-modifying effects, which include long-term and treatment-free remission of allergic symptoms or potential prevention of asthma and new sensitizations.43
A recent report on chronic inflammatory skin diseases defines DMTs as inducing deep and therapy-free remission beyond drug discontinuation.35 In asthma, DMTs are defined as treatments that can potentially achieve the goal of asthma remission, defined as the sustained absence of asthma symptoms and exacerbations, stable lung function, and no need for systemic corticosteroids.44 Long-lasting remission after treatment discontinuation should also be a goal of DMTs in CSU.
Of note, CSU is characterized by the occurrence of spontaneous remission. The cessation of symptom-preventing treatment in patients with a complete response can be followed by long-term remission; this alone is insufficient to regard treatment as disease-modifying. However, like allergic rhinitis, disease modification does not necessarily require remission. In allergic rhinitis, the complete absence of symptoms is only rarely achieved with immunotherapy. There is a therapeutic algorithm in CSU, and disease modification can also be achieved if a step-down approach in this algorithm is possible.16 As with long-term remission, whether this disease modification is based on the previous drug treatment or whether individual patients have spontaneous remission is unclear.
4.3 Targeting and affecting the underlying disease-driving mechanisms, as demonstrated by an effect on disease-driving signals and/or a biomarker
In Alzheimer's disease, disease modification requires that the intervention impacts the underlying pathology and pathophysiology.45 Drugs showing the most promise in disease modification in Alzheimer's disease are the targeted intravenous monoclonal antibodies, lecanemab, donanemab and aducanumab.36 These targeted therapies retard the underlying pathophysiology of the disease, leading to cell death or dysfunction.45 The targeted disease process must be in the pathway responsible for producing clinical decline, and there must be a clinical response corresponding to the disease intervention; this aligns with the view of the FDA, which defines DMTs as agents that exert their effects on the disorder's underlying pathology and pathophysiology.46
Biomarkers which assess changes in disease-driving signals are important for evaluating responses to DMTs. In RA, for example, interleukins (IL) play a significant role in disease progression; reductions in plasma IL-6 levels can be used as a biomarker in RA patients treated with methotrexate because post-treatment levels strongly predict radiographic progression.47 In atopic dermatitis, lowering the levels of IL-4/IL-13 could potentially be used as a biomarker to measure the efficacy of dupilumab and assess the progressive improvement in disease activity and modification.48
The same principles should be applied to CSU DMTs; treatments should target disease-driving mechanisms upstream of MC activation, such as autoantibodies, activation of immune cells, or secretion of cytokines, and the effects of these mechanisms should relate to clinical benefits reflected in biomarker changes. For example, markers of aiCSU, a positive basophil histamine release assay (BHRA) and autologous serum skin testing (ASST) decreased after treatment with cyclosporine.49 Targeting autoantibody production in CSU should reduce disease activity linked to decreased autoantibody levels or biomarkers such as ASST. Similarly, a treatment that targets disease-driving cytokines should provide a clinical benefit linked to reduced levels of the targeted cytokine or a biomarker of this effect.
5 DEFINING DISEASE MODIFICATION IN CSU
The concepts of disease modification developed in other chronic inflammatory diseases include one or more of three features: (1) slowing of disease progression, (2) inducing long-lasting remission beyond drug discontinuation, and (3) targeting disease-driving mechanisms. All three of these features are relevant for disease modification in CSU. We, therefore, propose that disease modification in CSU be defined as shown in Box 1.
BOX 1. The Definition of Disease Modification in CSU
Disease modification in CSU is a favorable treatment-induced change in the underlying pathophysiology and therefore, the disease course, which is clinically beneficial and enduring.
6 THE CRITERIA FOR TARGETED DISEASE-MODIFYING TREATMENTS IN CSU
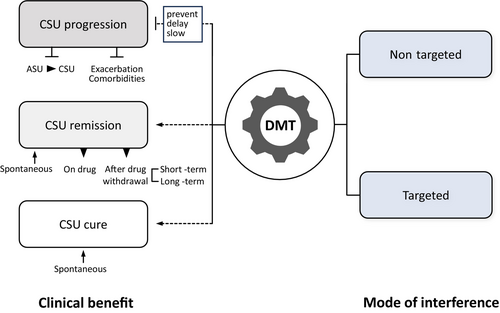
DMTs in CSU aim to positively change the pathophysiological processes that drive CSU to prevent disease progression and achieve clinical remission (Figures 7 and 8). Thus, DMTs need to fulfill the three criteria outlined in Box 2.
BOX 2. Criteria for DMTs in CSU
(1) Preventing, delaying, or slowing the progression of CSU.
(2) Inducing long-term clinical improvement with a step-down in the algorithm or long-term therapy-free remission, that is, the sustained absence of CSU signs and symptoms without the need for treatment.
(3) Affecting the underlying mechanism of CSU, as demonstrated by an effect on disease-driving signals and/or a biomarker.
7 THE DISEASE-MODIFYING POTENTIAL OF EMERGING TARGETED CSU TREATMENTS USED OFF-LABEL AND/OR UNDER DEVELOPMENT
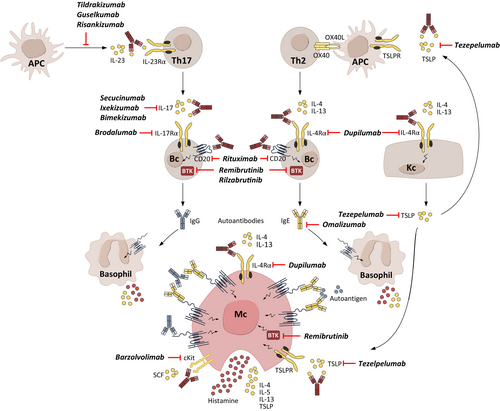
Mechanisms upstream of MC activation include IgE and IgG autoantibodies, proinflammatory cytokines, and possibly dysbiotic microbiota that compromise the gut barrier.10, 28, 50, 51 Some of the targeted treatments currently used off-label for the treatment of CSU and/or under development for CSU aim to disrupt these mechanisms and, therefore, have the potential to be disease-modifying (Table 2 and Figure 8).
| Treatment | Mechanism of action | Level of evidence | Data on long-term clinical remission |
|---|---|---|---|
| Targeted therapies used off-label and/or under development for CSU | |||
| Remibrutinib | Interferes with BTK signaling by inhibiting the pathways leading to skin MC degranulation. | Phase 3 | A 52-week study showed good long-term safety and sustained efficacy during treatment with remibrutinib in patients with CSU, but no long-term, treatment-free remission has been demonstrated.118 However, the treatment-free follow-up period was short, so more evidence is required to investigate any longer-term disease-modifying effects. |
| Dupilumab | A humanized IgG4 monoclonal antibody that targets the IL-4 receptor α chain.119 Dupilumab affects MCs and infiltrating cells in CSU skin lesions,120 may act upon B cells, and downregulates IgE.68 | Phase 3 | A case series of patients with CSU who had failed to respond to antihistamines and more than one year of omalizumab therapy up to 600 mg monthly demonstrated that two-thirds of patients remained in complete remission (UAS = 0) at 14–22 months post-discontinuation with dupilumab.69 |
| Rilzabrutinib | Interferes with BTK signaling by inhibiting the pathways leading to skin MC degranulation. | Phase 2 | Rilzabrutinib 400 mg thrice daily demonstrated a significant reduction from baseline in itch severity score at Week 12 compared to placebo.121 It also significantly reduced UAS7 from baseline at Week 12 compared to placebo. A 40-week open-label extension trial is ongoing to determine if rilzabrutinib induces any long-term changes or remission in patients with CSU. |
| Tezepelumab | A humanized immunoglobulin G1 monoclonal antibody that binds to the ε chain of CD3 on T-cells with high affinity.122 | Phase 2 | In a 32-week study with tezepelumab (210 mg every 4 weeks or 420 mg every 2 weeks), omalizumab (300 mg every 4 weeks), or placebo, a sustained treatment effect was observed in both tezepelumab arms, and was not achieved with omalizumab or placebo. Sustained improvements in UAS7 with tezepelumab occurred alongside continued reductions in IL-5 and IL-13, and were independent of changes in IgE. In anti-IgE-naïve patients, tezepelumab treatment led to continued reductions in CSU disease activity and biomarkers after treatment discontinuation, suggesting a sustained effect from TSLP blockade after treatment cessation. |
| Rituximab | An anti-CD20 monoclonal antibody that targets and depletes CD20+ B-lymphocytes.78 | Case reports | A limited number of case reports in patients with recalcitrant CU have shown potential long-term, treatment-free remission after discontinuation of the drug. However, no randomized, controlled trials have been conducted to support its use in CU.123 One report described a 51-year-old woman with refractory autoimmune urticaria who received four weekly infusions of rituximab 375 mg/m2 and methotrexate 15–25 mg/week (to prevent the development of antibodies against rituximab).79 The patient achieved complete CU remission six weeks after her last infusion and could permanently discontinue oral glucocorticoids and antihistamines.79 |
| Secukinumab | An anti-IL-17 therapy. IL-17 is highly expressed in peripheral blood and the skin of patients with CSU.124 | Phase 2 | Eight patients with antihistamine- and/or omalizumab-refractory CSU treated with 150 mg secukinumab once a week for four weeks, then 150 mg every two weeks achieved significant improvements in symptoms, assessed via UAS7. After three months, UAS7 was reduced by 82%. CSU relapsed when treatment was stopped after 6–8 months, suggesting that anti-IL-17 is required for longer periods.61 |
| Tildrakizumab | Monoclonal anti-IL-23 antibody. | Case series | Small case series have shown successful treatment in three patients with omalizumab-refractory type IIb aiCSU.73 |
| Ruxolitinib | Janus kinase inhibitor. | Case report | A patient with chronic urticaria refractory to several treatments, including antihistamines and cyclosporine, was successfully treated with ruxolitinib. After one week, urticarial symptoms improved markedly, and the patient was almost asymptomatic at Week five of treatment.77 However, further controlled data are needed to confirm these results. |
| Older, non-targeted therapies | |||
| Cyclosporine | Inhibits cell-mediated autoimmune responses by downregulating the Th1 lymphocyte response and T-cell-dependent antibody formation in B-lymphocytes.125 | Developed | It was shown to induce possible lasting remission after treatment is discontinued.119 This treatment can be especially effective for patients with markers of aiCSU, that is, low total IgE, positive anti-thyroid antibodies and/or positive BHRA.24, 91 |
| Mycophenolate | Unknown disease-modifying mechanism of action, but it possibly prevents lymphocyte and monocyte glycoprotein glycosylation, inhibiting the recruitment of leukocytes to inflammatory sites and impairing antigen presentation.95 | Developed | A small study of nine patients with severe CSU over 12 weeks showed the potential of mycophenolate as a DMT.94 All patients discontinued glucocorticoids by the end of the trial, with the improvement persisting for ≥6 months after discontinuation. |
| Dapsone | Unknown disease-modifying mechanism of action, but it has anti-inflammatory effects, which include interference with the function of lysosomal enzymes, disruption of integrin-mediated neutrophil adhesiveness, and inhibition of prostaglandin and leukotriene activity.126 | Developed | One trial involving 22 patients with antihistamine refractory CSU showed improvement when treated with dapsone within the first week, with three patients achieving complete resolution of their CSU.96 A larger retrospective review including 79 patients with CSU refractory to four times the standard dose of H1 antihistamines showed that tapering down the drug after two months of controlled symptoms led to ten patients having sustained remission for up to 16 months.97 |
| Sulfasalazine | The mechanism of action in CSU is unknown, but the salicylate component acts as an anti-inflammatory agent.127 | Developed | In a retrospective review of 39 patients with CSU refractory to antihistamines and other therapies, sulfasalazine treatment added to existing therapies resulted in 84% of patients improving within three months, and 51% of patients became asymptomatic on sulfasalazine plus antihistamines by si x months.127 Ninety per cent of steroid-dependent patients could discontinue glucocorticoids; sulfasalazine was gradually withdrawn once symptoms were controlled, and 28% remained asymptomatic after sulfasalazine was discontinued, requiring only antihistamine therapy.98 |
| High-dose intravenous immunoglobulin | Inactivates autoreactive T cells and can downregulate B cell production of antibodies by neutralizing pathogenic antibodies. On macrophages, dendritic cells and neutrophils, it can block Fcε receptor-mediated activity.100 | Developed |
Clinical benefits were observed in 9/10 patients with severe, recalcitrant CU, three of whom had prolonged complete remissions at three years follow-up.128 |
- Abbreviations: aiCSU, autoimmune chronic spontaneous urticaria; BHRA, basophil histamine release assay; BTK, Bruton's tyrosine kinase; CSU, chronic spontaneous urticaria; CU, chronic urticaria; DMT, disease-modifying treatment; Ig, immunoglobulin; IL, interleukin; MC, mast cell; TSLP, thymic stromal lymphopoietin; UAS, Urticaria Activity Score; UAS7, weekly Urticaria Activity Score.
7.1 Reducing mast cell-activating IgG autoantibodies
The presence of IgG autoantibodies that activate skin MCs through binding to FcεRI or IgE-FcεRI complexes is a defining feature of aiCSU. The reduction of these autoantibodies has been linked to the efficacy of the CSU treatment Bruton's tyrosine kinase (BTK) inhibitors. In a clinical trial with the BTK inhibitor fenebrutinib, IgG-anti-FcεRI+ CSU patients showed more reduction in disease activity with a low-dose treatment than IgG-anti-FcεRI− patients. In IgG-anti-FcεRI+ patients, fenebrutinib markedly reduced IgG-anti-FcεRI levels at all doses compared to placebo, and greater reductions in IgG-anti-FcεRI were associated with greater decreases in disease activity.52, 53 Fenebrutinib is no longer under development for CSU due to unwanted off-target effects; however, other BTK inhibitors, including remibrutinib and rilzabrutinib, can also be expected to reduce autoantibody levels in CSU, but this needs further investigation. In patients with features of aiCSU, such as insufficient responses to omalizumab, very low total IgE levels, or a positive CU index (an in vitro BHRA), the BTK inhibitor remibrutinib was shown to be equally effective compared to those without these features.54, 55 Furthermore, inhibition of BTK with rilzabrutinib reduced pathogenic IgG autoantibodies in pemphigus vulgaris56 and with remibrutinib in Sjögren's syndrome.57
IL-17 promotes the production of autoantibodies in several autoimmune diseases, such as pemphigus vulgaris and psoriatic arthritis.58, 59 In patients with CSU, serum levels of IL-17 are raised and correlate with disease activity and ASST, a marker of aiCSU, compared with healthy controls.60 In a single report, treatment with secukinumab, an anti-IL-17A antibody, improved CSU disease activity within 90 days in eight non-responders to antihistamines and omalizumab. All reached a weekly Urticaria Activity Score (UAS7) of 6–8.61 BTK inhibitors and possibly IL-17-targeted treatments have disease-modifying potential in aiCSU.
7.2 Reducing mast cell-activating IgE autoantibodies
Emerging evidence suggests that many chronic inflammatory diseases present IgE autoantibodies against autoantigens.62 Up to 80% of patients with CSU have IgE autoantibodies, which, along with their autoantigens, activate and degranulate skin MCs.62 Th2 cytokines drive the production of IgE.62 IL-4 promotes the differentiation of B cells into plasma cells that produce IgE, and stimulates the class switching of immunoglobulin production from IgM or IgG to IgE.63 IL-13, similar to IL-4, promotes IgE production and class switching and often works in conjunction with IL-4 to enhance the generation of IgE-secreting plasma cells.64 Dupilumab is a monoclonal antibody that inhibits the action of IL-4 and IL-13 by binding to the IL-4 receptor α chain. In atopic dermatitis, dupilumab treatment reduced IgE levels by up to 76%.65-67 In a recent case study, dupilumab effectively treated CSU by acting on B cells and downregulating IgE, with subsequent effects on IgE receptor expression.68 Another case report has demonstrated that dupilumab maintains CSU remission in 67% of treated patients for up to 22 months following therapy discontinuation.69 Together, these data highlight the disease-modifying potential of dupilumab in aaCSU (Figure 8).
7.3 Targeting cytokine profile polarization
Therapies that target signaling pathways or cytokines which promote the production of IL-17 and IL-4/13, that is, IL-23 and thymic stromal lymphopoietin (TSLP), or target them directly, have the potential to induce sustained remission and disease modification by inhibiting cytokine profile polarization and upregulating cytokine production.70
IL-23 is a proinflammatory cytokine that promotes the survival, maintenance, and activation of Th17 cells; patients with CSU have been reported to exhibit high IL-23 serum levels linked to a positive ASST, a defining marker for aiCSU.60, 71, 72 Treatment with tildrakizumab, an anti-IL-23 monoclonal antibody, markedly improved disease activity and control in three patients with CSU.73 Before treatment, all patients had high disease activity, angioedema, non-responsiveness to standard treatments, very low total IgE levels, increased IgG-anti-TPO antibodies, anti-nuclear antibodies and/or IgG-anti-FcεRI/IgE antibodies, that is, markers of aiCSU.
Tezepelumab is a monoclonal antibody that targets TSLP receptors, preventing TSLP/TSLP receptor interaction. Importantly, TSLP receptors are expressed in MCs; the TSLP ligand contributes to the development of MCs and plays an important role in preventing apoptosis of cutaneous MCs.74 Tezepelumab is currently being developed to treat CSU. Previous studies revealed an upregulation of TSLP in urticaria lesions,75 while tezepelumab was shown to reduce serum IgE levels, indicating the disease-modifying potential of this drug.76
The Janus kinase signal transducer and activator of transcription (JAK/STAT) pathway is paramount for intracellular signaling, promoting inflammation in various skin conditions. Cytokines associated with the JAK/STAT pathway can play a role in the pathophysiology of CSU; blocking the JAK/STAT pathway with JAK inhibitors may thus constitute a therapeutic approach with disease-modifying potential.71 In a case report, a patient with primary myelofibrosis and chronic urticaria refractory to several treatments, including antihistamines and cyclosporine, was successfully treated with ruxolitinib (JAK 1/2 inhibitor). After one week, urticarial symptoms improved markedly, and the patient was almost asymptomatic at Week five of treatment.77 Controlled studies with JAK inhibitors are needed to assess the efficacy of this drug class in CSU. Two JAK inhibitors, TLL018 (Phase 1, NCT05373355) and povorcitinib (Phase 2, NCT05936567) are in clinical trials for CSU.
B cell depletion with an anti-CD20 monoclonal antibody,78 such as rituximab, may have potential DMT abilities in CSU. No randomized, controlled trials support the idea, but limited case reports have shown potential long-term, treatment-free remission after discontinuation of the drug in patients with aiCSU.79
7.4 Normalizing the gut microbiome and barrier
The role of the intestinal microbiome, barrier and permeability in the pathogenesis of chronic diseases—also beyond the gastrointestinal tract—has gained increasing importance in recent years.80 Composition alterations in the intestinal microbiome have been observed in patients with CSU, with a shift in the balance from beneficial to opportunistic bacteria.81 It is not yet entirely understood why the activation threshold of skin MCs from CSU patients is reduced. However, proinflammatory cytokines, such as IL-33, and microbial byproducts, such as lipopolysaccharide (LPS), contribute to this phenomenon.82
One finding that emphasizes the role of the intestinal microbiome and barrier in the pathogenesis of CSU is that a reduction in the concentration of short-chain fatty acids (SCFAs) was detected in metabolome analyses of patients with CSU.28, 83 SCFAs are produced by intestinal bacteria and play a role in the homeostasis of epithelial barrier function.84 Furthermore, they can inhibit MC activation85 and regulate the colonization of opportunistic bacteria.86 There is increasing evidence that therapy with probiotics, which increases SCFA-producing bacteria, positively affects the development of allergic diseases87 and the activity of chronic urticaria.88 Achieving a balance between beneficial and opportunistic bacteria within the microbiome of patients with CSU could, therefore, contribute to the normalization of SCFAs and LPS concentrations and has potential use as a DMT.
7.5 Modulating neuroinflammation
Evidence shows that certain neuroinflammatory receptors, such as the Mas-related G protein-coupled receptor X2 (MRGPRX2), are highly expressed in skin MCs.89 These receptors' responses to stimuli triggers MC degranulation and the release of proinflammatory mediators, leading to whealing, itching and stimulation of sensory neurons. Patients with CSU have upregulated expression of MRGPRX2 and its agonists by skin MCs, meaning these could serve as potential biomarkers for disease progression.89 Additionally, these biomarkers could represent promising targets for treating CSU, but DMTs in this field are still under development.
8 NON-TARGETED TREATMENTS WITH DISEASE-MODIFYING POTENTIAL
Several non-targeted agents used to treat CSU have shown disease-modifying potential (Table 2). Cyclosporine, the third-line treatment recommended in the urticaria guidelines,16 has been shown to induce possible lasting remission after treatment is discontinued and has good supporting data.90-92 This treatment can be especially effective for patients with aiCSU markers, such as low total IgE, elevated levels of anti-thyroid antibodies, and/or a positive BHRA.24, 91, 92 However, cyclosporine has potentially serious adverse effects such as hypertension and renal insufficiency, although these are uncommon at the doses used for CSU.93 Cyclosporine acts on MCs, which is unlikely to cause disease-modifying effects, and on T cells, which could be disease-modifying.49
Mycophenolate has shown efficacy and possible disease modification in a small study of nine patients with severe CSU over 12 weeks.94 Two-thirds of patients experienced markedly improved Urticaria Activity Scores (UAS) during treatment. All patients could discontinue glucocorticoids by the end of the trial, with the improvement persisting for ≥6 months after discontinuation. The disease-modifying mechanism of mycophenolate is unknown, but possible mechanisms include the prevention of lymphocyte and monocyte glycoprotein glycosylation, inhibiting the recruitment of leukocytes to inflammatory sites, and impairing antigen presentation.95
Dapsone is another agent that has shown potential disease-modifying effects. A trial involving 22 patients with CSU showed improvement in itch and urticaria scores within the first week, with three patients achieving complete resolution of their CSU.96 A larger review of patients with CSU showed that 13% of patients had sustained remission for up to 16 months after tapering down the drug after two months of controlled symptoms.97 Close monitoring is required when taking this treatment as it can cause serious adverse effects, such as haematological changes. Additionally, the observed disease-modifying impact may be due to the natural, spontaneous remission of the disease, and there is little other evidence of a true modifying effect.
Sulfasalazine can be useful as an add-on to standard therapies in patients with refractory CSU.98 In a retrospective review of 39 patients with CSU refractory to antihistamines and other therapies, sulfasalazine treatment added to existing therapies improved CSU in 84% of patients within three months, and 51% of patients became asymptomatic on sulfasalazine plus antihistamines by six months.98 In steroid-treated patients, 90% could discontinue glucocorticoids; sulfasalazine was gradually withdrawn once symptoms were controlled, and 28% remained asymptomatic after sulfasalazine was discontinued, requiring only antihistamine therapy.98 A possible disease-modifying mechanism is that sulfasalazine inhibits cytokine release, including IL-1, IL-2, IL-6, IL-12, and TNF, thereby creating an anti-inflammatory environment.99
High-dose intravenous immunoglobulins (IVIGs) have the potential to inactivate autoreactive T cells and can downregulate B cell antibody production by neutralizing pathogenic antibodies. On macrophages, dendritic cells, and neutrophils, IVIGs can block FcεR-mediated activity.100 Clinical benefits were observed in 9/10 patients with severe, recalcitrant chronic urticaria, three of whom had prolonged complete remissions at three years follow-up.100
9 TARGETED TREATMENTS WITH DISEASE-MODIFYING POTENTIAL THAT SHOULD BE DEVELOPED FOR CSU
Treatments that deplete plasma cells producing MC-activating autoantibodies are more likely to result in long-lasting remission and disease modification than treatments that transiently reduce the secretion of autoantibodies by plasma cells (see Table 2). The challenge lies in creating treatments that specifically target plasma cell-producing autoantibodies responsible for activating MCs while sparing those that have protective antibodies. Treatments that deplete plasma cells, such as bortezomib101 or daratumumab,102 are effective but also target protective immune functions. Immunoablation procedures followed by autologous stem cell transplantation, wherein immune memory is eradicated, and the immune system is effectively reset, indicate that the loss of pathogenic immune memory can lead to long-term remission or even a cure.103-106 However, it is important to note that these treatments are typically applied for life-threatening autoimmune conditions and may not be suitable for CSU. A recent study in mice provided promising proof of concept, demonstrating the feasibility of targeting long-lived plasma cells in vivo.107 This study labelled plasma cells with antibodies containing a known antigen. Subsequently, these antigen-specific plasma cells secreted antibodies targeting the labeled antigen, triggering processes like antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity. This approach offers a valuable opportunity for developing therapeutic interventions against autoantibody-mediated diseases, including CSU.
Drugs targeting the lymphocyte surface and adhesion molecules OX40/OX40 ligand and Th2-promoting cytokine IL-13 pathways in CSU could have potential disease-modifying roles, but clinical data is not yet available.75, 108
Anti-IL-25 is another potential DMT. IL-25 can increase Th2 cytokines, resulting in enhanced Th2-type immune responses. Thus, inhibiting IL-25 can dampen Th2-type immune responses.109 C5a inhibition might lead to disease modification by diminishing the activation of MCs and reducing the inflammatory infiltrate in the skin of CSU patients.
Finally, a novel approach to disease modification that could be further developed is vaccine therapy, such as targeting IgE or type 2 cytokines by inducing endogenous neutralizing antibodies. These techniques have been used successfully in animal models.110
10 CONCLUSION AND OUTLOOK
By treating CSU directly at the immune system level, DMTs could provide valuable alternatives to pharmacotherapy in CSU management. Specific DMTs in CSU are yet to be developed, but future therapies could potentially prevent CSU signs and symptoms and associated comorbidities. Treating the underlying pathophysiology ultimately aims to achieve long-term clinical benefits after discontinuing therapy, thus lowering the need for additional pharmacotherapies. Our proposed definition for disease modification in CSU is a favorable treatment-induced change in the underlying pathophysiology and, therefore, the disease course, which is clinically beneficial and enduring.
Regarding the best time to treat patients to achieve the best possible disease-modifying outcome, there is evidence from other chronic conditions that early treatment in patients whose urticaria is not yet chronic, that is, those with ASU, could potentially prevent the disease from becoming chronic. Indeed, this has been demonstrated in plaque psoriasis, another chronic skin condition, where treatment with secukinumab in patients with new-onset disease (≤12 months) resulted in high and sustained skin clearance, indicating that treatment may be more effective if used early in the disease course.111 Patients with ASU who progress towards CSU have markers for positive ASST results, thyroid autoimmunity, and basopenia at baseline; these could be used as prognostic biomarkers in patients who would benefit from early treatment interventions.112, 113
The most promising data for disease modification are seen in aiCSU; at least 8% of patients with CSU have aiCSU, and almost all of them also have autoallergy.14 Cyclosporine, BTK inhibitors and rituximab have all shown good efficacy and indicators of long-term remission in patients with aiCSU.52, 79, 91
Longer-term longitudinal studies are required to assess the impact of each treatment, including follow-up assessments after drug discontinuation to identify DMTs that are effective in CSU. Disease remission of CSU is the absence of signs or symptoms after treatment withdrawal, which should be the aim of any DMT.40
AUTHOR CONTRIBUTIONS
All authors contributed to the consensus process that led to this manuscript, reviewed the literature, critically reviewed and revised the article, and proofread and approved the final version. Marcus Maurer, Pavel Kolkhir, Manuel P. Pereira, Frank Siebenhaar, Ellen Witte-Händel, and Martin Metz conceived the project, drafted the article, and coordinated and supervised its finalization.
ACKNOWLEDGEMENTS
We thank Gillian Brodie from Orbit Medical Communications Ltd. for writing and editorial support. We thank Julie McLaren, Jessica Gereige, and Philip Sugerman for critical input and helpful discussions. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
M. Maurer is or recently was a speaker and/or advisor for and/or has received research funds from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL, Behring, FAES, Genentech, GI Innovation, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Roche, Sanofi/Regeneron, Third Harmonic Bio, UCB, and Urich. P. Kolkhir has been a speaker for Novartis, ValenzaBio and Roche. M. P. Pereira has received research funding from Almirall; he is an investigator for Allakos, Celldex Therapeutics, Incyte, Sanofi, and Trevi Therapeutics; he has received consulting fees, speaker honoraria and/or travel fees from AbbVie, Beiersdorf, Celltrion, Doctorflix, Eli Lilly, GA2LEN, Galderma, Menlo Therapeutics, Novartis, P.G. Unna Academy, Sanofi, StreamedUP, and Trevi Therapeutics. F. Siebenhaar is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Blueprint, Celldex, Cogent, Escient, Granular, GSK, Invea, Noucor, Novartis, Moxie, Sanofi/Regeneron, and Third Harmonic Bio. H. Bonnekoh was a speaker/consultant and/or advisor for and/or has received research funding AbbVie, Novartis, Sanofi Aventis and ValenzaBio outside of submitted work. T. Buttgereit is or recently was a speaker and/or advisor for Aquestive, AstraZeneca, Biocryst, CSL-Behring, GlaxoSmithKline, Hexal, KalVista, Medac, Novartis, Pharming, Roche, Sanofi, and Takeda. K. Krause has received grants/research support or served as a consultant for Bayer, Beiersdorf, CSL Behring, Moxie, Novartis, Roche/CHUGAI, SOBI, and Takeda. She has no conflicts of interest related to the content of this article. M. Magerl is or recently was a speaker and/or advisor for and/or has received research funding from Biocryst, Pharming, Takeda, CSL Behring, Ionis Pharmaceuticals, KalVista, and Pharvaris. M. Muñoz has received personal fees from AstraZeneca, Celldex Therapeutics, Takeda, and GA2LEN, UNEV, and has received research funding from Roche outside the submitted work. J. Scheffel has conducted studies for, received research funds from and advised Allakos, Ascilion, AstraZeneca, CSL Behring, Escient, Evommune, Novartis, Sanofi, Third Harmonic Bio, Third Rock, and Thermo Fisher. R. Treudler has received honoraria for lectures, advisory boards and/or research funding from AbbVie, ALK-Abello, Almirall, CSL-Behring, Leo Pharma, Novartis, Pfizer, Sanofi-Genzyme, Takeda, Viatris, all outside this paper. K. Weller is or recently was a speaker and/or advisor for and/or has received research funds from Moxie, Novartis, Pharvaris, Shire, Takeda, and Uriach. T. Zuberbier has received institutional funding for research and/or honoraria for lectures and/or consulting from Amgen, AstraZeneca, AbbVie, ALK, Almirall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, FAES, HAL, Henkel, Kryolan, Leti, L'Oreal, Meda, Menarini, Merck, MSD, Novartis, Pfizer, Sanofi, Stallergenes, Takeda, Teva, UCB, and Uriach. In addition, he is a member of ARIA/WHO, DGAKI, ECARF, GA2LEN, and WAO. M. Metz is or recently was a speaker and/or consultant for Amgen, AstraZeneca, Argenx, Celldex, Celltrion, Escient, Jasper Therapeutics, Novartis, Pharvaris, Regeneron, Sanofi, and Third Harmonic Bio. E. Witte-Händel, J. W. Fluhr, L. Herzog, L. Alice Kiefer, S. Neisinger, P. Pyatilova, N. Nojarov, D. Terhorst-Molawi, K-C. Bergmann, S. Prins, S. Frischbutter, E. Maria Grekowitz, and A. Ramanauskaité declare no conflicts of interest regarding this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




