Sounding the alarmins—The role of alarmin cytokines in asthma
Abstract
The alarmin cytokines thymic stromal lymphopoietin (TSLP), interleukin (IL)-33, and IL-25 are epithelial cell-derived mediators that contribute to the pathobiology and pathophysiology of asthma. Released from airway epithelial cells exposed to environmental triggers, the alarmins drive airway inflammation through the release of predominantly T2 cytokines from multiple effector cells. The upstream positioning of the alarmins is an attractive pharmacological target to block multiple T2 pathways important in asthma. Blocking the function of TSLP inhibits allergen-induced responses including bronchoconstriction, airway hyperresponsiveness, and inflammation, and subsequent clinical trials of an anti-TSLP monoclonal antibody, tezepelumab, in asthma patients demonstrated improvements in lung function, airway responsiveness, inflammation, and importantly, a reduction in the rate of exacerbations. Notably, these improvements were observed in patients with T2-high and with T2-low asthma. Clinical trials blocking IL-33 and its receptor ST2 have also shown improvements in lung function and exacerbation rates; however, the impact of blocking the IL-33/ST2 axis in T2-high versus T2-low asthma is unclear. To date, there is no evidence that IL-25 blockade is beneficial in asthma. Despite the considerable overlap in the cellular functions of IL-25, IL-33, and TSLP, they appear to have distinct roles in the immunopathology of asthma.
1 INTRODUCTION
The epithelial cell-derived alarmins thymic stromal lymphopoietin (TSLP), interleukin (IL)-33, and IL-25 are pleiotropic cytokines found to regulate infection, inflammation, and metabolic homeostasis. They play important roles in many diseases including cancer, allergic and immune-mediated diseases, and diseases of the central nervous system1-7; however, clinical trials blocking TSLP, IL-33, and IL-25 have mostly been conducted in diseases driven by type 2 (T2) inflammation, and most extensively in patients with asthma. The alarmin cytokines have emerged as key players in asthma pathogenesis, driving T2 cytokines, most notably IL-4, IL-5 and IL-13, from multiple effector cells.8-10 T2 cytokine expression initiates allergic mechanisms including eosinophilic inflammation, immunoglobulin E (IgE) class switching, B-cell growth, and goblet cell metaplasia.11 Randomized controlled trials (RCTs) have evaluated the efficacy of anti-alarmin monoclonal antibody (mAb) therapies for the treatment of asthma. This review will focus on the immunopathogenic role of alarmins in asthma, and their unique contribution to asthma pathophysiology as evidenced by alarmin-targeted drugs in clinical trials.
2 ALARMIN EXPRESSION AND ASTHMA
Airway epithelial cells (AECs) represent the first line of host defense against environmental pathogens and serve as a major source of alarmins IL-25, IL-33, and TSLP. Alarmins are constitutively expressed in AECs and released following activation of transmembrane pattern recognition receptors (PRRs) including toll-like receptors (TLR) 1, 2, 4-6 and 10, C-type lectin receptors (CLRs) such as dectin-1, mannose receptors (MRs), and protease-activated receptors (PARs) such as PAR-2. TLRs 3, 7-9 and other PRRs are found intracellularly on organelle membranes (e.g., endosomes), and CLRs including MRs and dendritic cell-specific ICAM-3 (DC-SIGN) are also present intracellularly (Figure 1).2, 12, 13 Innate immune cells, adaptive immune cells, and structural cells are also capable of releasing alarmins; however, the mechanisms are not well-understood yet.
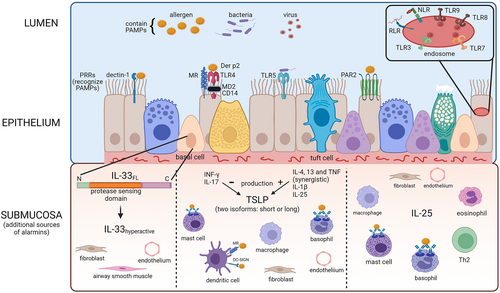
TSLP, a member of the IL-2 family of cytokines, was initially identified as a pre-B-cell growth factor.14 The TSLP receptor heterodimer consisting of TSLPR and IL-7Rα is broadly expressed within a wide variety of hematopoietic cell populations.14-22 In response to exogenous and endogenous danger signals, epithelial cells at barrier surfaces as well as immune cells secrete TSLP,23-32 which promotes T2 inflammation through activation of myeloid dendritic cells (DC), type 2 innate lymphoid cells (ILC2s) and polarization of naïve T helper (Th) cells to Th2 cells.15-19
IL-33 a member of the IL-1 cytokine family mediates early immune development and polarization toward T2 inflammation.33, 34 IL-33 is constitutively expressed in the nuclei of epithelial and vascular endothelial cells and other structural cells exposed to the environment35-38 and immune cells; murine models show that innate immune cells such as macrophages and mast cells are important producers of IL-33 that contribute to ILC2 activation.39, 40 Damage to bronchial epithelial cells at mucosal barrier interfaces by allergen-associated protease activity, pollutants, viral and fungal pathogens leads to further increases in IL-33 release, which serves as a damage-associated molecular pattern (DAMP) signal.41, 42 In murine models, epithelial cells exposed to fungal allergens show extracellular accumulation of ATP, activation of P2 purinergic receptors and increases in intracellular Ca2+ levels driving the IL-33 secretion signaling cascade.42 This, however, remains to be fully described in human cells as quantification is complicated by rapid posttranslational modifications that reduce IL-33 levels.41
Two isoforms of IL-33 are described, a full-length and a processed form, which are both upregulated during airway inflammatory responses.41 IL-33 is active in its full-length form and passively released while cleavage of the N-terminal by the inflammasome complex and caspases-1, −3, and −7 inactivates this isoform.41 When oxidized, IL-33 undergoes a rapid conformational change forming two disulfide bonds that convert IL-33 to an inactive form Additionally, IL-33 is sensitive to serine protease activity exhibited by fungi (Aspergillus fumigatus), allergens such as house dust mite (Dermatophagoides pteronyssinus) and released by mast cells, eosinophils, and Th2 cells that cleave IL-33 in the sensor domain.41, 43 The shorter isoform exhibits increased affinity for the IL-33 receptor; additionally, neutrophil proteases enhance IL-33 activity in virally induced exacerbations of asthma.41 Thus oxidation and proteolytic cleavage are potentially major players in regulating IL-33 activity.
A rare genetic variant of the IL-33 gene has been described that prevents the formation of bio-active splice variants of lL-33. Subjects who carry this rare allele have a 40% lower IL-33 expression and a 50% reduction in asthma risk. As such, amino acids 66–111 on IL-33 are an important functional domain, and interference with IL-33 cleavage may be a useful strategy to reduce IL-33–mediated responses in asthmatic conditions.44 Furthermore, a report of a patient with a rare chromosomal duplication encompassing the entire IL-33 gene is described as having many atopic disease manifestations including elevated IgE, hypereosinophilia, eosinophilic skin infiltrations, food allergy, and mild asthma, demonstrating for the first time the effects of IL-33 overexpression in humans.45
IL-33 binds to a membrane-bound heteromeric receptor consisting of ST2 (IL-1RL1) and its coreceptor IL-1 receptor accessory protein (IL1RAcP). ST2 is predominantly expressed by CD4+ and CD8+ T cells, mast cells, ILC2s, macrophages, eosinophils, dendritic cells, basophils, natural killer (NK) T cells, and NK cells.42 Under basal conditions, ST2 expression has been observed in subsets of CD4+Foxp3+ Tregs in the lung.41 IL-33 induces expression of the epidermal growth factor-like molecule amphiregulin (AREG) in Tregs, promoting immune regulation.42 IL-33 also indirectly regulates Tregs by inducing IL-2 production by ILC2s, DCs, or mast cells, that subsequently promote Treg expansion. Therefore, IL-33 can both promote or regulate CD4+ T cell immunity depending on the tissue microenvironmental cues.
A soluble form of ST2 (sST2) may function as a decoy receptor.46 IL-33 and ST2 levels are markedly increased in the sputum of eosinophilic asthmatics47 and in those experiencing acute exacerbations.48-50 IL-33 is one of the earliest cytokines released in response to inhaled allergens in patients with allergic asthma and is elevated in lung epithelium,35 airway smooth muscle,49 and bronchoalveolar lavage,51 correlating positively with asthma disease severity.36
IL-25 (also known as IL-17 E) is a member of the IL-17 family but has distinct biological effects compared with the rest of the IL-17 family.52, 53 IL-25 is preformed and stored in the cytoplasm of airway epithelial cells. Other IL-25-secreting cells include endothelial cells, activated Th2 cells, alveolar macrophages, eosinophils, basophils, bone marrow–derived mast cells, and fibroblasts.54-60 The IL-25 receptor is a disulphide-linked dimer comprised IL-17Rα and IL-17Rβ subunits61 and expressed mainly on innate immune cells.62-69 IL-17Rβ expression is increased on myeloid and plasmacytoid DCs in blood and sputum of allergic asthmatic patients postallergen challenge, and IL-25 stimulation mediates TLR9 expression on plasmacytoid DCs suggesting a potential additional role in aeroallergen-induced immune responses in asthma.63 IL-25 directly augments T2 cytokine production by TSLP-DC-activated Th2 memory cells, by enhancing the production of Th2 transcription factors in an IL-4-independent manner.54
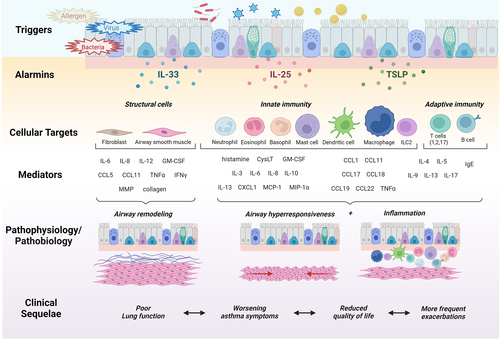
Together, the epithelial cell alarmins present an effective defense against pathogens encountering the airway mucosal surface; however, when dysregulated, they can orchestrate immune cell functions that drive pathological responses in the asthmatic airways leading to clinical sequelae (Figure 2).
3 CLINICAL TRIALS OF ANTI-ALARMINS IN ASTHMA
Clinical models have demonstrated a role for the alarmins in the pathogenesis of allergic asthma.27, 70-72 This hypothesis was initially tested using a mAb, AMG157 (now known as tezepelumab), which binds to TSLP preventing engagement with its receptor.73 The double-blind, randomized study examined mild, allergic asthmatic subjects challenged with an inhaled allergen known to induce both an early asthmatic response (EAR), a late asthmatic response (LAR), eosinophilic airway inflammation and airway hyperresponsiveness (AHR) to inhaled methacholine (Figure 3). The participants had evidence of baseline T2 airway inflammation, with elevated blood eosinophils, sputum eosinophils, and exhaled nitric oxide (FeNO). Treatment with AMG157 normalized these biomarkers after the first dose, indicating that constitutive release of TSLP is necessary for the maintenance of T2 inflammatory biomarkers in these mild asthmatic subjects. In addition, treatment with AMG157 significantly attenuated the allergen-induced EAR, LAR, sputum eosinophilia, and methacholine AHR.
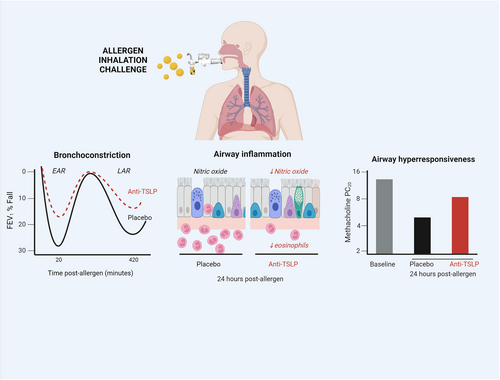
These results were, in part, replicated in another study using an inhaled antibody fragment, which also binds TSLP (CSJ117; ecleralimab).74, 75 Using a similar design, CSJ117 significantly attenuated allergen-induced bronchoconstrictor responses and airway inflammation. Collectively, these results demonstrate that inhaled allergens induce the release of TSLP, presumably from the airway epithelium, which can then drive, at least in part, allergen-induced inflammatory and bronchoconstrictor responses, as well as increased AHR. IL-25 and IL-33 have not yet been carefully investigated in a similar model of allergic asthma.
4 ASTHMA EXACERBATION
Outcomes in asthma clinical trials include assessments of asthma control, forced expiratory volume (FEV1), quality of life, and frequency of severe asthma exacerbations. It is widely accepted that reducing the risk of asthma exacerbations is the most important of these. Exacerbations are the time of risk in terms of asthma-related mortality; have significant impacts on patients and their families; are associated with loss of time at work or at school; and are costly to the health care system, especially when patients need hospitalization or intensive care management. Asthma exacerbations are frequent occurrences, with even patients with the mildest asthma at risk.76 Exacerbations are driven by viral and bacterial infections, and environmental allergen exposures.77 Patients with severe asthma can experience two or more exacerbations per year, and this can lead to progressive loss of lung function.78 Frequency of exacerbations is reduced but not fully prevented with inhaled corticosteroid (ICS) ± long-acting β2-agonists (LABA), hence the need for therapies providing additional protection.79 All the currently approved biological therapies for asthma have used severe exacerbation frequency as the primary outcome variable for their pivotal clinical trials; significant reduction is demonstrated by blocking IgE,80 TSLP,81, 82 or IL-5 or IL-4/IL-13 pathways,83-89 and it is widely recognized that patients with higher blood eosinophil counts respond best to these biologics.90, 91 The alarmins are an attractive target for preventing exacerbations, acting upstream of T2 cytokines and triggering a broad range of proinflammatory processes that are orchestrated through dendritic cells and key effector cells that drive eosinophilia.
4.1 Mechanism of TSLP in exacerbations
Clinical trials targeting IL-5 and IL-13 pathways have provided irrevocable evidence that T2 cytokines, at least in part, drive asthma exacerbations.83-89 TSLP, located upstream in the inflammatory cascade, plays a key role in the induction of T2 responses by stimulating myeloid DC maturation and upregulation of MHC class II, and the co-stimulatory molecules CD40, CD86, CD54, CD80 CD83 and CD-LMAP20 (Figure 4). In turn, TSLP-primed DCs promote the upregulation of OX40L on naïve T cells driving an inflammatory T2 phenotype that secretes IL-4, IL-5, IL-13, and TNFα but not IL-10.92 TSLP can also induce DC production of chemokines that induce recruitment of Th2 cells, eosinophils and neutrophils to the inflamed airways,27, 93, 94 and primes DCs to induce the expansion and functionality of CRTH2+ CD4+ Th2 memory cells, driving differentiation of Tregs and hindering developments of FOXP3+ Tregs.95-97 Although CD4+ and CD8+ T cells do not directly respond to TSLP under resting conditions, TSLPR surface expression increases on these cells following activation,98 facilitating TSLP signaling and TCR stimulation to promote proliferation and differentiation to Th2 cells.70, 99, 100 TSLP directly supports B-cell lymphopoiesis101, 102 from multilineage-committed CD34+ progenitor cells103 and suppresses the production of IL-10 by pulmonary Tregs104 altogether maintaining a T2 inflammatory airway environment that is conducive to exacerbating asthma.
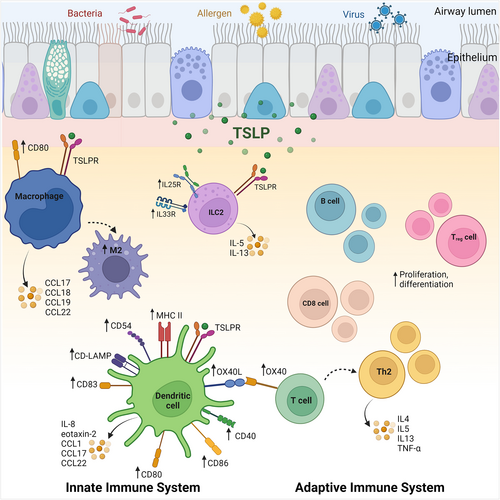
In some preclinical asthma models, TSLP drives a predominant ILC2-driven airway inflammatory response.105 This is also observed in severe asthmatics where increased airway levels of TSLP alone or with TL1A have been shown to mediate the corticosteroid insensitivity of airway ILC2s.106 In murine models, TSLP, IL-33, and IL-25 signaling reciprocally enhance each other's receptor protein expression on lung ILC2s, enhancing ILC2 activation, and possibly driving ILC2 plasticity.107, 108
4.2 Anti-TSLP and exacerbation outcomes in clinical trials
The most compelling data regarding the clinical efficacy of anti-alarmin therapies come from the phase 3 Navigator study evaluating the anti-TSLP mAb tezepelumab, administered at a dose of 210 mg subcutaneously (sc) q4wk for 52 weeks. In this study of 1061 patients with severe, uncontrolled asthma and at least two asthma exacerbations in the past 12 months, tezepelumab substantially and significantly reduced the annualized asthma exacerbations rate (AAER) and lengthened the time to first exacerbation.109 Moreover, this reduction in AAER was maintained even in patients with low baseline blood eosinophil counts of <150 cells/μl. Patients receiving tezepelumab also had significantly better asthma control and health-related quality of life (HRQOL); while improvements in asthma control questionnaire (ACQ)-6 and asthma quality of life questionnaire (AQLQ) scores were statistically significant, these aggregate changes did not meet the minimal clinically important difference for these patient-reported outcome measures.
The Navigator study data lend credence to earlier publications reporting on the Pathway study, a phase 2b double-blind study of tezepelumab administered at 70 mg and 210 mg sc q4wks; 280 mg sc q2wks versus placebo for 52 weeks in 550 asthmatics not controlled by high doses ICS + LABA. This study reported a >50% reduction in AAER, and improvements in most other endpoints measured.81 These results are similar, and perhaps slightly better than other mAb intervention studies using anti-IL-5, anti-IL-5R, and anti-IL-4Rα,83, 85, 88 all of which are now approved for the management of severe asthma; however, no head-to-head comparisons are available. The reduction in AAER was similar in participants with blood eosinophils above and below 250 cells/μl and in those defined as T2 high and T2 low. Although AAER differed by season, tezepelumab reduced AAER across all seasons.110 Furthermore, evaluating the effect of tezepelumab in severe uncontrolled asthma with perennial allergy over a full year, including periods of seasonal allergies, showed the improvement in exacerbation rate was comparable in patients with and without perennial allergy, including those patients meeting or not meeting eligibility criteria for the anti-IgE biologic omalizumab.111 Interestingly, a higher reduction in exacerbation (84%) in those with 3 or more allergen reactivities demonstrates a clear positive effect in T2 asthma and effectiveness in the presence of allergy. Additional post-hoc evaluations of these data demonstrated a comparable effect of tezepelumab in patients with and without self-reported nasal polyps112 suggesting, again, that tezepelumab may work in a wider range of subjects.
4.3 Mechanism of IL-33 in exacerbations
Like TSLP, IL-33 plays a central role in activating both the innate and adaptive arms of immunity. IL-33 can activate resident DC to mature and induce differentiation of T cells into Th2 polarized cells9, 113, 114 and promote naïve CD4+ T cells to produce IL-5 and IL-3,33 which in turn stimulates eosinophil survival, adhesion, and degranulation.115 IL-33-activated DCs also promote mast cell survival, adhesion, and increased cytokine production and alveolar macrophage-mediated secretion of IL-13 (Figure 4).116, 117 Macrophages express both the membrane-bound ST2 and sST2 and upon IL-33 stimulation are either activated to promote M1 chemokine generation (CCL3) in naïve macrophages or to enhance the expression of M2 chemokine markers (CCL17, CCL18, CCL24) in previously polarized macrophages.117, 118 IL-33 is also a potent activator of ILC2s, upregulating the co-stimulatory molecules OX40L and PD-L1 and inducing T2 cytokine production,119, 120 including up to 100-fold more IL-5 and IL-13 compared with activated Th2 cells.121 IL-33 also regulates IL17A and IL-31 secretion indicating a role for IL-33/ST2 axis in Th2/IL-31 and Th17 immune responses.122
IL-33 also plays a role in initiating the adaptive response. Memory Th2 cells express higher levels of ST2 than effector Th2 cells and IL-33 induces the expression of IL-4, IL-5, and IL-13 in both.123, 124 Additionally, IL-33 can initiate IL-9 secretion from CD4+ T cells.125 These functions of IL-33 collectively drive T2 inflammation in a multifactorial manner. Larger clinical trials will be needed to determine whether IL-33 blockade indeed performs better in patients with low blood eosinophil levels, as this finding is somewhat counterintuitive in the context of IL-33 biology.
Rhinoviruses are major players in asthma exacerbations in both children and adults.126, 127 In models of viral-induced asthma exacerbations using mice infected with influenza virus or human cells exposed to rhinovirus-16, type II alveolar cells in mice and bronchial ciliated cells in human subjects were a major source of IL-33.126 Exacerbations characterized by increased airway hyperresponsiveness and T2 inflammatory response were inhibited by blocking ST2, highlighted a role for IL-33, and not TSLP.126 In addition, using a human exposure model of viral infection IL-33 induced type 2 cytokine generation by ILC2s during rhinovirus-induced asthma exacerbation.127 Additionally, IL-33 dampened both innate and adaptive type 1 inflammatory responses126 by causing epithelial and dendritic cells of mice to decrease IFN-β expression. Together, these data highlight the potential of therapies targeting the IL-33/ST2 axis for treating viral-induced asthma exacerbations.
4.4 IL-33 pathway and exacerbation outcomes in clinical trials
Recent clinical trials blocking components of the IL-33/ST2 axis have examined the effects on asthma exacerbations. In patients with moderate-to-severe asthma (GINA step 4–5) for at least 12 months, the anti-IL-33 mAb, itepekimab was administered over a 12-week period, alone at 300 mg sc q2wks, or together with the anti-IL4Rα mAb dupilumab 300 mg sc q2wks versus placebo.128 The study used a medication withdrawal study design, where treatments with LABAs were discontinued at week 4, and ICS tapered at weeks 6 through 9. At week 12, itepekimab significantly improved asthma control, an effect similar to that seen with dupilumab. Compared with dupilumab alone and the combination itepekimab plus dupilumab, the odds ratio for itepekimab was lowest in the <300 eosinophils/μl subgroup (0.46), and highest in the ≥300 eosinophils/μl subgroup (0.39), with the combination itepekimab plus dupilumab not as good as either treatment alone for asthma control. Itepekimab and dupilumab monotherapies significantly improved pre-bronchodilator FEV1 versus placebo by 0.14 L and 0.16 L, respectively. Itepekimab demonstrated the largest improvement (0.06 L vs 0.02 L for the other treatment groups) in the <300 eosinophils/μl subgroup, and monotherapies dupilumab and itepekimab showed significant improvements (0.30 L and 0.18 L, respectively, and 0.15 L for combination) in the ≥300 eosinophils/μl subgroup. Other endpoints ACQ-5 and AQLQ(S) improved to a similar degree in all active treatment groups, and T2 biomarkers including FeNO and serum IgE, eotaxin, and periostin were reduced by itepekimab, but the effect was generally less than that of duplilumab or combination therapy.
Astegolimab, a human ST2 mAb was studied at three doses (70 mg, 210 mg, 490 mg) sc q4wk for 52wks versus placebo in 502 patients with severe asthma. Overall, lower and higher doses significantly reduced AAER by 37% and 42%, respectively, with no significant improvement (22%) observed at the mid-range dose.129 Moreover, when the patients were stratified by eosinophil subgroups with cutoffs at 300 cells/μl and 150 cells/μl, the reduced AAER was still only seen at the lower and higher doses; in patients with eosinophil counts <300 cells/μl the AAER reduction was 35% and 54%, respectively; in patients with eosinophil counts ≥150 cells/μl the AAER reduction was 42% for both lower and higher doses. At the 210 mg dose, there was no significant AAER reduction overall or in any eosinophil subgroups. These results suggest that astegolimab, unlike tezepelumab, may be of lesser benefit in patients characterized as having a T2-high asthma phenotype. It is intriguing that there was no dose response for exacerbations and no effect in patients with eosinophils >300 cells/μl, although this might simply reflect the small sample size. Further confirmatory phase 3 randomized controlled trials blocking IL-33 and ST2 in patients with severe asthma are warranted.
5 LUNG FUNCTION
Variable bronchoconstriction is a hallmark of asthma, with inflammation contributing to persistent airflow obstruction in part due to irreversible remodeling of the airway wall.130 Periods of intense airway inflammation during asthma exacerbations are associated with a decline in lung function.78
5.1 Mechanisms of alarmins in lung function and AHR
The alarmin cytokines are believed to contribute to bronchoconstriction and airway remodeling (Figure 5). TSLP and IL-33 induce expression of smooth muscle actin, collagen, and fibronectin131, 132 Airway smooth muscle (ASM) cell proliferation and expression of IL-6, IL-8, and eotaxin-1 is mediated by TSLP.133, 134 Stimulation with IL-25 can modulate bronchial airway smooth muscle hyperplasia and collagen deposition leading to airway remodeling,135 and is reflected in asthmatic patients with high IL-25 transcripts having greater AHR than those with low transcript levels.136
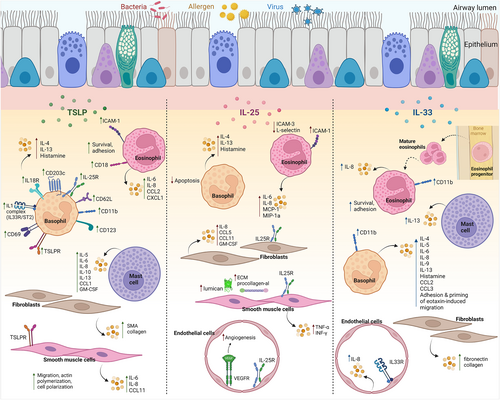
Alarmins act upstream of cellular pathways that ultimately trigger the release of mediators of bronchoconstriction. TSLP stimulation of mast cells induces the release of T2 cytokines including IL-5 and IL-13.24, 137 Basophils are a significant source of TSLP16 and express high levels of TSLPR16, 138; overnight incubation of basophils with TSLP upregulates epithelial alarmin cytokine receptor expression (TSLPR, IL-25R, ST2), activation markers (CD203c), T2 cytokines (IL-4, IL-13) and enhances degranulation of histamine.138 In mice, TSLP promotes bone-marrow-resident precursor cells to differentiate into basophils independently of IL-3.16, 139 This axis is not yet fully elucidated in humans and warrants further study. IL-33 promotes basophil IgE-dependent and IgE-independent release of histamine and secretion of IL-4, IL-5, IL-6, IL-8, IL-9, IL-13, CCL2 and CCL3.125, 140-142 IL-33 also induces basophil CD11b expression, adhesion, and primes eotaxin-induced migration.143, 144 IL-33 also induces production of IL-13 from mast cells.145 IL-25 inhibits basophil apoptosis and augments IgE-mediated basophil degranulation, but has no effect on inflammatory cytokine release.66 Mast cells are shown to express equal levels of IL-25R to those of eosinophils, T cells, and endothelial cells58; however, the effect of IL-25 stimulation of mast cells has not been reported.
5.2 Anti-TSLP and measures of airway hyperresponsiveness in clinical trials
AHR is a cardinal feature of asthma and is measured by increased bronchoconstrictor responses to inhaled direct-acting stimuli, such as methacholine,146 or inhaled indirect-acting stimuli, such as mannitol.147 AHR is not regularly tested in large clinical trials of severe asthma patients. A small mechanistic study in mild allergic asthmatics reported a significant 1.24 doubling dose (dd) improvement in methacholine PC20 after treatment with tezepelumab 700 mg IV q4wks for 12 weeks, versus 0.66 with placebo.73 Moreover, two recent publications examined the effect of tezepelumab treatment on AHR to mannitol in asthmatic patients. Treatment with tezepelumab for 28 weeks in a subgroup of patients with moderate-to-severe asthma significantly improved mannitol PD15 by 1.41 dd versus 0.57 dd with placebo148 although the lung function of some patients may have been lower than that recommended for mannitol challenge. In 40 mild–moderate asthmatics, treatment with tezepelumab for 12 weeks improved mannitol PD15 by 1.9 dd versus 1.0 dd in placebo (p = 0.06) at 4 weeks after the last dose, and this benefit was maintained 13 weeks after the last dose.149 In both mannitol investigations, dose shift magnitude is likely limited by methodology with the top dose of 635 mg. Both studies also reported a greater proportion of negative mannitol responders after treatment, thereby supporting the potential underestimation of a dose shift endpoint. The improvement in AHR was greater in those with eosinophilic asthma. Together, these studies validate the initial proof-of-concept publication by Gauvreau and colleagues that identified tezepelumab as a potentially effective asthma treatment.73 To date, the effect of IL-25 and IL-33 blockade on AHR has not been evaluated.
5.3 Anti-TSLP and measures of lung function in clinical trials
In small studies of patients with mild asthma and nearly normal lung function, it is not surprising that tezepelumab does not improve baseline FEF25-75%149 or FEV1.73 As discussed earlier, in mild allergic asthmatic patients undergoing allergen bronchoprovocation, the early and late bronchoconstriction responses significantly improved by 28% and 45%, respectively, after 12 weeks tezepelumab 700 mg IV q4wks,150 and 17% and 48%, respectively, after 12 weeks CSJ117 4 mg inhaled once daily.74, 75 In both studies inhibition of allergen-induced bronchoconstriction was accompanied by a major reduction in sputum eosinophils that did not correlate to the inhibition of the attenuation of fall in FEV1, suggesting that other cells, such as mast cells may be driving changes in lung function.
Treatment with the anti-TSLP mAb tezepelumab has consistently improved lung function. The Pathway study, conducted in patients selected for having reversible airflow obstruction and resting bronchoconstriction, demonstrated that tezepelumab improved pre-bronchodilator FEV1 by 120–150 ml greater than placebo, and this improvement was observed by the first measurement at week 4.81 When patients were stratified by sensitivity to perennial aeroallergen, the improvement in pre-bronchodilator FEV1 was found to be independent of allergy status.111 Similar improvements were reported in the Navigator study where patients with reversible airflow obstruction and resting bronchoconstriction randomized to tezepelumab experienced pre-bronchodilator FEV1 improvement 130 ml greater than placebo after 52-week treatment; improvements were seen by the first measurement at 2 weeks.109 Stratified by blood eosinophil counts, there were smaller improvements versus placebo seen in those with eosinophils <150 cells/μl (30 ml), compared to those with eosinophils >150 cells/μl (170 ml). The effect of tezepelumab on FEV1/FVC ratio and postbronchodilator FEV1 was not reported.
5.4 IL-33 pathway blockade and measures of lung function in clinical trials
Improvements in lung function have also been reported in clinical trials blocking the IL-33/ST2 axis. Treatment with the anti-IL-33 mAb, itepekimab, for 12 weeks in asthmatic patients improved FEV1 by 140 ml more than placebo. Stratification by eosinophil levels showed FEV1 improvement was greater in the group with the highest eosinophils (>300 cells/μl).128 Similarly, blockade of ST2 with astegolimab for 52wks in asthmatic patients improved FEV1 by 130 ml compared with 100 ml in the placebo group.129 This FEV1 improvement appeared greater in the high eosinophil group; however, none of these changes was statistically significant. Thus, blockade of IL-33 and ST2 both show some improvement in FEV1, which is more evident in patients with high eosinophil levels, while astegolimab demonstrated no impact on exacerbation rates in this group, demonstrating a dissociation between the two outcomes.
5.5 IL-25 blockade and measures of lung function in clinical trials
To date, there is no convincing evidence that blocking the IL-25 pathway improves lung function. Brodalumab blocks IL-17RA, inhibiting several IL-17 molecules including IL-17 E (IL-25). In a dose-response study of 12 weeks treatment with brodalumab 140, 210, and 280 mg or placebo sc q2wk, patients with mean FEV1 65%, with 20% reversibility were evaluated. The primary endpoint of ACQ had a small but not statistically significant improvement in the high reversibility (>20%) group.151 However, there was no overall effect on ACQ, pre-bronchodilator FEV1, nor was there any signal in the high reversibility group or trend toward a dose response, which may be a result of the small sample size. To date, no clinical trials have been conducted that specifically block IL-25, or the IL-25 receptor subunit, IL-17RB, which is mainly expressed in cells associated with T2 inflammation.
6 ALARMINS AND INFLAMMATORY BIOMARKERS
TSLP has a wide complement of direct functions on eosinophils, leading to their accumulation in the airways. TSLP stimulates eosinophil differentiation in human blood and cord blood-derived CD34+ cells that express the TSLP receptor and IL-7α chain subunit.152, 153 TSLP stimulation of CD34+ progenitor cells also causes a dose-dependent release of T2 cytokines, IL-5, IL-13, GM-CSF, as well as chemokines CCL22, CCL17, CXCL8, and CCL1, and increased expression of IL-5Rα.150, 153 TSLP stimulation prevents apoptosis of mature eosinophils, upregulates adhesion molecule CD18, intercellular adhesion molecule-1 and induces IL-6, CXCL8, CXCL1, CCL2 cytokine secretion while downregulating L-selectin expression.154
Several clinical trials have included measures of inflammation as study endpoints. In the Navigator study, tezepelumab reduced blood eosinophils and FeNO at the first measurement at week 2, and this benefit persisted throughout the 52-week treatment period.109 This suppression of eosinophils largely mimicked results reported from the Pathway study and post-hoc analyses, showing reduction in blood eosinophils and FeNO irrespective of atopic status or season.81, 110, 111 Emson et al. reported significant reductions in levels of eosinophils, FeNO, IL-5 and IL-13 by tezepelumab, regardless of nasal polyposis status.112
Mechanistic studies have been conducted to understand the anti-inflammatory mechanisms of TSLP blockade. The Cascade study demonstrated 28 weeks of treatment of tezepelumab significantly reduced submucosal eosinophils compared with placebo and irrespective of T2 status at baseline. However, the number of neutrophils, CD3+ T cells, CD4+ T cells, tryptase+ mast cells, and chymase+ mast cells and indicators of airway remodeling as measured by the change in reticular basement membrane thickness and epithelial integrity were not reduced by tezepelumab treatment, suggesting that improvements in clinical outcomes are likely a reflection of reduced airway eosinophils.148 In a study by Sverrild and colleagues, 12 weeks of treatment with tezepelumab significantly decreased airway tissue and BAL eosinophils by 74% and 75%, respectively,149 and reduced mast cell numbers by 25%, which reached statistical significance in a subpopulation of eosinophilic patients. Mechanistic studies have also been performed in patients with milder disease. Gauvreau et al. reported inhibition of allergen-induced levels of blood and sputum eosinophils, plus an overall reduction in baseline FeNO by 1 week and blood eosinophils by 1 month,73 suggesting constitutive TSLP production in airways drives a low level of T2 inflammation in mild disease. Another allergen bronchoprovocation study examining an inhaled anti-TSLP fragment, CSJ117, reported similar reductions in FeNO and allergen-induced sputum eosinophils74 without reducing systemic eosinophilia, reflecting the local delivery of drug, and providing evidence that blocking TSLP in the lungs alone may provide adequate protection from environmental allergens.
Similar to TSLP, IL-33 also has direct effects on eosinophils. IL-33 induces CD11b expression, adhesion to albumin, fibronectin, ICAM-1, and VCAM-1, as well as enhancing eosinophil survival and cytokine production.47, 143, 155, 156 ST2 is highly expressed in hematopoietic progenitor cells and like TSLP, IL-33 primes the migration to the chemoattractant CXCL12.157 Although effects of IL-25 blockade on eosinophilic inflammation has not been measured in humans, IL-25 has been shown to have direct effects on eosinophils in vitro by increasing expression of chemokines CCL2 and CCL3,158 regulating the expression of adhesion molecules ICAM-1, ICAM-3 and L-selectin,65 and priming mobilization of eosinophil progenitors to CXCL12.159
Treatment with the anti-ST2 mAb, astegolimab, led to a substantial and consistent decrease in blood eosinophil counts throughout a 52-week treatment period; however, there was no corresponding improvement in FeNO across any of the doses studied.129 In a corticosteroid withdrawal study, the anti-IL-33 mAb itepekimab prevented an increase in blood eosinophils and blunted the increase in FeNO that was observed in the placebo group.128
These preliminary studies blocking the IL-33 pathways both show improvement in blood eosinophils without a significant improvement in FeNO, in contrast to tezepelumab, which consistently reduces both, suggesting that despite the many overlapping pathways of TSLP and IL-33, these alarmins drive asthma in different ways160 (Figure 5) (Table 1).
| Target | Phase | Patients | Drug | Primary outcome | Result/Status | NCT | Reference |
|---|---|---|---|---|---|---|---|
| TSLP | 1 | Mild allergic asthma | AMG 157/MEDI9929/Tezepelumab | Late asthmatic response | Positive | NCT01405963 | 72 |
| TSLP | 1 | Moderate–severe AR | TNSS AUC | Positive | NCT02237196 | 160 | |
| TSLP | 2 | Uncontrolled asthma | Mannitol PC15 | Negative | NCT02698501 | 148 | |
| TSLP | 2 | Uncontrolled asthma | AAER | Positive | NCT02054130 | 80 | |
| TSLP | 2 | Moderate–severe asthma | Airway submucosal inflammation | Positive | NCT03688074 | 147 | |
| TSLP | 2 | Moderate–severe AD | EASI | Negative | NCT02525094 | 161 | |
| TSLP | 3 | Physician diagnosed asthma | AAER | Positive | NCT03347279 | 81 | |
| TSLP | 1 | Mild allergic asthma | CSJ117 | Late asthmatic response | Positive | NCT03138811 | 73 |
| TSLP | 2 | Physician diagnosed asthma | FEV1 | Terminated | NCT04410523 | ||
| TSLPR | PC | Non-human primate | RG7258 | T2 inflammation | Positive | 162 | |
| TSLPR | PC | Non-human primate | ASP7266/UPB-101 | Skin allergic reaction | Positive | 163 | |
| TSLPR | 1 | Mild asthma | Safety | In progress | NCT05448651 | ||
| IL-33 | 1 | Mild allergic asthma | REGN3500/SAR440340/Itepekumab | Sputum inflammation | Not posted | NCT03112577 | |
| IL-33 | 2 | Physician diagnosed asthma | Loss of asthma control | Positive | NCT03387852 | 127 | |
| IL-33 | 2 | Moderate–severe AD | ANB020/Etokimab | EASI | Negative | NCT03533751 | |
| IL-33 | 2 | Chronic rhinosinusitis wNP | Nasal Polyp Score | Negative | NCT03614923 | ||
| IL-33 | 2 | Early-onset uncontrolled asthma | MEDI3506/Tozorakimab | pre-BD FEV1 | In progress | NCT04570657 | |
| IL-33 | 2 | COPD and chronic bronchitis | pre-BD FEV1 | In progress | NCT04631016 | ||
| IL-33 | 2 | Chronic AD | EASI | In progress | NCT04212169 | ||
| ST2 | 1 | Chronic rhinosinusitis wNP | RG6149/ MSTT1041A/ AMG282/Astegolimab | Safety | Not posted | NCT02170337 | |
| ST2 | 1 | Mild asthma | PK/PD | Not posted | NCT01928368 | ||
| ST2 | 2 | Severe asthma | AAER | Positive | NCT02918019 | 128 | |
| ST2 | 2 | Moderate–severe AD | EASI | Negative | NCT03747575 | 164 | |
| ST2 | 2 | Severe asthma with AFAD | GSK3772847/Melrilimab | Blood Eosinophils | Positive | NCT03393806 | |
| ST2 | 2 | Moderate–severe asthma | Loss of asthma control | Not posted | NCT03207243 | ||
| IL-17RA | 2 | Moderate–severe asthma | Brodalumab | ACQ composite score | Negative | NCT01902290 | 150 |
7 CONCLUSION
Both mechanistic studies and clinical trials in asthmatic patients have emphasized a critical role in the release of TSLP and IL-33, likely from the airway epithelium, and engagement of their receptor complexes in the pathogenesis of asthma. The strongest evidence has been provided by studies with mAbs, which bind to these alarmins and which, in addition to establishing safety, have demonstrated clinically important benefits, particularly in patients with severe asthma. The mAb, which is the most advanced in clinical development, and already approved for clinical use in some countries, is tezepelumab, which not only reduces asthma exacerbation risk, but also improves all other asthma outcomes measured in the clinical trials, including biomarkers of inflammation, and AHR. In contrast to other asthma biologics, which target IL-5, IL-5Rα, or IL-4Rα, tezepelumab has been demonstrated to provide these clinical benefits in severe asthma patients with blood eosinophil counts <150 cells/μl, and who do not have biomarker evidence of T2-high asthma. Another interesting approach to blocking TSLP has been the development of a mAb fragment delivered by inhalation, rather than subcutaneously or intravenously, which has demonstrated clinical benefit in early studies. Interestingly, the effects of these approaches span the range of severity of asthma, from very mild (in the proof-of-concept studies) to severe asthma, characterized by persistent poor asthma control despite standard of care treatment.
Antibodies against IL-33 and its receptor ST2 have also suggested that the IL-33 axis is important in severe asthma. The development of these mAbs is less advanced than for TSLP, but larger clinical trials are underway. The third epithelial alarmin, IL-25 does not have any clinical trial evidence to support a biological role in severe asthma; however, the studies reported with a mAb against its receptor were not sufficiently powered to confidently assess efficacy against asthma outcomes, such as severe exacerbations, where other anti-alarmins have shown benefit.
The recognition that the release of TSLP, and possibly IL-33, is critical to the development of severe asthma exacerbations, and blockade of the alarmins improves all asthma-related clinical outcomes represents a major advance in the understanding of asthma pathogenesis and has resulted in new treatment options to treat severe asthma. Positioning of the alarmin cytokines upstream of the inflammatory cascade targeted by other asthma biologics may be additionally advantageous, but more studies are needed to fully elucidate this potential benefit.
ACKNOWLEDGMENTS
All authors contributed to the content of this manuscript, reviewed, and approved the final version. The authors would like to acknowledge AstraZeneca Canada for financial support in publishing this manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interest related to this manuscript.
Outside of this work, GMG reports receiving consulting or speaker fees from AstraZeneca. Sanofi-Regeneron and research grants from Biohaven, Genentech, BioGaia, Novartis; CB reports receiving consulting or speaker fees from Valeo, AstraZeneca, GSK, Grifols, Takeda, and research grants (clinical trials paid to Vancouver Coastal Health Research Institute/University of British Columbia) from Biohaven, AstraZeneca, Regeneron, GSK, Novartis, Sanofi, Teva; LPB reports receiving consulting or speaker fees from AstraZeneca, Covis, Cipla, GlaxoSmithKline, Novartis, Merck, Sanofi-Regeneron, and research grants from Amgen, AstraZeneca, GlaxoSmithKline, Merck, Novartis, Sanofi-Regeneron, Biohaven; DWC reports receiving research grants from Department of Medicine University of Saskatchewan, AstraZeneca, Biohaven, Novartis, CSACI, and AllerGen NCE; AC reports receiving consulting or speaker fees from AstraZeneca, Covis, Valeo pharma, GlaxoSmithKline, Sanofi-Regeneron, and research grants from GlaxoSmithKline; RL reports receiving consulting or speaker fees from Novartis, AstraZeneca, GlaxoSmithKline, Sanofi Genzyme, and Valeo Pharma Inc research grants from Biohaven; POB reports personal fees for consulting or speaker fees from AstraZeneca, GSK, Medimmune, Chiesi, Menarini, and Covis and research grants from AstraZeneca, Medimmune, Biohaven, Merck, and Bayer. RL reports receiving consulting or speaker fees from AstraZeneca, GlaxoSmithKline, Regeneron, Sanofi Genzyme and Valeo Pharma Inc. RS reports speaker fees from AstraZeneca, Teva, Genentech and Grants-In Aid from AstraZeneca, GlaxoSmithKline, Teva, Genentech.




