Dietary digestible carbohydrates are associated with higher prevalence of asthma in humans and with aggravated lung allergic inflammation in mice
Stephanie Musiol and Carla P. Harris equal contribution.
Abstract
Background
Dietary carbohydrates and fats are intrinsically correlated within the habitual diet. We aimed to disentangle the associations of starch and sucrose from those of fat, in relation to allergic sensitization, asthma and rhinoconjuctivitis prevalence in humans, and to investigate underlying mechanisms using murine models.
Methods
Epidemiological data from participants of two German birth cohorts (age 15) were used in logistic regression analyses testing cross-sectional associations of starch and sucrose (and their main dietary sources) with aeroallergen sensitization, asthma and rhinoconjunctivitis, adjusting for correlated fats (saturated, monounsaturated, omega-6 and omega-3 polyunsaturated) and other covariates. For mechanistic insights, murine models of aeroallergen-induced allergic airway inflammation (AAI) fed with a low-fat-high-sucrose or -high-starch versus a high-fat diet were used to characterize and quantify disease development. Metabolic and physiologic parameters were used to track outcomes of dietary interventions and cellular and molecular responses to monitor the development of AAI. Oxidative stress biomarkers were measured in murine sera or lung homogenates.
Results
We demonstrate a direct association of dietary sucrose with asthma prevalence in males, while starch was associated with higher asthma prevalence in females. In mice, high-carbohydrate feeding, despite scant metabolic effects, aggravated AAI compared to high-fat in both sexes, as displayed by humoral response, mucus hypersecretion, lung inflammatory cell infiltration and TH2-TH17 profiles. Compared to high-fat, high-carbohydrate intake was associated with increased pulmonary oxidative stress, signals of metabolic switch to glycolysis and decreased systemic anti-oxidative capacity.
Conclusion
High consumption of digestible carbohydrates is associated with an increased prevalence of asthma in humans and aggravated lung allergic inflammation in mice, involving oxidative stress-related mechanisms.
Graphical Abstract
Epidemiological study discovers novel associations between high intake of dietary sucrose and starch with current asthma in males and females, respectively. High-carbohydrate feeding in mice aggravates allergic outcomes: serum IgE, lung inflammatory cell infiltration, TH2- or TH2-TH17 profiles and mucus hypersecretion. Dietary carbohydrate-driven enhanced pulmonary oxidative stress and decreased systemic anti-oxidative capacity are involved in this context.Abbreviations: APE, aqueous pollen extract; EOS, eosinophils; GINIplus, German Infant study on the Influence of Nutrition Intervention plus environmental and genetic influences on allergy development; HDM, house dust mite; IgE, immunoglobulin E; LISA, life-style related factors on the development of the Immune System and Allergies in East and West Germany; M, macrophages; NEU, neutrophils; Th, T helper
Abbreviations
-
- AAI
-
- Allergic airway inflammation
-
- ALA
-
- alpha-linolenic acid
-
- ALA
-
- alpha-linolenic acid = ALA
-
- APE
-
- Aqueous pollen extract
-
- BAL
-
- Bronchoalveolar lavage
-
- eos
-
- eosinophils
-
- FACS
-
- Fluorescence activated cell sorting
-
- GAE
-
- Gallic acid equivalents
-
- GINIplus
-
- German Infant study on the Influence of Nutrition Intervention plus environmental and genetic influences on allergy development
-
- GTT
-
- Glucose tolerance test
-
- HDM
-
- House dust mite
-
- HFD
-
- High-fat diet
-
- IgE
-
- Immunoglobulin E
-
- LA
-
- linoleic acid
-
- LA
-
- linoleic acid, and
-
- LF-HA
-
- Low fat, high starch (Amylum)
-
- LF-HS
-
- Low fat, high sucrose
-
- LISA
-
- Life-style related factors on the development of the Immune System and Allergies in East and West Germany
-
- M
-
- macrophages
-
- MUFA
-
- monounsaturated fatty acids
-
- NEFA
-
- non-esterified fatty acids
-
- neu
-
- neutrophils
-
- PAS
-
- Periodic acid–Schiff
-
- PBS
-
- Dulbecco''s phosphate-buffered saline, no calcium, no magnesium
-
- SFA
-
- saturated fatty acids, linoleic acid
-
- TH
-
- T helper
-
- tIgE
-
- total immunoglobulin E
-
- Treg
-
- regulatory T cell
-
- VAT
-
- Visceral adipose tissue
1 INTRODUCTION
The incidence of allergic diseases, such as rhinoconjuctivitis and asthma, has been steadily increasing in the last decades, especially in children.1, 2 Currently, an interaction of genetic, host, and environmental factors is discussed to be involved in asthma development.3 Westernized countries have experienced a substantial increase in asthma incidence, mainly attributed to changes in lifestyle, including diets rich in fats, added sugars, and highly processed foods.4, 5 Whilst the effects of dietary fats have been widely investigated, pointing towards a functional link between obesity and asthma,6-8 the role of digestible carbohydrates (sugars and starches) in allergic diseases remains unclear. Addressing this evidence gap is crucial, as the two macronutrients are tightly interrelated, limiting our understanding of their isolated effects.
Indeed, prolonged high-sugar intake has been shown to be associated with obesity, especially if combined with a sedentary lifestyle.9 Additionally, high-sugar intake has been linked to the development of cardiovascular diseases, pointing to oxidative stress as one key mechanism in this process.10 With respect to allergic airway inflammation (AAI), recent studies have shown associations between sugar-sweetened beverage intake and asthma in both children11-14 and adults.15, 16 However, these effects are believed to result from the excess free fructose present in such beverages.16, 17 The role of sucrose, a common source of added sugar in the human diet,18 calls for further research, especially considering that the already elevated consumption of sugar-rich foods has increased even further during the COVID-19 pandemic.19 Additionally, starchy foods with a high glycemic load, such as potatoes, or refined grains (processed to remove the protein- and fat-rich germ and fibre-rich bran), have also been associated with increased risk of cardiometabolic diseases, and contribute substantially more calories to the typical Western diet than do added sugars.20 In addition, while reducing total dietary fat tends to be accompanied by an increased intake of carbohydrates,21 many high-fat products are also rich in added sugars, leading to expected correlations between starch and sucrose with fat intake. Therefore, the aims of the present study were to disentangle the effects of these two types of digestible carbohydrates from that of dietary fat on the development of aeroallergen-induced allergic disease. Therefore, we carried out epidemiological analyses among participants of two German birth cohort studies, testing the association of sucrose and starch, as well as their major dietary sources, with allergic sensitization, asthma and rhinoconjunctivitis, adjusting for dietary fat intake. Furthermore, to investigate possible underlying mechanisms, we used mouse models of aeroallergen-induced AAI and compared lung allergic responses following low-fat, high-sucrose (or high-starch) with high-fat diet feeding. Here we demonstrate that consumption of a diet rich in digestible carbohydrates is associated with higher asthma prevalence in humans and increased risk of AAI in mice. Moreover, mice on a low-fat, high-carbohydrate diet display increased pulmonary oxidative stress, signals of metabolic switch favoring glycolysis and decreased systemic anti-oxidative capacity, which may support the increased allergic response. These findings call for further dietary intervention studies investigating whether reducing the consumption of digestible carbohydrates serves to support the prevention and therapy of asthma.
2 METHODS
2.1 Epidemiological data
Data from participants of two German birth cohorts, GINIplus22 and LISA,23 were obtained through questionnaires or medical examinations carried out at the 15-year follow-up assessment. Detailed descriptions of the food frequency questionnaire used for dietary assessment, and the assessment of sensitization status, asthma, rhinoconjunctivitis and covariates, are provided in the online supplement.
2.2 Murine experimental protocols
C57BL/6J mice were fed a low-fat diet containing high-sucrose (LF-HS), high-starch (LF-HA) or a high-fat diet (HFD) for 11 weeks. Balb/c mice were fed LF-HA, HFD or a regular chow diet for 20 weeks (Table S2). To induce AAI, Dermatophagoides farinae (HDM)24 or ragweed aqueous pollen (APE)25 extracts were instilled in C57BL/6J and Balb/c mice, respectively, in the last 2 weeks of experiments. At sacrifice, serum was tested for total immunoglobulin E (tIgE) and metabolites, and lungs were subjected to bronchoalveolar lavage (BAL) analysis.25 Lung and visceral adipose tissue (VAT) were processed for flow cytometric analysis and lung tissue for additional RNA/protein isolation and histology. For methodological details, see the online supplement.
2.3 Statistical analysis
Epidemiological analyses in humans were performed stratified by sex using the statistical software R, version 4.4.1.26 Descriptive characteristics of the study population were presented as medians (25th; 75th percentile) for continuous variables, and counts (%) for categorical variables. Dietary variables were presented as their relative contribution to total energy intake (%EI). Sex-differences in population characteristics were tested by Wilcoxon rank-sum test or Pearson's chi-squared test (for continuous and categorical variables, respectively). Correlations of each carbohydrate variable with total fat, and different fat subtypes (saturated = SFA, monounsaturated = MUFA, linoleic acid = LA, and alpha-linolenic acid = ALA) were tested by the Spearman's rank correlation. The association of dietary carbohydrates, namely sucrose, sugary foods, sugary drinks, starch, starchy vegetables, and refined grains (independent variables) with allergic sensitization, asthma, and rhinoconjunctivitis (dependent variables), were assessed by logistic regression. In order to determine whether associations with asthma were mainly driven by participants with an allergic phenotype (allergic asthma), sensitivity analyses were performed in a subset of the study population with positive aeroallergen sensitization (N = 715). Unadjusted analyses, then analyses adjusting for pre-selected covariates were performed. For independent variables presenting a significant correlation with dietary fats (at p-values<0.001), the respective regression model was further adjusted for the relevant fat subtypes. Effects are presented as odds ratios and 95% confidence intervals (OR (95% CI)) for an interquartile range increase in the respective independent variable.
For murine studies, statistical analyses were performed by one-way or two-way analysis of variance (ANOVA) with Bonferroni's post-hoc test or by Student's unpaired two-tailed t-test. Data were analyzed using Prism software version 6 (GraphPad software Inc). Threshold of significance: p < 0.05.
3 RESULTS
3.1 Digestible carbohydrates are associated with increased odds of allergic sensitization and asthma
Descriptive characteristics of the study population are presented in Table 1. Males presented significantly higher levels of allergic sensitization (50.7%) and asthma (6.9%) than females (40.9% and 4.1%, respectively). There was no sex difference in the prevalence of rhinoconjunctivitis (21.0% in females and 20.2% in males). Total sucrose intake represented around 10% of total energy intake (10.6% and 9.98% in females and males, respectively), and total starch intake was around 27% (27.8% and 26.2% in females and males, respectively). Correlations between carbohydrates and fat variables are presented in Table 2. Sugary drinks were negatively correlated with fat intake in males only, whereas all other correlations were mostly similar in both sexes. While sucrose presented significant negative correlations with MUFA and LA, sugary foods were positively correlated with SFA. Starch was negatively correlated with SFA, MUFA and ALA, and the same was observed for refined grains (except for ALA in females). Starchy vegetables were positively correlated with LA. The various associations with different fat types, each type having unique effects on health, show that simply adjusting analyses for total fat intake would not be sufficient to account for the complex interrelations between different dietary carbohydrates and fats. For example, while both starch and sucrose were inversely correlated with total fat, LA accounts largely for this association in the case of sucrose, whereas in the case of starch, the correlation is mostly due to SFA.
| Females (N = 830) | Males (N = 743) | p-value | |
|---|---|---|---|
| Dietary carbohydrates | |||
| Total sucrose (%EI) | 10.6 (8.20; 13.4) | 9.98 (7.89; 12.8) | 0.028 |
| Sugary foods (%EI) | 10.7 (6.60; 15.7) | 9.73 (6.58; 14.4) | 0.031 |
| Sugary drinks (%EI) | 0.79 (0.17; 2.64) | 2.24 (0.86; 6.11) | <0.01 |
| Total starch (%EI) | 27.8 (22.9; 32.5) | 26.2 (21.7; 31.5) | 0.001 |
| Starchy vegetables (%EI) | 1.87 (1.17; 3.02) | 1.70 (1.16; 2.75) | 0.057 |
| Refined grains (%EI) | 28.1 (22.1; 34.1) | 27.1 (20.9; 33.7) | 0.019 |
| Allergic outcomes (yes) | |||
| Aeroallergen sensitizationa | 340 (41.1) | 375 (50.5) | <0.01 |
| Asthma | 34 (4.1) | 52 (7.0) | 0.016 |
| Subset: allergic asthma b | 23 (6.8) | 44 (11.7) | 0.032 |
| Rhinconjunctivitis | 172 (20.7) | 149 (20.1) | 0.790 |
| Covariates | |||
| Age (years) | 15 (14.9; 15.1) | 15 (14.9; 15.1) | 0.198 |
| BMI (kg/m2) | 20.3 (18.8; 22.1) | 20 (18.5; 22.1) | 0.058 |
| Total energy intake (kcal) | 1731(1342; 2154) | 2348 (1883; 2804) | <0.01 |
| Fruit & vegetables (%EI) | 5.87 (3.72; 9.28) | 3.73 (2.14; 5.59) | <0.01 |
| Whole grains (%EI) | 291 (92.2; 798) | 273 (82.9; 716) | 0.296 |
| Meat (%EI) | 11.4 (7.1; 15.9) | 13.8 (10.0; 19.0) | <0.01 |
| Fish (%EI) | 1.01 (0.47; 1.77) | 1.19 (0.63; 1.84) | 0.001 |
| Dairy (%EI) | 13.9 (9.0; 20.1) | 14.8 (9.0; 21.3) | 0.142 |
| MVPA (min/day) | 7 (5.0; 10.5) | 9 (6.5; 13.0) | <0.01 |
| Study arm | |||
| GINI observation arm | 275 (33.1) | 247 (33.2) | 0.127 |
| GINI intervention arm | 264 (31.8) | 205 (27.6) | |
| LISA | 291 (35.1) | 291 (39.2) | |
| Study centre | |||
| Munich | 453 (54.6) | 425 (57.2) | 0.270 |
| Leipzig | 65 (7.8) | 71 (9.6) | |
| Bad Honnef | 37 (4.5) | 29 (3.9) | |
| Wesel | 275 (33.1) | 218 (29.3) | |
| Parental education (high) | 613 (73.9) | 533 (71.7) | 0.375 |
| Parental allergy (yes) | 596 (71.8) | 539 (72.5) | 0.788 |
- Abbreviations: %EI, percentage of total daily energy intake; BMI, body mass index; MVPA, moderate to vigorous physical activity. Values are presented as counts (%) for categorical variables, or median (25th; 75th percentile) for continuous variables. Differences between sexes were tested by Chi-squared test or Wilcoxon rank-sum test, for categorical or continuous variables, respectively (Significant differences are marked in bold, p-value<0.05).
- a IgE >0.35 kU/L.
- b Subset with positive aeroallergen sensitization (females = 340; males = 375).
| Females (N = 830) | Males (N = 743) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | SFA | MUFA | LA | ALA | Total | SFA | MUFA | LA | ALA |
| −0.19* | 0.02 | −0.26* | −0.33* | −0.23* | −0.19* | 0.00 | −0.27* | −0.30* | −0.20* |
| 0.18* | 0.33* | 0.10 | −0.10 | −0.08 | 0.16* | 0.24* | 0.08 | 0.02 | 0.05 |
| −0.09 | −0.06 | −0.11 | −0.06 | −0.09 | −0.19* | −0.19* | −0.17* | −0.11 | −0.09 |
| −0.36* | −0.37* | −0.33* | −0.09 | −0.17* | −0.41* | −0.48* | −0.35* | −0.03 | −0.13* |
| 0.02 | −0.03 | 0.02 | 0.15* | 0.06 | −0.02 | −0.08 | −0.01 | 0.14* | 0.06 |
| −0.25* | −0.26* | −0.24* | −0.03 | −0.06 | −0.36* | −0.42* | −0.34* | −0.06 | −0.08 |
- Abbreviations: ALA, alpha-linolenic acid (omega-3); LA, linoleic acid (omega-6); MUFA, monounsaturated fat; SFA, saturated fat; Total, total fat. Correlations presented as Spearmans Rho, tested by Spearman's rank correlation. *Significant correlation (p-value<0.001).
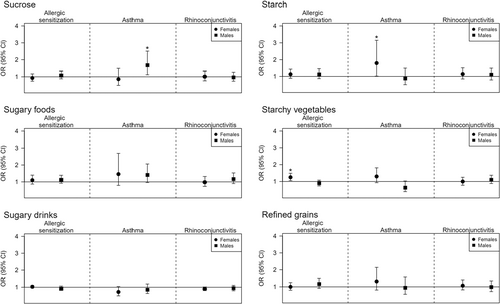
Associations of dietary intake variables (sucrose, sugary foods, sugary drinks, starch, starchy vegetables, and refined grains) with allergic sensitization, asthma, allergic asthma, and rhinoconjunctivitis, are displayed in Figure 1 and Table S1. A significant positive association was observed between sucrose intake and asthma in males (OR = 1.68, 95% CI = (1.12;2.51), p = 0.011), whereas no significant associations were observed with sugary foods or sugary drinks. In females, total starch was positively associated with asthma, (1.80 (1.02;3.15), p = 0.041), and starchy vegetables were positively associated with allergic sensitization (1.25 (1.05;1.49), p = 0.013). There were no significant associations with rhinoconjunctivitis in either sex. Sensitivity analyses assessing allergic asthma in the subset of aeroallergen sensitized participants indicated similar trends for sucrose in males (1.67 (1.02;2.75), p = 0.044); however, the OR of starch in females was reduced and not significant (1.36 (0.64;2.89), p = 0.420). In contrast, sugary foods were significantly associated with allergic asthma in females (2.36 (1.01;5.51), p = 0.047). Nevertheless, given the small sample size available for sensitivity analyses and large confidence intervals, these associations must be interpreted with caution.
3.2 High-sucrose diet exacerbates murine HDM-induced AAI
To mechanistically explore the results obtained in our epidemiological analyses, we employed a model of HDM-induced AAI in male and female mice fed either with a sucrose-rich LF-HS or a HFD as a control (Figure 2A). As expected, HFD-fed males showed a stronger increase in body weight, body fat mass and weight of fat pads, and a lower tolerance to injected glucose compared to both LF-HS-fed males and to HFD-fed females (Figure 2B; Figure S4A–C).27, 28 Accordingly, liver triglycerides, serum levels of insulin and glucose were increased in HFD-fed compared to LF-HS-fed male mice (Figure S4D). Notably, both LF-HS and AAI had no impact on metabolic parameters compared to respective diet- and PBS-controls. The increased body weight (and fat mass) in female HFD HDM should be disregarded as it was present from begin of sensitization (Figure 2B, right). FACS analysis of VAT (Figure S4E) revealed the expected increase of M1 macrophages in HFD-fed males,29 their slight decrease following AAI in both sexes, and no diet- or treatment-induced variations in M2 macrophages, CD4+T or CD8+T cells.
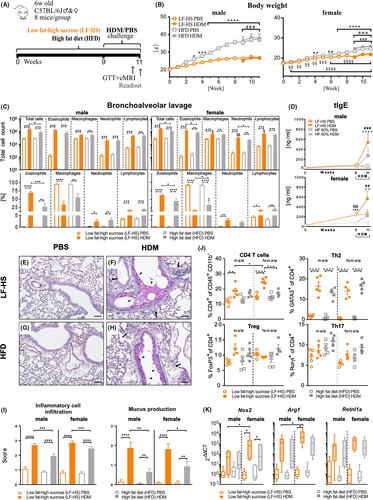
In lungs, AAI significantly increased BAL inflammatory cells numbers in LF-HS and HFD. Interestingly, total BAL cells and relative percentage of eosinophils were significantly higher in LF-HS compared to HFD in both sexes (Figure 2C; Figure S5A), whereby female mice showed higher infiltrating macrophages, lymphocytes and neutrophils compared to males. Analysis of BAL TH2, TH17, pro-inflammatory cytokines and chemokines revealed HDM-induced increased tendencies in both sexes, reaching significance only in a few parameters in LF-HS-fed animals (Figure S5C). BAL protein concentration, a reliable parameter of increased lung barrier permeability30 as expected31 increased significantly following HDM challenge in both sexes (Figure S5B). Serum tIgE, which in LF-HS-fed females was augmented compared to HFD-fed even before AAI induction, after HDM-challenge increased in both sexes, reaching significantly higher levels in LF-HS compared to HFD groups (Figure 2D). Lung histopathological analysis revealed increased perivascular and peribronchiolar inflammatory cell infiltration and mucus hypersecretion following AAI and, most importantly, both scores were significantly enhanced in LF-HS compared to HFD in both sexes (Figure 2E–I). To further characterize the type of lung immune response, we performed FACS analysis of lung tissue, which revealed a clear AAI-induced inflammatory type-2 phenotype, highlighted by a significant increase of CD4+T cell percentages in LF-HS HDM of both sexes, especially in females (Figure 2J). Contrarily, CD8+T cells varied minimally in males, but decreased significantly in LF-HS HDM females compared to their diet and PBS controls (Figure S3B). Whilst the percentage of GATA3+ cells strongly increase in both sexes following AAI, FoxP3+ and RORγt+ cells underwent only minor variations. Furthermore, analysis of M1- and M2-BAL macrophages markers indicated an AAI-induced upregulation of both markers in LF-HS, stronger in females compared to males, whereas in HFD only Nos2 was upregulated in females (Figure 2K).
Thus, consumption of a high-sucrose diet leads to an aggravated type-2 phenotype compared to HFD in both sexes, but especially in females.
3.3 A high-starch diet exacerbates murine HDM-induced AAI
To clarify if the observed high-sucrose-induced AAI-enhancing effect applied also to starch, we compared AAI outcomes in mice fed a sucrose-rich LF-HS with mice fed a low-sucrose, high-starch (Amylum) (LF-HA) diet, using HFD-fed animals as reference (Figure 3A). LF-HA-fed mice gained more weight compared to LF-HS mice (Figure 3B), but no difference was observed in their glucose tolerance (Figure 3C). Serum clinical chemistry parameters did not differ between the two diets or treatment, only triglycerides and non-esterified fatty acids (NEFA) were slightly higher in LF-HA compared to LF-HS (Figure S6A). Similarly, no difference in macrophage or T cell populations was detected between LF-HA and LF-HS in fat tissue (Figure S6B). As for the lung response to AAI, no difference in BAL cell infiltration (Figure 3D), histopathological alterations and scores (Figure 3E–I), serum tIgE (Figure 3J), BAL protein and cytokines (Figure S6C) was detected between LF-HA and LF-HS, whereby most of these parameters were strongly increased in high-carbohydrate groups compared to HFD. Lung FACS analysis revealed an AAI-induced TH2-driven response similar in LF-HA and LF-HS whereas the TH17 response was higher in LF-HA compared to HFD and to LF-HS, reflected also by higher IL-17A levels in BAL supernatant in this group (Figures 3K, S6C).
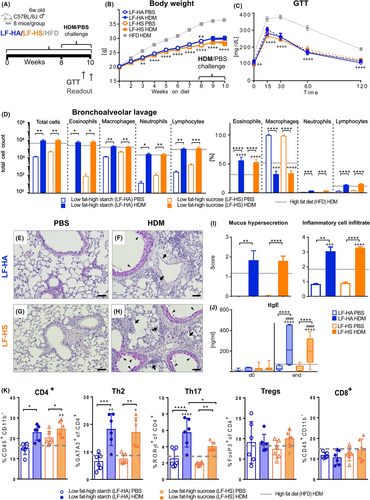
Herewith, we could demonstrate that a diet rich in starch potentiates a HDM-induced allergic response similarly to sucrose, but favoring mixed TH2-TH17 profiles.
3.4 Effects of a high-starch diet are not restricted to a specific allergen
To evaluate the allergen specificity of our findings, we employed an aero-allergen other than HDM in an AAI model previously established with ragweed-APE in female BALB/c mice25 at the end of a 20-week feeding period with LF-HA or HFD as a control (Figure 4A). Whereas at week 7 LF-HA-fed mice showed, additionally to a lower body weight, higher glucose tolerance compared to HFD (Figure S7A,C), at week 13 also mice fed LF-HA developed glucose intolerance, maintaining a lower body weight compared to HFD (Figure S7B,C). Accordingly, perigonadal fat pads were significantly increased in sham-sensitized HFD- compared to LF-HA-fed mice (Figure S7D,E). Additionally, serum cholesterol, triglyceride, NEFA and glucose were higher in HFD compared to LF-HA (Figure S7F). AAI induction had no impact on the metabolic parameters evaluated.
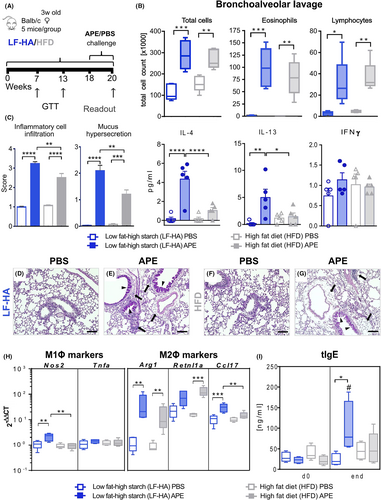
APE-challenge increased lung inflammatory cell infiltration of both LF-HA- and HFD-fed mice, dominated by eosinophils and lymphocytes. Total BAL cells and eosinophils showed only a higher tendency in LF-HA compared to HFD (Figure 4B, above), BAL total protein was similar in the two challenged groups whereas the Th2 cytokines IL-4 and IL-13 were significantly higher in LF-HA- compared to HFD-fed animals, together with no IFN-γ alterations (Figure 4B, below). Also histopathological scoring of lungs revealed increased perivascular and peribronchiolar inflammatory cell infiltration and mucus hypersecretion in allergic LF-HA compared to HFD (Figure 4C–G). Additionally, BAL macrophages retrieved from LF-HA APE showed a significant upregulation of Nos2, and to a higher extent of Arg1 and Ccl17 (Figure 4H). The expression of both Nos2 and Ccl17 was higher in LF-HA compared to HFD, whereas for Retnl1a no diet-related difference was shown. Finally, LF-HA displayed a near-to-significantly stronger increase in serum tIgE compared to HFD (Figure 4I).
Thus, although the overall allergic response in the APE model was lower compared to HDM, we could confirm that high amounts of digestible carbohydrates exacerbates features of AAI in a non-allergen-specific manner.
3.5 High-carbohydrate diet increases pulmonary and systemic oxidative stress and induces a metabolic switch to glycolysis
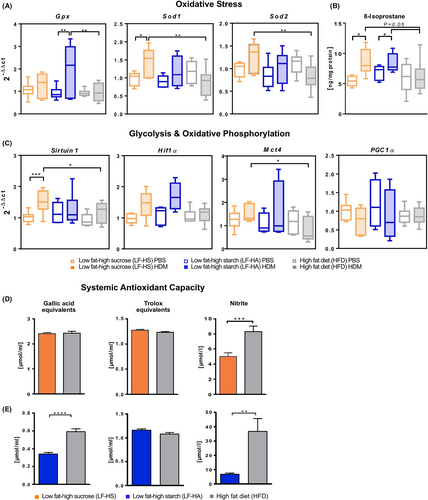
To obtain insights into the mechanism of how carbohydrate-rich diets can aggravate AAI, and considering the important role of oxidative stress in AAI in both mice and humans,32, 33 we measured key indicators of oxidative stress in lung tissue and serum. Our results reveal an allergen-induced increased expression of Gpx in LF-HA and of Sod1 and Sod2 in LF-HS compared to HFD (Figure 5A). On the same line, 8-isoprostane, a key marker of oxidative stress,34 was increased in lung homogenates after challenge in both LF-HS and LF-HA and not in HFD. Noticeably, after challenge this marker was near-to-significantly increased in LF-HS and LF-HA compared to HFD (Figure 5B). Next, we investigated whether a glycolytic reprogramming may play a role in our model. The lung expression of Sirtuin 1, HIF-1α and Mct4, important for sensing hypoxia and promoting glycolysis,35, 36 were higher in allergen-challenged LF-HS and LF-HA compared to HFD, although for HIF-1α the difference was not significant. Contrarily, lung expression of PGC1α, an indicator of mitochondrial oxidative metabolism,37 was slightly downregulated in allergen-challenged LF-HS and LF-HA compared to PBS controls (Figure 5C). To investigate potential systemic effects of the increased pulmonary oxidative stress, we quantified serum anti-oxidative capacity in both C57BL/6J (LF-HS, Figure 5D) and Balb/c (LF-HA, Figure 5E) compared to respective HFD groups. Whilst peroxyl radical scavenging capacity (trolox equivalents) was not altered by diet, nitrite concentrations were significantly lower in both LF-HS and LF-HA compared to HFD. Additionally, total phenolic antioxidant content (gallic acid equivalents, GAE) was significantly lower in LF-HA- compared to HFD-fed Balb/c animals, whereas no difference was reported between LF-HS- and HFD-fed C57BL/6J mice. Taken together, these results suggest that high levels of dietary carbohydrates induce augmented lung oxidative stress, signals of metabolic switch to glycolysis combined to systemic decreased anti-oxidative capacity compared to HFD.
4 DISCUSSION
This study uses epidemiological data from two population-based cohorts of female and male adolescents to evaluate associations of different types of digestible carbohydrates with aeroallergen sensitization, asthma and rhinoconjunctivitis, and to investigate underlying mechanisms using murine models.
The observational study demonstrates associations between higher consumption of starch and sucrose and greater asthma prevalence in females and males, respectively. Given the inclusion of correlated dietary fats as covariates, the associations observed appear to be independent of inherent variations in fat intake. According to our findings in males, sucrose intake increases the risk for asthma. Except one study reporting increased atopic respiratory outcomes in the offspring of mothers consuming more sugar during pregnancy,11 existing literature linking sugar intake to asthma focuses on consumption of sugary drinks.12, 15, 16, 38 In contrast, we observed no associations of asthma with sugary drinks. The low amount of sugary drink consumption (0.8 %EI in females and 2.8 %EI in males) and lower relative fructose content in drinks produced in Europe compared to the US39 may account for this discrepancy. Furthermore, the weak correlation between sugary drinks and total sucrose in our male population (rho = 0.09) shows that sugary drinks were not major contributors towards total sucrose intake. Additionally, we demonstrate that, among female adolescents, higher total starch intake is associated with asthma, and a similar non-significant trend is seen for refined grains (both are highly correlated, rho = 0.83). Sensitivity analysis among a subset of aeroallergen sensitized individuals indicated a smaller effect magnitude, suggesting that dietary starch increases the odds of having asthma irrespective of allergic sensitization. Nevertheless, given the reduced power due to the smaller sample size, this should be interpreted with caution. Our findings on total starch differ from those reported in an ecological study of asthma and allergies in 13–14 year-olds.40 However, the analyses in that study were not adjusted for positively correlated protective foods such as fruit, vegetables, fibers, and whole grains, nor for inversely correlated fats. Despite accounting for numerous confounding factors in the present study, including known dietary, lifestyle, socioeconomic and anthropometric risk factors of allergic disease development, the possibility of residual confounding through other uncontrolled influences remains a limitation common to all epidemiological studies. Furthermore, the cross-sectional nature of our analysis reduces our ability to establish causal associations. First, we cannot exclude the possibility of reverse causation, whereby symptomatic individuals might have purposefully altered the composition of their diet. Furthermore, dietary behaviors may change in adolescence due to physiological demands resulting from pubertal development, as well as increasing autonomy. Nevertheless, when comparing average carbohydrate intakes among a subset of the study population who also had available dietary data at age 10 years (shown in Table S5), we observed only small changes over time (≤1.5 %EI). In previous work addressing changes in diet from ages 10 to 15 years in the GINIplus cohort, we also found that although average intake changes occurred overall, intake levels relative to the rest of the study population often remained constant.41 Interestingly, and in contrast to total starch, starchy vegetables were associated with allergic sensitization in females of our study. Indeed, prevalence of sensitization to potato has been reported at around 10%,42 and sensitization to cooked potatoes has been considered a risk factor for early development of pollen allergy.43 Overall, sex-specific relationships were observed in our epidemiological data, but not in the rodent models. These sex differences are difficult to explain yet not unexpected, given the age of the cohort participants, and the sex-specific trajectories of allergic diseases evident around the time of puberty, such as the known switch in asthma prevalence from male to female dominance.44 Different trajectories of different allergic diseases would also explain the lack of consistent associations with other outcomes, since for example, asthma prevalence increases around late childhood, while rhinoconjunctivitis prevalence increases steadily over time.45 Besides offering the above reflections, we cannot currently explain why in our cohorts total dietary starch is associated with asthma only in females. Given the lack of studies specifically assessing the role of starch in relation to asthma, there is little comparable evidence. Particularly, given the prominence of starch in the Western diet, this finding would have important public health implications. Nevertheless, further studies, including human intervention trials, are needed to confirm these findings before any definitive conclusions can be reached. The observation that sucrose was associated with asthma more clearly in males could be due to sex differences concerning glucose handling, as well as body fat storage and distribution, altering effects on systemic metabolism. In this context, males are more sensitive to sucrose and fructose compared to females, who store more lipids and have higher whole-body insulin sensitivity than males.46 On the other hand, it is also possible that females and males simply differ in the type of foods regularly consumed, the details of which may not be fully captured in the epidemiological data, and which obviously cannot be mimicked in mice experiments. Overall, we should also consider that the underrepresentation of participants from lower social classes constitutes a non-random loss-to-follow-up, which may limit the generalizability of our findings.
Rodent models of antigen-driven allergy are successfully used in mechanistic studies for preclinical research, mimicking specific aspects of human disease.47 To disentangle the effects of sucrose and starch from that of dietary fat on AAI development, we induced aeroallergen-induced AAI combined with LF-HS or LF-HA diets, and used HFD as a control, despite existing literature demonstrating controversial effects of HFD on allergies.24, 48, 49 Notably, by using HFD as a control we intended to avoid non-digestible carbohydrates contained in regular chow diets.50 Nevertheless, we controlled for response in main allergic parameters following APE challenge and depicted no difference between HFD- and regular chow diet-fed animals. Our results show that high dietary sucrose and starch enhance typical TH2- or TH2-TH17-driven lung allergic inflammatory milieu, respectively,47, 51, 52 both comprising increased inflammatory infiltrate, mucus hypersecretion and serum tIgE levels compared to HFD. These data are in line with a recent study using an Alternaria alternata allergy model which demonstrates increased BAL and eosinophil cytokine content in high-sucrose- compared to high-fat-fed animals.53 High dietary starch, besides enhancing an HDM-driven TH17 response compared to high sucrose, affected glucose tolerance, but only after longer-term feeding. Notably, starch possesses a high glycaemic index, which can probably explain both inflammatory and metabolic effects.54 Whereas male mice showed the anticipated higher susceptibility to high-fat, but not to high-carbohydrate feeding, female mice confirmed their higher susceptibility to allergy.27, 28, 55, 56 Consequently, in female mice, both M1 and M2 alveolar macrophages markers significantly increased following AAI in both sensitization models, indicating the presence of a mixed M1/M2 phenotype in the high-carbohydrate groups,57 consistently with the critical role of both phenotypes in asthma development.58 To shed light on the mechanism responsible for disease aggravation, we further investigated which source of excessive energy supply (i.e. sucrose, starch or fat) may lead to an increased oxidative activity that, along with insufficient antioxidant defense, causes local or systemic overproduction of reactive oxygen species (ROS).59, 60 Increased expression of Gpx, Sod1 and Sod2 in mouse lungs following high-carbohydrate-feeding indicates increased activation of the antioxidant defense and thus, enhanced lung oxidative challenge compared to HFD-feeding. This was confirmed by the measurements of 8-isoprostane, which clearly demonstrate increased oxidative stress in lung tissue of high-carbohydrate- compared to HFD-fed animals. Notably, under hypoxic or inflammatory conditions, HIF-1α activity can trigger a shift from oxidative phosphorylation to glycolysis, thus supporting immune cell activation and inflammation under high cost of glucose consumption (Warburg effect).61, 62 The slight upregulation of HIF-1α combined to the downregulation of PGC1α, involved in Krebs-Cycle induction and mitochondrial ROS detoxification37, 63 point to a glycolytic reprogramming in the lungs of LF-HS- and LF-HA-fed animals. Furthermore, the increased expression of both Sirtuin1 and Mct4 in LF-HS- compared to HFD further indicate the occurrence of a metabolic switch to glycolysis in lung tissue with consecutive lactate production, a process known to be important in allergic asthma.64-67 Furthermore, LF-HA-fed Balb/c mice sera revealed lower GAE, applied as a sum parameter of anti-oxidative capacity.68 Contrarily, GAE in C57BL/6J mice was very high concentrated independently of diets. This likely represents a side-effect caused by artificial colorants present in the specific diet, some of which possess anti-oxidative capacities.69 Conversely, nitrite concentrations were lower in sera of both LF-HS- and LF-HA-fed compared to HFD-fed mice, independently of the mouse strain. Noteworthy, food nitrate/nitrite-derived nitric oxide (NO) is a readily bioavailable supplementation of endogenous NO, which possesses protective properties exerted through the attenuation of oxidative stress in immune cells70, 71 and may therefore play an important role in our model. Summing up, a systemic reduction of anti-oxidative capacity in high-carbohydrate-fed mice may increase glycolytic reprogramming and susceptibility to oxidative stress, promoting disease exacerbation.
Taken together, our study demonstrates for the first time a direct association of dietary sucrose and starch with asthma in humans, as well as the harmful effect of a diet rich in these carbohydrates on AAI development in mice. Following confirmation in human intervention trials, these findings could have important implications for dietary recommendations in the prevention and/or treatment of asthma.
AUTHOR CONTRIBUTIONS
Epidemiological data collection: SK, CPB, TS, DB, AvB, GH. Epidemiological study design: CPH, CF, MS and FA. Epidemiological data analysis: CPH, MS. Mouse experimental design: SM, RK, BR, JR, CBS-W, SU and FA. Conduction of experiments: SM, RK, JMG, BR, BS, ES, LM, EEV and FA. Experimental data analysis: SM, RK, JMG, BR, EEV, FA. Supervision: MS, CBS-W, SU, FA. Writing original draft: SM, CPH and FA. Review&Editing: All Authors. Funding acquisition: MHdA, MS, CBS-W, SU and FA.
ACKNOWLEDGMENTS
GINIplus Cohort: The authors thank all the families for their participation in the GINIplus study. Furthermore, we thank all members of the GINIplus Study Group for their excellent work. The GINIplus Study group consists of the following: Institute of Epidemiology, Helmholtz Zentrum München, German Research Center for Environmental Health, Neuherberg (Heinrich J, Brüske I, Schulz H, Flexeder C, Zeller C, Standl M, Schnappinger M, Ferland M, Thiering E, Tiesler C); Department of Pediatrics, Marien-Hospital, Wesel (Berdel D, von Berg A, Filipiak-Pittroff B); Ludwig-Maximilians-University of Munich, von Hauner Children's Hospital (Koletzko S, Werkstetter K); Child and Adolescent Medicine, University Hospital rechts der Isar of the Technical University Munich (Bauer CP, Hoffmann U); IUF-Environmental Health Research Institute, Düsseldorf (Schikowski T, Link E, Klümper C, Krämer U, Sugiri D). LISA Cohort: The authors thank all the families for their participation in the LISA study. Furthermore, we thank all members of the LISA Study Group for their excellent work. The LISA Study group consists of the following: Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Epidemiology, Munich (Heinrich J, Schnappinger M, Brüske I, Ferland M, Schulz H, Zeller C, Standl M, Thiering E, Tiesler C, Flexeder C); Department of Pediatrics, Municipal Hospital “St. Georg”, Leipzig (Borte M, Diez U); Marien Hospital Wesel, Department of Pediatrics, Wesel (von Berg A, Berdel D, Stiers G, Maas B); Pediatric Practice, Bad Honnef (Schaaf B); Helmholtz Centre of Environmental Research-UFZ, Department of Environmental Immunology, Leipzig (Herberth G, Schilde M, Bauer M, Röder S); Technical University Munich, Department of Pediatrics, Munich (Hoffmann U, Paschke M, Marra S); Clinical Research Group Molecular Dermatology, Department of Dermatology and Allergy, Technische Universität München (TUM), Munich (Ollert M, J. Grosch). For the experimental study, the authors wish to thank the animal caretakers of the Helmholtz Center Munich, Johanna Grosch and the technicians of the GMC clinical chemistry laboratory for technical assistance. We are very grateful to Dr. Annette Schuhmacher, Ssniff Spezialdiäten GmbH and to Mr. Steven Yeung, Research Diets, Inc. for excellent assistance regarding the diets used in the study. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
GINIplus Funding: The GINIplus study was mainly supported for the first 3 years of the Federal Ministry for Education, Science, Research and Technology (interventional arm) and Helmholtz Zentrum Munich (former GSF) (observational arm). The four-year, six-year, 10-year, and 15-year follow-up examinations of the GINIplus study were covered from the respective budgets of the five study centers (Helmholtz Zentrum Munich (former GSF), Research Institute at Marien-Hospital Wesel, LMU Munich, TU Munich, and from 6 years onwards also from IUF – Leibniz Research-Institute for Environmental Medicine at the University of Düsseldorf), and a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). Furthermore, the 15-year follow-up examination of the GINIplus study was supported by the Commission of the European Communities, the seventh Framework Program: MeDALL project, and as well by the Mead Johnson and Nestlé companies. LISA Funding: The LISA study was mainly supported by grants from the Federal Ministry for Education, Science, Research, and Technology, and in addition from Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research—UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef for the first 2 years. The four-year, six-year, 10-year and 15-year follow-up examinations of the LISA study were covered from the respective budgets of the involved partners (Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research–UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef, IUF—Leibniz-Research Institute for Environmental Medicine at the University of Düsseldorf) and in addition by a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). Furthermore, the 15-year follow-up examination of the LISA study was supported by the Commission of the European Communities, the seventh Framework Program: MeDALL project. Mouse studies: Mouse studies were supported by the HMGU Allergy Projects, Project #8 - G-509000-018, from the Helmholtz Zentrum München. German Mouse Clinic was supported by the German Federal Ministry of Education and Research (Infrafrontier grant 01KX1012); German Center for Diabetes Research (DZD).
CONFLICT OF INTEREST
The authors declare no conflict of interest.





