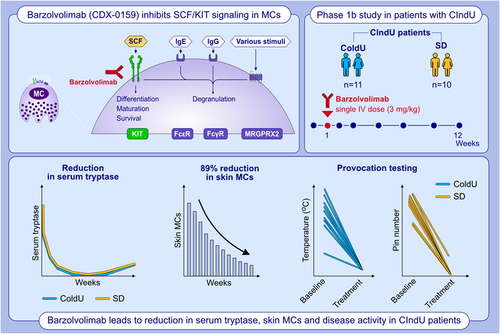
Anti-KIT antibody, barzolvolimab, reduces skin mast cells and disease activity in chronic inducible urticaria
Dorothea Terhorst-Molawi and Tomasz Hawro contributed equally (co-first authors).
Abstract
Background
Chronic inducible urticaria (CIndU) is characterized by mast cell (MC)-mediated wheals in response to triggers: cold in cold urticaria (ColdU) and friction in symptomatic dermographism (SD). KIT receptor activation by stem cell factor (SCF) is essential for MC function. Barzolvolimab (CDX-0159) is a humanized antibody that inhibits KIT activation by SCF and was well tolerated in healthy volunteers with dose-dependent plasma tryptase suppression indicative of systemic mast cell ablation.
Methods
This is an open-label, trial in patients with antihistamine refractory ColdU or SD, receiving one IV dose of barzolvolimab (3 mg/kg), with a 12-week follow-up. Primary endpoint was safety/tolerability; pharmacodynamic (PD)/clinical endpoints included serum tryptase, plasma SCF, skin MC histology, provocation tests, urticaria control test (UCT), and dermatology life quality index (DLQI).
Results
Analysis populations were safety (n = 21) and pharmacodynamics/clinical activity (n = 20). Barzolvolimab was well tolerated; most adverse events were mild and resolved. Treatment resulted in significant depletion of skin MCs, decreased tryptase (<limit of detection), and increased soluble SCF through Week 12. Complete responses (negative provocation test) occurred in 95% (n = 19/20) of patients (n = 10/10 ColdU; n = 9/10 SD), and all (n = 20/20) showed improvement in urticaria control (UCT ≥ 12). The kinetics of clinical activity mirrored that of MC and tryptase reduction. DLQI-measured impairment significantly decreased to minimal/none in 93% of patients on study.
Conclusion
In CIndU patients, barzolvolimab was well tolerated, demonstrated marked, rapid, durable depletion of skin MCs, circulating tryptase, and reductions in clinical activity with significant improvements in disease control and quality of life (QoL) demonstrating potential therapeutic effects for MC-mediated disorders.
Graphical Abstract
In this Phase 1b study of CIndU (ColdU and SD) patients, barzolvolimab, a humanized antibody that inhibits KIT activation by SCF, was well tolerated. Barzolvolimab demonstrated marked, rapid, durable depletion of skin MCs, circulating tryptase, reductions in clinical activity, and significant improvements in disease control and QoL. Barzolvolimab has potential as a therapy for MC-mediated diseases.Abbreviations: CIndU, chronic inducible urticaria; ColdU, cold urticaria; FcR, Fc receptor; Ig, immunoglobulin; KIT, KIT proto-oncogene, receptor tyrosine kinase; MC, mast cell; MRGPRX2, mas-related G protein-coupled receptor-X2; QoL, quality of life; SCF, stem cell factor; SD, symptomatic dermographism
1 INTRODUCTION
Chronic inducible urticaria (CIndU), a form of chronic urticaria (CU), is characterized by the occurrence of urticarial signs and symptoms as a response to a specific and definite trigger.1 Two of the most frequent forms of CIndU are cold urticaria (ColdU) and symptomatic dermographism (SD).2, 3 Patients with typical ColdU develop itchy wheals when their skin is exposed to a cold stimulus; these patients may also have systemic reactions associated with systemic cold exposure (e.g., aquatic activities).4 Those with SD develop itching and/or burning wheals, often strip-shaped, from shearing forces on the skin, including rubbing, scratching, or scrubbing.4
Both ColdU and SD can be debilitating conditions, the latter of which is the most common form of CIndU occurring in up to 0.5% of the general population.3 The mean disease duration ranges from 3.6 to 6.9 years, although some patients may experience SD over decades.5 As a result, these patients experience significantly impaired quality of life (QoL).5 ColdU is a common and often dangerous form of CU accounting for one-third of all physical urticarias.6 It can result in loss of consciousness and death due to extensive cold contact and up to 72% of patients experience systemic reactions.6
The treatment options for CIndU are limited. While patients are advised to avoid triggers, consensus recommendations in the latest international guideline for urticaria, as well as the EAACI/GA2LEN/EDF/UNEV guidelines for CIndU, recommend a nonsedating second-generation antihistamine (SG-H1AH) for first line with a higher dose of the same for second line treatment.1, 4 In a recent study, the response of patients with CIndU was lower vs. chronic spontaneous urticaria (CSU) using standard doses of SG-H1AH (20.9% vs 37.9%, respectively).7 In addition, a high percentage of patients may not be receiving any treatment for their condition as noted in the AWARE study, where only 1 in 5 patients with CIndU were receiving non-sedating SG-H1AH.8 Beyond antihistamines, there are no licensed drugs indicated for CIndU. Omalizumab may be used off-label,4, 9 but many (~50%) CIndU patients have experienced insufficient responses.6, 10
Although the pathophysiology of ColdU and SD is not fully understood, activation and degranulation of skin mast cells and their release of histamine are held to be involved in both of CIndUs in the development of signs and symptoms.11-13 Mast cells (MCs) are powerful inflammatory tissue-resident immune cells implicated in numerous disorders spanning several disease areas, including allergy, inflammation, auto-immunity, fibrosis, and neurodegeneration. MCs are considered the main effectors in the pathogenesis of various inflammatory diseases including CU and systemic mastocytosis.1, 13, 14 In ColdU, SD, and all other forms of CU, skin MC activation results in sensory nerve stimulation, vasodilation, and extravasation, as well as the development of whealing, itch, and angioedema.13 IgE-dependent MC activation can drive activity in a subset of ColdU and SD as evidenced by the clinical activity of the anti-IgE mAb omalizumab and passive transfer experiments.6, 10, 15-17 Additionally, type IIb autoimmune mechanisms may be implicated, potentially through MC activation by IgG autoantibodies against IgE or its receptor (FceR1).5
Given the central role that MCs are believed to play in CU, agents that inhibit or deplete MCs may provide broad clinical benefit independent of the underlying causes and relevant triggers. Activation of the KIT (CD117, c-KIT) receptor tyrosine kinase by its only ligand stem cell factor (SCF) is required for the differentiation, chemotaxis, maturation, and survival of MCs.18 Importantly, genetic and pharmacological inhibition of KIT or SCF, in mice, results in tissue MC depletion, suggesting that KIT targeting may be an effective approach for treating CU.19, 20
To date, KIT-targeting therapies have generally been limited to small molecule tyrosine kinase inhibitors (TKIs), which are often tailored to inhibit activating mutations in the KIT kinase that drive certain disorders such as mastocytosis, and gastrointestinal stromal tumors. In mastocytosis, activating mutations in the KIT receptor drive MC expansion and symptoms, although a role for SCF and wild-type KIT has not been ruled out. By contrast to antibody-based therapies, TKIs that target wild-type KIT generally exhibit significant reactivity to related kinases and relatively low potency, which can limit their clinical utility.
Barzolvolimab (CDX-0159) is a humanized immunoglobulin G1 kappa (IgG1κ) monoclonal antibody (mAb) that binds the extracellular domain of KIT with high specificity and sub-nanomolar affinity, and allosterically inhibits activation by SCF.21 Modifications to the Fc fragment of barzolvolimab eliminated FcγR binding and the potential for significant infusion-related reactions through Fc-mediated MC activation, and enhanced antibody serum exposure in non-human primates. We have recently demonstrated that a single-dose of barzolvolimab in healthy human subjects was generally well tolerated and significantly suppressed plasma tryptase—a marker of tissue MC numbers—indicative of systemic MC ablation and underscoring its potential utility in MC-driven disorders.21 In this study, we examined the safety and clinical activity of barzolvolimab in patients with CIndU.
2 METHODS
2.1 Overall study design
This is an ongoing single center, open-label Phase 1b study evaluating the safety/tolerability and clinical efficacy of a single dose of barzolvolimab in patients with CIndU refractory to SG-H1AHs. Four cohorts of patients are planned: (1) ColdU (n = 10) with 3 mg/kg; (2) SD (n = 10) 3 mg/kg; (3) Cholinergic urticaria (n = 10) 3 mg/kg; and (4) ColdU (n = 10) 1.5 mg/kg. Single dose of barzolvolimab is administered intravenously on Day 1, and patients are followed for 12 weeks for safety, PD, and clinical activity assessments. Doses were selected based on the pharmacokinetics and profound and durable effect of tryptase suppression observed with healthy subjects. After completing the initial 12-week follow-up period, patients are invited to continue into a long-term safety follow-up period to 36 weeks.
2.2 Subpopulation focus
Here, we report data from two cohorts of patients with ColdU and SD treated with a single dose of barzolvolimab (3 mg/kg) followed for 12 weeks as detailed below.
The study consisted of three distinct parts over 14 weeks: (1) screening (Day −14 to Day 1): duration of ≤2 weeks where patients who provided written informed consent were assessed for study eligibility and baseline signs and symptoms; (2) treatment (Day 1): following confirmation of eligibility, baseline assessments were conducted and treatment with barzolvolimab 3 mg/kg IV was administered over 60 min; and (3) post-treatment follow-up (Day 2–Day 85; 12 weeks): follow-up assessment visits occurred at weeks 1, 2, 4, 6, 8, and 12.
2.3 Patient selection
Adults 18–75 years old with a diagnosis of ColdU or SD for ≥3 months were eligible. Symptoms included both wheal and itch/burning/painful sensation despite concurrent use of non-sedating SG-H1AH (up to four times the approved dosage); patients had to be on stable doses of these treatment ≥4 weeks prior to the screening visit. Patients who were unresponsive to, did not tolerate, or discontinued treatment with omalizumab were allowed to enter the study after a washout period of 3 months.
For ColdU, patients had a positive cold provocation test at ≥4°C using TempTest® (wheal and itch/burning/painful sensation) during screening.22 For SD, patients had a positive reaction with at least one FricTest® pin (wheal and itch) during screening.23 Those with concomitant CSU were eligible provided that symptoms at screening were consistent with ColdU or SD and that ColdU or SD were the dominant form of CU. Patients continued to use SG-H1AH as background medication in stable doses during the study; rescue on demand, that is, additional antihistamine tablets, not exceeding fourfold dosage, was allowed.
2.4 Outcomes
The primary endpoint was safety/tolerability. Secondary/exploratory outcomes were pharmacodynamic and clinical activity and included provocation test, serum tryptase, skin MC numbers assessed using skin biopsies, urticaria control test (UCT), and the dermatology life quality index (DLQI).
2.5 Provocation tests
For patients with ColdU, the critical temperature threshold (CTT) was assessed and reported in °C using TempTest® (Courage + Khazaka), which is an electronic testing device with a metallic cooling element that maintains precise pre-set temperatures from 4° to 44°C applied to the skin. The patients placed the inner forearm on TempTest® for 5 min. The CTT, that is, the highest temperature that produced a wheal response, was recorded 10 min after the end of exposure. A negative TempTest® result occurred where there were no wheals present at any temperature along the gradient 10 min after termination of the provocation test. A negative value for the TempTest® was conservatively set to 3°C, one degree below the lowest level evaluated.
For patients with SD, the change in provocation threshold was assessed with FricTest® (Moxie, Berlin, Germany). The instrument was held perpendicular to the skin on the upper back or volar forearm, and a light stroking pressure was applied for 10 cm; then, the test was read at 10 min.23 A positive reaction occurred when a wheal developed and itch was reported at the site of the provocation with at least the longest pin. If all four pins induced wheals, the reaction was considered severe. A negative FricTest® occurred where there were no wheals present at any of the 4 pins 10 min after the provocation test. A complete response (CR) was defined as a negative provocation test at CTT ≤4°C or for SD 0 pins.
2.6 Pharmacokinetics
Whole blood was collected and processed to serum for barzolvolimab quantitation at pre-infusion, 15 min, 1, and 4 h after infusion and at 1, 7, 14, 28, 42, 56, and 84 days post-infusion. The barzolvolimab quantitation assay utilized a direct electrochemiluminescent (ECL) assay format. In this assay, a barzolvolimab capture antibody was coated on a Meso Scale Discovery (MSD) plate and after blocking non-specific binding sites on the plate's surface, barzolvolimab was captured from the serum sample. A solution composed of the detection antibody (biotinylated-goat anti-human IgG) and Streptavidin-Sulfo–TAG was added to the plate, and then, MSD Read Buffer was added, and the plate was read on the MSD Sector™ QuickPlex SQ 120. The relative light unit (RLU) signal was proportional to the amount of barzolvolimab bound to the plate.
2.7 Mast cells, tryptase, and SCF assessments
Non-lesional 3 mm skin punch biopsies were taken at baseline, and Weeks 1, 4, 8, and 12 Visits to assess the change in MCs over time. Mast cells were quantified by a blinded investigator using quantitative histomorphometry on tryptase and CD117 (KIT)-stained sections.
Immunohistochemistry for tryptase and CD117 was performed on paraffin-embedded skin sections. In brief, after deparaffinization, epitope retrieval in citrate buffer at 55°C and protein blocking, sections were incubated with a primary antibody for tryptase (mouse anti-human, dilution 1:2,000; Dako Agilent) or CD117 (mouse anti-human, dilution 1:300; Dako Agilent), followed by a secondary anti-mouse antibody (Dako Agilent). Sections were then counter stained with Mayer's Hematoxylin and analyzed under a light microscope by a blinded investigator. Number of positive cells were evaluated in consecutive microscopic fields at 100× magnification directly underneath the basal membrane of the epidermis with 4–7 microscopic fields assessed per patient, and the data are presented as the mean of these microscopic fields.
Biomarker samples including tryptase and SCF were drawn at each timepoint. An additional serum sample for tryptase was drawn at ~1 h following onset of symptoms for any patients who experienced an infusion reaction. Total serum tryptase was analyzed using an ImmunoCAP™ fluorescent enzyme immunoassay and was performed by Mayo Laboratories. Tryptase measurements below the level of assay detection (1 ng/ml) were assigned a value of 0. Plasma SCF was measured with a quantitative ECL immunoassay using a sandwich immunosorbent assay format and performed using an MSD instrument.
2.8 Urticaria control test
The UCT is a validated and highly reliable disease-specific patient-reported outcome measure consisting of four questions that retrospectively assesses disease control over the previous 4 weeks.24, 25 The UCT was specifically developed and validated to measure disease control in all forms of CU, including CIndU.26 Each of the four questions, covering disease activity, QoL survey, disease control, and therapy, was scored on a scale of 0–4. The UCT score was derived by adding up the scores from each of the four questions. A total score from 0 (no control) to 16 points (complete control) was derived, with a score of ≥12 indicating well-controlled disease; the minimal clinically important difference (MCID) was 3 points.
2.9 Quality of life: dermatology life quality index
Quality of life (QoL) was measured using the DLQI which consists of 10 items addressing patients' perception of their QoL over the last week such as dermatology-related symptoms and feelings, daily activities, leisure, work or school, personal relationships, and the treatment. Each item was scored on a 4-point Likert scale (0 = “not at all/not relevant”; 1 = “a little”; 2 = “a lot”; and 3 = “very much”). The total score was the sum of the 10 items (0–30). A high score was indicative of a poor QoL; MCID was a score of ≥4, and 0–1 indicated no impact of disease on patients' QoL.
2.10 Safety/tolerability
Severity of events were rated by the investigator as mild (usually transient in nature and generally not interfering with normal activities); moderate (sufficiently discomforting to interfere with normal activities); and severe (prevents normal activities). Per protocol, AE related to study drug are being followed beyond 12 weeks, to resolution or stabilization.
2.11 Statistical analysis
The safety population included all patients who received a dose; the PD/clinical activity population included anyone who received a full dose of barzolvolimab. Descriptive summary statistics were used for demographics and other baseline characteristics as well as all other endpoints at each visit and as changes from baseline for all treated subjects.
2.12 Trial oversight
The study (NCT04548869; EUDRA-CT 2020-002792-35) was approved by an IEC and was conducted in accordance with the ethical principles of the International Council for Harmonisation Guideline for Good Clinical Practice and the World Medical Association Declaration of Helsinki 2013, and in accordance with applicable local regulations. All participants provided written consent prior to study screening.
3 RESULTS
3.1 Patient disposition
Twenty-one patients were included in the safety analysis. Since one patient received only a partial dose, 20 were included in the PD and clinical activity analyses. Median age (range) was 41 (25–65) years, about half (48%) were female, and ~57% had disease duration for ≥5 years (Table 1). At baseline, patients' mean scores (range) for DLQI (10.8 [2–21]) and UCT (6.0 [0–13]) indicated marked impairment of quality of life and poorly controlled disease, respectively. Three patients (1 with ColdU and 2 with SD) were previously treated with omalizumab and chose to discontinue that treatment because they remained symptomatic. At baseline, provocation thresholds, on average (range), were 18.9 (5–27) °C for ColdU patients and 3.5 (2–4) pins for SD patients.
| ColdU (N = 11) | SD (N = 10) | All (N = 21) | |
|---|---|---|---|
| Age, median (range) years | 43 (27–65) | 39 (25–56) | 41 (25–65) |
| Gender female, n (%) | 6 (54.5%) | 4 (40.0%) | 10 (47.6%) |
| Race, n (%) | |||
| White | 10 (90.9%) | 10 (100%) | 20 (95.2%) |
| Asian | 1 (9.1%) | 0 | 1 (4.8%) |
| Ethnicity. n (%) | |||
| Hispanic or Latino | 1 (9.1%) | 0 | 1 (4.8%) |
| Weight median (range) kg | 77.0 (61.0–93.0) | 85.7 (57.0–122.0) | 81.5 (57.0–122.0) |
| Disease duration. n (%) | |||
| <5 years | 5 (45.5%) | 4 (40%) | 9 (42.9%) |
| ≥5 years | 6 (54.5%) | 6 (60%) | 12 (57.1%) |
| History of angioedema, n (%) | 6 (54.5%) | 0 | 6 (28.6%) |
| Provocation threshold mean (range) | 18.9 (5–27) °C | 3.5 (2–4) Pins | |
| UCT mean (range) | 7 (2–13) | 4.9 (0–10) | 6.0 (0–13) |
| DLQI mean (range) | 10.1 (3–17) | 11.2 (2–21) | 10.8 (2–21) |
| Prior medication H1AH, n (%) | 11 (100%) | 10 (100%) | 21 (100%) |
| Biologics (omalizumab) | 1 (9%) | 2 (20%) | 3 (14.3%) |
| Tryptase median (range), ng/ml | 3.8 (2.4–5.5) | 4.6 (1.3–8.6) | 4.2 (1.3–8.6) |
- Abbreviations: AH, antihistamines; ColdU, cold urticaria; DLQI, Dermatology Life Quality Index; H1AH, non-sedating H1 antihistamines; SD, symptomatic dermographism; UCT, urticaria control test.
3.2 Outcomes
3.2.1 Safety/tolerability
Barzolvolimab was well tolerated (Table 2). All 21 patients reported AE. Most AEs were mild, and the most common (≥3 patients) were hair color changes (areas of hair lightening) (76%; n = 16/21), infusion reactions (43%; n = 9/21), taste changes (38%; n = 8/21), nasopharyngitis (24%; n = 5/21), malaise (24%; n = 5/21), and headache (19%; n = 4/21).
| Adverse event n (%) | ColdU N = 11 | SD N = 10 | Total N = 21 |
|---|---|---|---|
| Any adverse event | 11 (100) | 10 (100) | 21 (100) |
| Hair color changes | 8 (72.7) | 8 (80.0) | 16 (76.2) |
| Infusion-related reactionsa | 8 (72.7) | 1 (10.0) | 9 (42.9) |
| Taste changesb | 3 (27.3) | 5 (50.0) | 8 (38.1) |
| Nasopharyngitis | 2 (18.2) | 3 (30.0) | 5 (23.8) |
| Malaisec | 4 (36.4) | 1 (10.0) | 5 (23.8) |
| Headache | 3 (27.3) | 1 (10.0) | 4 (19.0) |
- Note: Preferred Term MedDRA Version 23.1. >1 adverse event could have occurred in one patient.
- Abbreviations: ColdU, cold urticaria; SD, symptomatic dermographism.
- a Infusion-related reactions include abdominal discomfort, dizziness, erythema, feeling hot, headache, hypoesthesia, oropharyngeal pain, paranesthesia oral, pruritus, rash, infusion-related reaction, and urticaria.
- b Taste changes include ageusia and hypogeusia.
- c Malaise includes malaise and fatigue.
First appearance of hair color change was reported 4–6 weeks post-treatment. Affected areas were mainly head hair, eyebrows, eyelashes and in men beard hair as well as body hair in some patients. The hair color change was transient and improved over time with new hair growth at about 10–12 weeks post-treatment, with complete resolution over time during a follow-up. Overall, the hair color changes were considered mild and not burdensome to patients.
Taste changes were reported by patients as being selective for umami and salty flavor. All patients experiencing taste changes reported the ability to taste, but less intensively and the loss of intensity varied between patients. Changes were all transient and improved gradually with complete resolution within 12 weeks after treatment in most patients. Given the quick resolution, patients did not report impairment of quality of life by the taste changes.
Infusion reactions, which manifested as localized hives and itching as well as erythema and feeling hot, resolved spontaneously. One patient with a history of fainting experienced loss of consciousness during infusion; this event was not associated with MC activation based on decreasing tryptase values shortly after the event.
Overall, mean hematology parameters remained within the normal ranges. Mild, transient, and asymptomatic decreases in hemoglobin and white blood cell parameters occurred for some patients (Figure S1).
3.2.2 Pharmacokinetic and pharmacodynamic outcomes
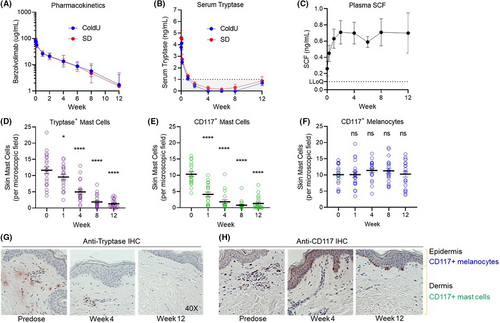
Single-dose barzolvolimab administration exhibited a slow rate of clearance (2.7 ± 0.9 ml/day/kg) and a terminal half-life of 20.1 ± 7.1 days, consistent with engineered half-life extending mutations.21 The volume of distribution in the terminal phase was 70.9 ± 7.1 ml/kg or approximately 4.9 L in a 70 kg human. There were no differences in PK parameters between CU and SD patients and exposure was very similar to that observed in healthy volunteers (Figure 1A).21 Barzolvolimab administration resulted in a rapid and profound depletion of serum tryptase that was followed by a continued decrease in skin MCs over the 12-week study. The concomitant increase in circulating SCF as a result of KIT blockade by barzolvolimab is indicative of KIT engagement in tissues (Figure 1B,C). Notably, baseline serum tryptase levels were within the normal range but rapidly decreased after dosing, consistent with findings in healthy human subjects (Figure 1B).21 Similarly, the reduction of skin MCs was significant at Weeks 1, 4, 8, and 12 after dosing as assessed by immunohistochemistry (Figure 1D,E). Skin MC reduction was observed and independently assessed by staining for tryptase (89% decrease at Week 12) and CD117 (KIT; 87% decrease at Week 12; Figure 1G,H). The kinetics of serum tryptase reduction correlated with that of CD117-positive and tryptase-positive skin MC numbers, demonstrating circulating tryptase is a surrogate marker for skin MC number (Figure S2). There was no change from baseline in epidermal CD117 (KIT+) melanocytes at any timepoint (Figure 1F).
3.2.3 Clinical outcomes
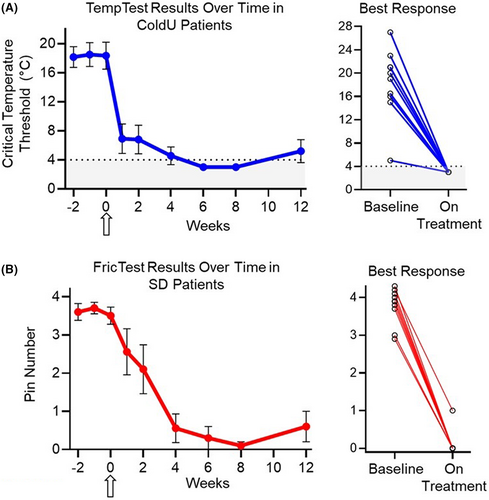
Rapid (as early as 1 week) and durable responses were observed in all CIndU patients as assessed by provocation testing (Figure 2). A complete response (CR) was achieved in 95% (n = 19/20) of patients (n = 10/10 ColdU; n = 9/10 SD; Figure 2). The median duration (range) of CR through the 12-week observation period was 77+ days (29–86; n = 10) or ColdU and 57+ days (16–70; n = 9) for SD patients. Across timepoints (Weeks 4, 8, and 12), 80% (n = 8/10), 100% (n = 10/10), and 80% (n = 8/10) of ColdU and 78% (n = 7/9), 90% (n = 9/10), and 70% (n = 7/10) of SD patients, respectively, experienced a CR. All three patients who had insufficient response to omalizumab treatment had a CR after treatment with barzolvolimab. Following a single dose of barzolvolimab, rapid improvements in urticaria control, a UCT score of ≥12 (well controlled) was achieved by 80% (n = 16/20) of the patients within Week 4 post-treatment (Figure 3A,B). By Week 8, all patients (100%; n = 20/20) achieved well-controlled urticaria, which was sustained to Week 12 post-dose by 80% (n = 16/20) of patients. Complete urticaria control (UCT = 16) was achieved by 35% (n = 7/20), 65% (n = 13/20), and 40% (n = 8/20) at Weeks 4, 8, and 12, respectively (Figure 3A,B).
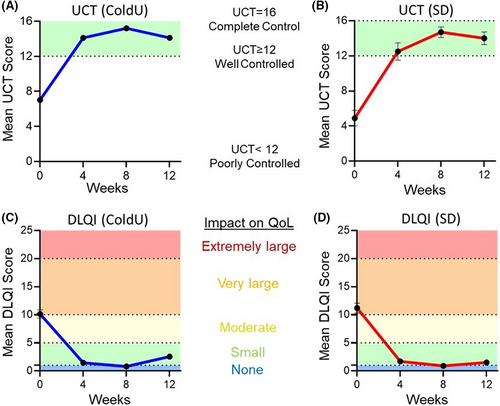
At baseline, patients in both treatment groups reported disease impact on their QoL (Table 1; Figure 3C,D). Disease impact significantly decreased after dosing; a score of 0–1 (minimal/none) was achieved by 80% (n = 16/20) for all patients who completed the DLQI during the study.
Additionally, clinically meaningful improvements in QoL were attained and sustained to Week 12. All patients (100%; n = 14/14) at Week 4, 93% (n = 13/14) at Week 8, and 86% (n = 12/14) at Week 12 showed a minimally clinically important difference (MCID) in QoL as assessed by a ≥4-point reduction from baseline (or a score decrease to 0 or 1 [no disease impact on QoL]) for that week. Only patients who had a baseline score of ≥4 were included in the MCID analysis.
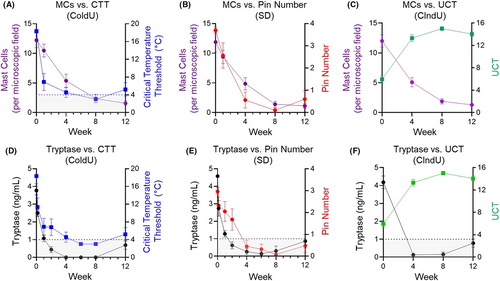
Overall, the kinetics of circulating tryptase and skin MC reduction correlated with improvements in provocation testing (Figure 4A–E; Figure S3) and clinical activity (Figure 4C,F; Figure S4), consistent with a central role for MCs in the pathogenesis of ColdU and SD.
4 DISCUSSION
Mast cells have been implicated in the etiology of many disorders in several disease areas, but direct evidence for their true involvement in the manifestation of clinical symptoms is lacking. Here, we demonstrate, for the first time, that a single dose of the anti-KIT inhibitory mAb, barzolvolimab, rapidly depletes skin MCs leading to profound decreases in disease activity in SD and ColdU, the two most common forms of CIndU. This study also highlights the striking reliance of skin mast cells on KIT/SCF signaling and provides the first direct demonstration that skin MCs are the key effector cells in ColdU and SD. Indeed, we observed a strong correlation between the kinetics of KIT inhibition (evidenced by SCF increases) with MC depletion (seen in cutaneous tissues and manifested by reductions in serum tryptase) and clinical activity. A single dose of barzolvolimab was sufficient to induce rapid and prolonged PD responses due to its potency and consistent with the slow rate of antibody clearance. Our data also establish serum tryptase as a convenient biomarker for skin MC number and disease activity in CIndU, which should greatly facilitate dose selection and schedule in future studies of barzolvolimab.
The results from this study were consistent with recent data in healthy subjects where the high exposure achieved with barzolvolimab resulted in long-lasting pharmacodynamic effects. The terminal half-life of barzolvolimab at a single 3 mg/kg dose was approximately 20 days and was accompanied with tryptase suppression for >6 weeks. The extended half-life was attributed to modifications engineered into the Fc domain, which increased binding to the FcRn and reduced clearance of the antibody.21
In this study, barzolvolimab was generally well tolerated. The observed adverse effects of hair color and taste changes were consistent with KIT inhibition. KIT activation by SCF in follicular melanocytes participates in their terminal differentiation and maturation but is not expressed in melanocyte stem cells.27 In our study, hair color changes were reversible as expected based on findings that KIT inhibition only impacts follicular melanocytes in cycling hair. These observations are consistent with reversible hair depigmentation reported with other KIT-targeting mAbs and TKIs,28 and are in agreement with a specific role of KIT in the maintenance of differentiated follicular melanocytes.28 Similarly, KIT expression has been reported in mature T1R3-positive type II taste cells29 although its role in this context is not well defined, and recoverable taste alterations have also been reported with KIT-targeting TKIs.29 Consistent with observations in healthy subjects,21 single-dose barzolvolimab led to decreases in hematological parameters that were asymptomatic and reversible.
The clinical data from this study provide the first direct evidence that MCs are the key effector cells in ColdU and SD, as evidenced by the profound and durable clinical improvements over 12 weeks. Moreover, three patients with ColdU and SD who were symptomatic during prior treatment with omalizumab demonstrated complete responses after a single dose of barzolvolimab, suggesting that broader efficacy may be achieved through MC depletion via KIT inhibition than through inhibition of specific MC triggers.
Our data demonstrate a striking correlation in the kinetics of tryptase and MC depletion with changes in provocation testing and clinical activity. Interestingly, MCs are not fully depleted in all patients, and their numbers may start to return at Week 12. Similarly, it is unclear whether tryptase is fully depleted in all patients due to quantitative limitations in the assay, although it appears that full symptom control generally requires tryptase depletion below assay detection levels. It is therefore possible that longer term KIT suppression will drive full MC depletion. Alternatively, there may be a small population of MCs that can persist despite prolonged KIT inhibition. Longer-term multiple dose studies and off-treatment biopsy collection will provide a fuller picture of the kinetics of mast cell depletion and re-population. While we only evaluated skin MCs histologically in this study, the profound suppression of circulating tryptase observed—which reflects the sum total contribution of mature MCs in all tissues—suggests that barzolvolimab likely depletes MCs from other tissues as well. These findings justify exploring barzolvolimab in other diseases with MC involvement which may also help to further unravel the contribution of MCs to disease mechanism as well as its potential therapeutic utility.
Limitations of this study included the small sample size, and open-label, single center design. In this analysis, we only presented 12-week post-dose data from two cohorts of patients with SD or ColdU who were treated with the 3 mg/kg dose. Patients are being followed long term (to 36 weeks), and results from the entire study will be presented once the study is complete. To date, we do not know the effect of barzolvolimab on mast cell populations other than skin mast cells, although we do speculate in a prior study.21
Further studies are needed to assess lung mast cells, gut mast cells, and mast cells at other sites for changes in number and function in response to barzolvolimab. Overall, data from this study support further evaluation of barzolvolimab's safety and efficacy in larger multi-dose, randomized, controlled clinical trials to corroborate these results, ones that portend a novel treatment approach which could address unmet needs in CIndU and other chronic MC-mediated diseases.
AUTHOR CONTRIBUTIONS
M Metz, M Maurer, DTM, T Hawro, EC, MHC, and DA designed the study. M Metz, M Maurer, DTM, T Hawro, EG, LK, LJT, and DA were directly involved in acquisition of the data. EC, MHC, DA, and KM coordinated data collection from the investigators. MHC, EC, DA, and T Hawthorne led the analysis of the data with input from KM. Underlying data were available to and verified by all authors. All authors contributed to the writing of the manuscript with medical writing assistance from Leonard Lionnet, PhD. The authors alone are responsible for the views expressed in this article, and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
ACKNOWLEDGMENTS
The authors would like to thank the patients for their participation, Evelin Hagen for technical assistance, Katharina Dick for patient care, Theresa Belotti for data management and quality control, Asma Ejaz and Pamela Morani for bioanalytical support, Linda Crew for technical contributions, and Leonard Lionnet, PhD for providing medical writing assistance which was funded by Celldex Therapeutics. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
Funding was provided by Celldex Therapeutics. Design of the study was conducted by M Maurer, M Metz, DTM, TH, KM, DA, EC, and MHC. Celldex utilized the CRO, Proinnovera, to manage the collection of primary data. All authors interpreted the data, had full access to all the data, and had final responsibility for the decision to submit the manuscript for publication.
CONFLICT OF INTEREST
D Terhorst-Molawi has received research funds/was advisor for Celldex, Moxie, Novartis and Sanofi; T Hawro is or recently was a speaker for Moxie and Roche; E Grekowitz has received research funds/was advisor for Novartis, and L Keifer have no conflicts to report; M Metz is or recently was a speaker and/ or advisor for Amgen, Aralez, ArgenX, Celldex, Moxie, Novartis, Roche, Sanofi, Third Harmonic Bio, Uriach; M Maurer is or recently was a speaker and/or advisor for, and/or has received research funding from: Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Roche, Sanofi/Regeneron, Third HarmonicBio, UCB, and Uriach; K Merchant, D Alvarado, E Crowley, LJ Thomas, T Hawthorne, and M Heath-Chiozzi are employees of Celldex and may hold stock and/or stock options.