The impact of dupilumab treatment on SARS-CoV-2 T cell responses in atopic dermatitis patients
Benjamin Ungar, Susan Hartzell, and Daniel Lozano-Ojalvo, a Paolo Cravedi and Emma Guttman-Yassky contributed equally to this work.
Studies on the pathophysiology of immune responses in COVID-19 point at a critical role for Th1 cells in viral clearance. Therefore, it has been postulated that abnormally elevated Th2 cytokines in individuals with atopic dermatitis (AD), a dermatological condition characterized by Th2-driven skin inflammation, impairs appropriate Th1 responses to viral infection and that specific Th2-targeting therapies are corrective.1 In line with this hypothesis, we previously showed that dupilumab, a monoclonal antibody that blocks the IL-4Rα subunit, thereby inhibiting Th2-associated IL-4 and IL-13 signaling, is associated with milder COVID-19 severity in AD patients.1 Importantly, dupilumab does not affect SARS-CoV-2 IgG antibody levels after vaccination.2 However, the effect of dupilumab on T cell responses after infection or vaccination is not known.
We prospectively collected PMBC samples from ≥12 year old moderate-to-severe AD patients either after COVID-19 infection or after SARS-CoV-2 mRNA vaccination in the Department of Dermatology at the Icahn School of Medicine, New York, between June 2020 and October 2021. Fifty-five samples from patients with prior SARS-CoV-2 infection confirmed by positive anti-SARS-CoV-2 Spike IgG (unvaccinated at the time of sample collection), and 125 post-vaccination samples from different subjects were analyzed. PBMCs were incubated for 24 h with peptides covering the immune-dominant regions of the S glycoprotein of SARS-CoV-2 and IFNγ and IL-2 antigen-specific responses were quantified using IFNγ/IL-2 Double-Color FluoroSpot (see Methods S1 and Figure S1 for further details). Spike antigens were used to provide comparisons between treatment groups as a measure of T cell responses for both post-infection and post-vaccination samples (the vaccine contains only spike antigen of SARS-CoV-2). Comparisons on log10-transformed spot counts to minimize the impact of outliers were made with unpaired Student's t-tests and correlations were calculated with the Spearman correlation coefficient. Patients were stratified into three cohorts based on their treatment strategy: (1) Dupilumab alone (Dupilumab); (2) other systemics immunomodulators (JAK-inhibitors, prednisone, phototherapy; Systemic), and (3) untreated or topical medications only (Limited).
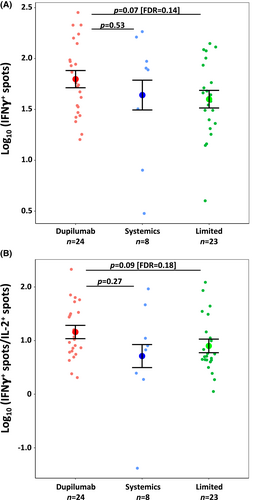
After the COVID-19 infection, IFNγ+ T cell-reactive counts were non-significantly higher in the Dupilumab (n = 24) vs. Limited (n = 23) groups (p = .072 [FDR = 0.144]; Figure 1A). IL-2+ and dual IFNγ+IL-2+ T cell counts did not differ between these two cohorts (not shown), but the IFNγ+/IL-2+ T cell count ratio trended toward an increase in the Dupilumab vs. Limited cohorts (p = .091 [FDR = 0.182]; Figure 1B). No differences were identified between Systemics (n = 8) and the other groups in terms of IFNγ+ or IL-2+ T cell counts or the IFNγ+/IL-2+ ratio.
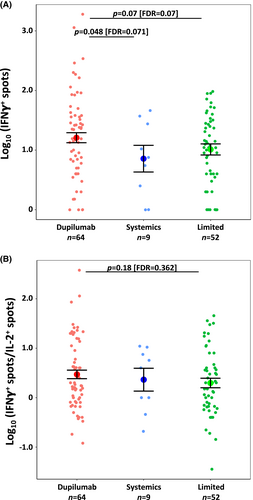
In the post-vaccination samples, there were significantly greater IFNγ+ T cell counts in Dupilumab vs. Systemics patients (p = .048 [FDR = 0.071]; Figure 2A) and a trend toward greater IFNγ+ T cell counts vs. Limited patients (p = .068 [FDR = 0.068]; Figure 2A). The IFNγ+/IL-2+ ratio was not significantly between Dupilumab vs. Limited (p = .181 [FDR = 0.362]; Figure 2B) or vs. Systemics. We observed no correlation between IFNγ+ or IL-2+ T cell counts or IFNγ+/IL-2+ ratio and time after vaccination, consistent with previous reports.3
To validate these results, in a subset of patients, we determined the specificity of the memory (CD45RA−) IL-2- and IFNγ-producing CD4+ T cells by flow cytometry using CD154 (CD40L) as an early activation marker upregulated upon Spike antigen recognition. While no significant differences were observed in CD154+IL-2+ T cells between groups, there was a higher percentage of IFNγ-producing Spike-specific (CD154+ IFNγ+) CD4+ T cells in dupilumab-treated patients vs. the Limited group after vaccination (Figure S2A,B). Consistent with previous studies,4 we observed an augmentation of IFNγ-producing Th1 cells after SARS-CoV-2 mRNA vaccination that was significantly higher in subjects treated with Dupilumab.
This study supports the hypothesis that specific Th2-targeting by dupilumab promotes a more balanced Th1/Th2 response to the COVID-19 infection in AD individuals (as shown in comparison to the Limited group, consisting of patients not receiving systemic treatments), with trends toward increased SARS-CoV-2-specific IFNγ+ T cell counts and a greater IFNγ/IL-2 ratio, potentially an indicator of a more specific Th1 response given that IL-2's involvement in multiple Th pathways.5 Furthermore, by blunting the abnormal activity of Th2 cells, dupilumab appears to promote Th1-prone T cell responses to mRNA vaccination. Of note, IFNγ counts were greater in the post-infection group than the post-vaccination group across all treatments. Further work is necessary to evaluate this, raising the question of whether Spike protein in the presence of other viral antigens may elicit a more robust response than Spike mRNA vaccination. Limitations of this study include small sample size, the unknown COVID-19 infection dates (prior infection was identified by IgG serologies), and lack of serial samples. Future studies in larger populations are needed to characterize T cell phenotypes more thoroughly.
Overall, this study suggests that Th2-inhibition with dupilumab does not hinder, and may possibly even improve, Th1-specific T cell responses to COVID-19 infection and mRNA vaccination.
ACKNOWLEDGMENTS
We thank the Mount Sinai Biorepository & Pathology Core for providing support in antibody sample processing.
FUNDING INFORMATION
This work was supported by the Department of Dermatology at the Icahn School of Medicine at Mount Sinai and a grant from Regeneron and Sanofi. Patients were recruited from within the Department of Dermatology at the Icahn School of Medicine. All funding sources reviewed and accepted the study design and the manuscript, with minimal input from Regeneron and Sanofi. Research reported in this publication was also supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01AI152036. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Paolo Cravedi was supported by a grant from the National Institute of Allergy and Infectious Diseases under Award Number 3U01AI063594-17S1.
CONFLICTS OF INTEREST
E. Guttman-Yassky is an employee of Mount Sinai and has received research funds (grants paid to the institution) from Abbvie, Amgen, AnaptysBio, AstraZeneca, Boehringer-Ingelhiem, Cara Therapeutics, Innovaderm, Janssen, KAO, Kyowa Kirin, Leo Pharma, Pfizer, Regeneron Pharmaceuticals, Inc., and UCB; and is a consultant for Abbvie, Almirall, Amgen, Arena, Asana Biosciences, AstraZeneca, Boehringer-Ingelheim, Bristol-Meyers Squibb, Cara Therapeutics, Connect Pharma, Eli Lilly, EMD Serono, Evidera, Galderma, Ichnos Sciences, Incyte, Janssen Biotech, Kyowa Kirin, Leo Pharma, Pandion Therapeutics, Pfizer, Ribon, RAPT Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi, SATO Pharmaceutical, Siolta Therapeutics, Target PharmaSolutions, UCB, and Ventyx Biosciences. B. Ungar is an employee of Mount Sinai and has received research funds (grants paid to the institution) from: Incyte, Rapt Therapeutics, and Pfizer. He is also a consultant for Arcutis Biotherapeutics and Castle Biosciences. A. Golant Is an employee of Mount Sinai and has served as a consultant or speaker for Regeneron, Sanofi, Abbvie, Incyte, Dermavant, Lilly, LEO Pharma, Evelo Biosciences, and Arcutis A. Pavel is an employee of Mount Sinai and conducts research sponsored by Regeneron. The other authors have no conflicts of interest to disclose.
Abbreviations
-
- AD
-
- atopic dermatitis
-
- COVID-19
-
- coronavirus disease 2019
-
- IgG
-
- Immunoglobulin G
-
- mRNA
-
- messenger RNA
-
- PBMC
-
- Peripheral blood mononuclear cell
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2




