Efficacy of FP-025: A novel matrix metalloproteinase-12 (MMP-12) inhibitor in murine allergic asthma
To the Editor,
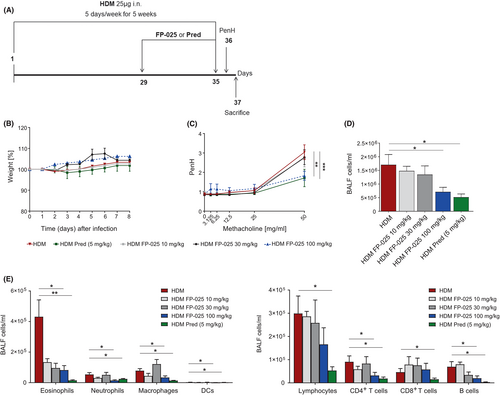
Asthma is a heterogeneous disease, as reflected by differences in age of onset, severity, treatment response and inflammatory profile. Matrix metalloprotease (MMP)-12 has been associated with inflammation in various subtypes of asthma.1 In allergic asthma, MMP-12 activity has been implicated in the accumulation of neutrophils and eosinophils, in regulating macrophage functions and in the development of airway hyperresponsiveness (AHR).1 To date, the underlying mechanisms by which MMP-12 may drive asthma pathophysiology have not been defined, nor has inhibition of MMP-12 as a targeted therapy for asthma been assessed in humans. FP-025 is a novel, potent, and highly selective non-hydroxamate inhibitor of MMP-12 with 90-fold selectivity over the closest family member (MMP-2) and two to three orders of magnitude over the seven other MMP family members. In an established mouse model of persistent house dust mite (HDM) allergic asthma,2 we explored the effect of increasing doses (0–100 mg/kg, daily for 7 days) of oral FP-025 on airway pathophysiology, with Prednisone (5 mg/kg) given intraperitoneally as control treatment (Figure 1A).3 FP-025 did not induce behavioral or physical signs of discomfort nor differences in body weight compared with controls, even at the highest dose (Figure 1B). FP-025 100 mg/kg abrogated airway hyperresponsiveness (AHR) to a similar extent as Prednisone (Figure 1C). Similarly, the highest dose of FP-025 significantly reduced numbers of total bronchoalveolar lavage (BAL) cells, comparable to Prednisone (Figure 1D). Numbers of eosinophils, neutrophils, macrophages, inflammatory-migratory dendritic cells (DCs), B lymphocytes, CD4+ T lymphocytes, but not CD8+ T lymphocytes, were significantly reduced in BAL by 100 mg/kg FP-025, like with Prednisone (Figure 1E). Prednisone, however, also attenuated CD8+ T lymphocytes.
FP-025 reduced peri-bronchial and peri-arterial cellular infiltrates in lungs of HDM-sensitized mice in a dose-dependent manner, without affecting total inflammation scores. Furthermore, both the alpha-smooth muscle actin (α-SMA) stain, as a marker of fibrosis, and mucus production by PAS stain were reduced by 100 mg/kg FP-025 in lungs of HDM-sensitized mice (Figure 2A).
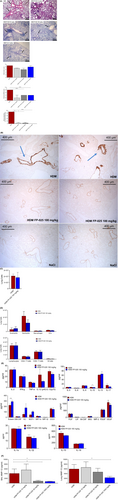
We found that the bronchial epithelial cells are the predominant source of MMP-12, and FP-025 highly reduced MMP-12 expression in lungs of HDM-sensitized mice, comparable to the stain for untreated mice (Figure 2B). Interestingly, and in line with total inflammation scores, in lung parenchyma, numbers of total inflammatory cells (Figure 2C), eosinophils, neutrophils, macrophages, inflammatory-migratory (CD11b+) dendritic cells (DCs), CD4+ lymphocytes, CD8+ lymphocytes and B lymphocytes were not affected (Figure 2D). Additionally, none of the mediators of innate, Th1- and Th2-responses, chemoattractant chemokines and growth factors (Figure 2E) were affected by FP-025. Importantly, FP-025 efficiently reduced levels of MMP-12 in BAL and lung parenchyma of HDM-sensitized mice in a dose-dependent manner (Figure 2F).
Respiratory viruses are the most frequent trigger of asthma exacerbations.4 As MMP-12 has a dualistic modulatory effect on the interferon (IFN)-α-mediated anti-viral response,5 we evaluated the efficacy of the anti-viral immune response to influenza upon FP-025 treatment (Figure S1A). No differences in AHR and IFN-α (below lower limit of detection) levels were noted between FP-025-treated and untreated mice, but FP-025 markedly reduced body weight loss and viral load (Figure S1B,C). Furthermore, FP-025 significantly reduced T lymphocyte numbers in lung parenchyma of infected mice, whereas myeloid cell numbers as well as that of lymphoid and myeloid BAL cells were unaffected. In parallel, FP-025 reduced BALF levels of IL-12 and CCL5 (Figure S1D–F). Also, in infected mice, FP-025 significantly reduced levels of MMP-12 in BAL and lung parenchyma (Figure S2).
In all, FP-025 in a dose-dependent manner significantly attenuated AHR, BAL inflammatory cell numbers and lung pathohistology in a mouse model of persistent HDM-allergic asthma. FP-025 markedly reduced MMP-12 expression in airway epithelial cells and levels of MMP-12 in lung parenchyma and airways, indicating that the enhanced expression in allergic asthma depends on an autocrine process. FP-025 did not negatively affect anti-viral responses, contributing to its safety profile. These findings warrant interventions with FP-025 in allergic asthma, also as FP-025 showed safety, tolerability, and good pharmacokinetics characteristic in a randomized, placebo-controlled, single, and multiple ascending dose study in healthy subjects.6
ACKNOWLEDGMENTS
We thank David Lau, Wenjin Yang, and Ben Chien (all Foresee Pharmaceuticals Co., Ltd) for sharing their knowledge and expertise on FP-025. This study was financially supported by Foresee Pharmaceuticals Co., Ltd.
CONFLICT OF INTEREST
L.R. receives funding from Foresee Pharmaceuticals, Chiesi Farmaceutici. Z.D. has received honoraria for serving on advisory boards or as a consultant with ALK, AstraZeneca, Boehringer-Ingelheim, GSK, HAL-Allergy, MSD, Sanofi-Genzyme. She acted as Research Director at QPS-NL: this institution has received research support from Foresee Pharmaceuticals, Novartis and Patara pharma. R.L. receives funding from Foresee Pharmaceuticals, Chiesi Farmaceutici, Astra Zeneca, MedImmune, GSK. The rest of the authors declare that they have no relevant conflicts of interest.




