Development and preclinical evaluation of virus-like particle vaccine against COVID-19 infection
Ismail Cem Yilmaz
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorEmre Mert Ipekoglu
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorArtun Bulbul
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorNilsu Turay
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorMuzaffer Yildirim
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorIrem Evcili
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorNaz Surucu Yilmaz
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorNese Guvencli
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorYagmur Aydin
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorBilgi Gungor
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorBerfu Saraydar
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorAsli Gulce Bartan
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorBilgehan Ibibik
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorTugce Bildik
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorİlayda Baydemir
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorHatice Asena Sanli
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorBasak Kayaoglu
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorYasemin Ceylan
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorTugce Yildirim
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorIrem Abras
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorIhsan Cihan Ayanoglu
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorSefa Burak Cam
Hacettepe University Faculty of Medicine Department of Histology and Embryology, Ankara, Turkey
Search for more papers by this authorEda Ciftci Dede
Hacettepe University Graduate School of Science and Engineering, Department of Bioengineering, Ankara, Turkey
Search for more papers by this authorMerve Gizer
Hacettepe University Graduate School of Health Sciences, Department of Stem Cell Sciences, Ankara, Turkey
Search for more papers by this authorOsman Erganis
Faculty of Veterinary Medicine, Selcuk University, Konya, Turkey
Search for more papers by this authorFahriye Sarac
Pendik Veterinary Research and Control Institute, Istanbul, Turkey
Search for more papers by this authorSerdar Uzar
Pendik Veterinary Research and Control Institute, Istanbul, Turkey
Search for more papers by this authorHakan Enul
Pendik Veterinary Research and Control Institute, Istanbul, Turkey
Search for more papers by this authorCumhur Adiay
Pendik Veterinary Research and Control Institute, Istanbul, Turkey
Search for more papers by this authorGamze Aykut
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorIsmail Selim Yildirim
Marmara Research Center, TUBITAK, Istanbul, Turkey
Search for more papers by this authorGulay Korukluoglu
Virology Laboratory, General Directorate of Public Health, Ankara, Turkey
Search for more papers by this authorHasan Ersin Zeytin
Biotechnology Research Center, Nobel Pharma, Istanbul, Turkey
Search for more papers by this authorPetek Korkusuz
Hacettepe University Faculty of Medicine Department of Histology and Embryology, Ankara, Turkey
Search for more papers by this authorCorresponding Author
Ihsan Gursel
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Correspondence
Mayda Gursel, Department of Biological Sciences, Middle East Technical University, Ankara, Turkey.
Email: [email protected]
Ihsan Gursel, Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey.
Email: [email protected]
Search for more papers by this authorCorresponding Author
Mayda Gursel
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Correspondence
Mayda Gursel, Department of Biological Sciences, Middle East Technical University, Ankara, Turkey.
Email: [email protected]
Ihsan Gursel, Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey.
Email: [email protected]
Search for more papers by this authorIsmail Cem Yilmaz
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorEmre Mert Ipekoglu
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorArtun Bulbul
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorNilsu Turay
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorMuzaffer Yildirim
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorIrem Evcili
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorNaz Surucu Yilmaz
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorNese Guvencli
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorYagmur Aydin
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorBilgi Gungor
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorBerfu Saraydar
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorAsli Gulce Bartan
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorBilgehan Ibibik
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorTugce Bildik
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorİlayda Baydemir
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorHatice Asena Sanli
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorBasak Kayaoglu
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorYasemin Ceylan
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorTugce Yildirim
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorIrem Abras
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorIhsan Cihan Ayanoglu
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Search for more papers by this authorSefa Burak Cam
Hacettepe University Faculty of Medicine Department of Histology and Embryology, Ankara, Turkey
Search for more papers by this authorEda Ciftci Dede
Hacettepe University Graduate School of Science and Engineering, Department of Bioengineering, Ankara, Turkey
Search for more papers by this authorMerve Gizer
Hacettepe University Graduate School of Health Sciences, Department of Stem Cell Sciences, Ankara, Turkey
Search for more papers by this authorOsman Erganis
Faculty of Veterinary Medicine, Selcuk University, Konya, Turkey
Search for more papers by this authorFahriye Sarac
Pendik Veterinary Research and Control Institute, Istanbul, Turkey
Search for more papers by this authorSerdar Uzar
Pendik Veterinary Research and Control Institute, Istanbul, Turkey
Search for more papers by this authorHakan Enul
Pendik Veterinary Research and Control Institute, Istanbul, Turkey
Search for more papers by this authorCumhur Adiay
Pendik Veterinary Research and Control Institute, Istanbul, Turkey
Search for more papers by this authorGamze Aykut
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Search for more papers by this authorIsmail Selim Yildirim
Marmara Research Center, TUBITAK, Istanbul, Turkey
Search for more papers by this authorGulay Korukluoglu
Virology Laboratory, General Directorate of Public Health, Ankara, Turkey
Search for more papers by this authorHasan Ersin Zeytin
Biotechnology Research Center, Nobel Pharma, Istanbul, Turkey
Search for more papers by this authorPetek Korkusuz
Hacettepe University Faculty of Medicine Department of Histology and Embryology, Ankara, Turkey
Search for more papers by this authorCorresponding Author
Ihsan Gursel
Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey
Correspondence
Mayda Gursel, Department of Biological Sciences, Middle East Technical University, Ankara, Turkey.
Email: [email protected]
Ihsan Gursel, Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey.
Email: [email protected]
Search for more papers by this authorCorresponding Author
Mayda Gursel
Department of Biological Sciences, Middle East Technical University, Ankara, Turkey
Correspondence
Mayda Gursel, Department of Biological Sciences, Middle East Technical University, Ankara, Turkey.
Email: [email protected]
Ihsan Gursel, Molecular Biology and Genetics Department, Bilkent University, Ankara, Turkey.
Email: [email protected]
Search for more papers by this authorMayda Gursel and Ihsan Gursel are joint senior authors.
Abstract
Background
Vaccines that incorporate multiple SARS-CoV-2 antigens can further broaden the breadth of virus-specific cellular and humoral immunity. This study describes the development and immunogenicity of SARS-CoV-2 VLP vaccine that incorporates the four structural proteins of SARS-CoV-2.
Methods
VLPs were generated in transiently transfected HEK293 cells, purified by multimodal chromatography, and characterized by tunable-resistive pulse sensing, AFM, SEM, and TEM. Immunoblotting studies verified the protein identities of VLPs. Cellular and humoral immune responses of immunized animals demonstrated the immune potency of the formulated VLP vaccine.
Results
Transiently transfected HEK293 cells reproducibly generated vesicular VLPs that were similar in size to and expressing all four structural proteins of SARS-CoV-2. Alum adsorbed, K3-CpG ODN-adjuvanted VLPs elicited high titer anti-S, anti-RBD, anti-N IgG, triggered multifunctional Th1-biased T-cell responses, reduced virus load, and prevented lung pathology upon live virus challenge in vaccinated animals.
Conclusion
These data suggest that VLPs expressing all four structural protein antigens of SARS-CoV-2 are immunogenic and can protect animals from developing COVID-19 infection following vaccination.
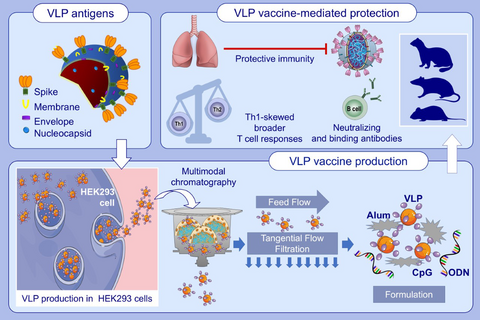
Graphical Abstract
SARS-CoV-2 VLP vaccine that incorporates the four structural proteins of SARS-CoV-2 is reproducibly produced in suspension adapted HEK293 cells. Alum adsorbed, K3-CpG ODN-adjuvanted VLPs elicit high titer anti-S, anti-RBD, anti-N IgG, and neutralizing antibodies in mice, rats, and ferrets. The VLP vaccine supports multifunctional Th1-biased T-cell responses and demonstrate immunoprotective activity against live SARS-CoV-2 challenge in vaccinated mice. Abbreviations: Alum, aluminum hydroxide; CpG, cytosine-phosphate-guanosine dinucleotide; HEK293, human embryonic kidney cell line; ODN, oligodeoxynucleotide; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Th, T-helper cell; VLP, virus-like particle.
CONFLICT OF INTEREST
There is no financial conflict of interest to declare by the Authors.
Supporting Information
| Filename | Description |
|---|---|
| all15091-sup-0001-FigS1-S4.docxWord 2007 document , 1.9 MB | Figure S1-S4 |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1 McGill COVID19 Vaccine Tracker Team, COVID19 Vaccine Tracker. https://covid19.trackvaccines.org/vaccines/
- 2Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384(5): 403-416. 10.1056/NEJMoa2035389
- 3Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27): 2603-2615. 10.1056/NEJMoa2034577
- 4Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397(10269): 99-111. 10.1016/S0140-6736(20)32661-1
- 5Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021; 397(10275): 671-681. 10.1016/S0140-6736(21)00234-8
- 6Sadoff J, Gray G, Vandebosch AN, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021; 384(23): 2187-2201. 10.1056/NEJMoa2101544
- 7Hoffmann M, Arora P, Groß R, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021; 184(9): 2384-2393.e12. 10.1016/j.cell.2021.03.036
- 8Tada T, Dcosta BM, Samanovic-Golden M, et al. Neutralization of viruses with European, South African, and United States SARS-CoV-2 variant spike proteins by convalescent sera and BNT162b2 mRNA vaccine-elicited antibodies. bioRxiv. 2021. 10.1101/2021.02.05.430003
10.1101/2021.02.05.430003 Google Scholar
- 9Garcia-Beltran WF, Lam EC, St. Denis K, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021; 184(9): 2372-2383.e9. 10.1016/j.cell.2021.03.013
- 10Ikegame S, Siddiquey MNA, Hung CT, et al. Qualitatively distinct modes of Sputnik V vaccine-neutralization escape by SARS-CoV-2 Spike variants. medRxiv. 2021. 10.1101/2021.03.31.21254660
- 11McCallum M, Bassi J, De Marco A, et al. SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science. 2021; 373: 648-654. 10.1126/science.abi7994
- 12Cele S, Gazy I, Jackson L, et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021; 593(7857): 142-146. 10.1038/s41586-021-03471-w
- 13Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021; 384(20): 1885-1898. 10.1056/NEJMoa2102214
- 14Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021; 397(10282): 1351-1362. 10.1016/S0140-6736(21)00628-0
- 15Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021; 184(9): 2348-2361.e6. 10.1016/j.cell.2021.02.037
- 16Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021; 27: 1379-1384. 10.1038/s41591-021-01413-7
- 17Davis C, Logan N, Tyson G, et al. Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. medRxiv 2021. 10.1101/2021.06.23.21259327
10.1101/2021.06.23.21259327 Google Scholar
- 18Liu C, Ginn HM, Dejnirattisai W, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021; 184: 4220-4236.e13. 10.1016/j.cell.2021.06.020
- 19Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021; 39: 4423-4428. 10.1016/j.vaccine.2021.05.063
- 20Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020; 21(11): 1336-1345. 10.1038/s41590-020-0782-6
- 21Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020; 183(4): 996-1012.e19. 10.1016/j.cell.2020.09.038
- 22Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications.Science. 2020; 369(6508):eabc8511. 10.1126/science.abc8511
- 23Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020; 183(1): 158-168.e14. 10.1016/j.cell.2020.08.017
- 24Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 Coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020; 181(7): 1489-1501.e15. 10.1016/j.cell.2020.05.015
- 25Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol. 2008; 181(6): 4168-4176. 10.4049/jimmunol.181.6.4168
- 26Caddy SL, Vaysburd M, Papa G, et al. Viral nucleoprotein antibodies activate TRIM21 and induce T cell immunity. EMBO J. 2021; 40(5):e106228. 10.15252/embj.2020106228
- 27Ke Z, Oton J, Qu K, et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020; 588(7838): 498-502. 10.1038/s41586-020-2665-2
- 28Hsieh C-L, Goldsmith JA, Schaub JM, et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020; 369(6510): 1501-1505. 10.1126/science.abd0826
- 29Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014; 32(48): 6377-6389. 10.1016/j.vaccine.2014.06.065
- 30Shirota H, Klinman DM. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev Vaccines. 2014; 13(2): 299-312. 10.1586/14760584.2014.863715
- 31Ezoe S, Palacpac NMQ, Tetsutani K, et al. First-in-human randomised trial and follow-up study of Plasmodium falciparum blood-stage malaria vaccine BK-SE36 with CpG-ODN(K3). Vaccine. 2020; 38(46): 7246-7257. 10.1016/j.vaccine.2020.09.056
- 32Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011; 44(5): 725-738. 10.1165/rcmb.2009-0210ST
- 33Gu H, Chen Q, Yang G, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020; 369(6511): 1603-1607. 10.1126/science.abc4730
- 34Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020; 586(7830): 567-571. 10.1038/s41586-020-2622-0
- 35Pallesen J, Wang N, Corbett KS, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci USA. 2017; 114(35): E7348-E7357. 10.1073/pnas.1707304114
- 36Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367(6483): 1260-1263. 10.1126/science.abb2507
- 37Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020; 181(2): 281-292.e6. 10.1016/j.cell.2020.02.058
- 38Huber VC, McKeon RM, Brackin MN, et al. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006; 13(9): 981-990. 10.1128/CVI.00156-06
- 39Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001; 166(12): 7381-7388. 10.4049/jimmunol.166.12.7381
- 40Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology. 2006; 352(2): 418-426. 10.1016/j.virol.2006.05.008
- 41Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007; 81(7): 3487-3494. 10.1128/JVI.02128-06
- 42Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969; 89(4): 422-434. 10.1093/oxfordjournals.aje.a120955
- 43Bolles M, Deming D, Long K, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011; 85(23): 12201-12215. 10.1128/JVI.06048-11
- 44Czub M, Weingartl H, Czub S, He R, Cao J. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine. 2005; 23(17–18): 2273-2279. 10.1016/j.vaccine.2005.01.033
- 45Tian JH, Patel N, Haupt R, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021; 12(1): 372. 10.1038/s41467-020-20653-8
- 46Han K, Blair RV, Iwanaga N, et al. Lung expression of human angiotensin-converting enzyme 2 sensitizes the mouse to SARS-CoV-2 infection. Am J Respir Cell Mol Biol. 2021; 64(1): 79-88. 10.1165/rcmb.2020-0354OC
- 47Meyers LM, Gutiérrez AH, Boyle CM, et al. Highly conserved, non-human-like, and cross-reactive SARS-CoV-2 T cell epitopes for COVID-19 vaccine design and validation. NPJ Vaccines. 2021; 6(1): 71. 10.1038/s41541-021-00331-6
- 48Matchett WE, Joag V, Stolley JM, et al. Nucleocapsid vaccine elicits spike-independent SARS-CoV-2 protective immunity. J Immunol. 2021; 207(2): 376-379. 10.4049/jimmunol.2100421
- 49Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv Drug Deliv Rev. 2009; 61(3): 248-255. 10.1016/j.addr.2008.12.012
- 50Weeratna RD, Brazolot Millan CL, McCluskie MJ, Davis HL. CpG ODN can re-direct the Th bias of established Th2 immune responses in adult and young mice. FEMS Immunol Med Microbiol. 2001; 32(1): 65-71. 10.1111/j.1574-695X.2001.tb00535.x
- 51Davis HL. Novel vaccines and adjuvant systems: the utility of animal models for predicting immunogenicity in humans. Hum Vaccin. 2008; 4(3): 246-250. 10.4161/hv.4.3.5318
- 52Lopez AM, Hecker R, Mutwiri G, van Drunen Littel-van den Hurk S, Babiuk LA, Townsend H. Formulation with CpG ODN enhances antibody responses to an equine influenza virus vaccine. Vet Immunol Immunopathol. 2006; 114(1–2): 103-110. 10.1016/j.vetimm.2006.07.013
- 53Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021; 27(7): 1205-1211. 10.1038/s41591-021-01377-8