IgE-cross-blocking antibodies to Fagales following sublingual immunotherapy with recombinant Bet v 1
Abstract
Background
Evidence has accumulated that birch pollen immunotherapy reduces rhinoconjunctivitis to pollen of birch homologous trees. Therapeutic efficacy has been associated with IgE-blocking IgG antibodies. We have recently shown that sera collected after 16 weeks of sublingual immunotherapy with recombinant Bet v 1 (rBet v 1-SLIT) display strong IgE-blocking bioactivity for Bet v 1. Here, we assessed whether rBet v 1-SLIT-induced IgG antibodies display cross-blocking activity to related allergens in Fagales pollen.
Methods
IgE, IgG1 and IgG4 reactivity to recombinant Bet v 1, Aln g 1, Car b 1, Ost c 1, Cor a 1, Fag s 1, Cas s 1 and Que a 1 were assessed in pre- and post-SLIT samples of 17 individuals by ELISA. A basophil inhibition assay using stripped basophils re-sensitized with a serum pool containing high Bet v 1-specific IgE levels was established and used to assess CD63 expression in response to allergens after incubation with pre-SLIT or post-SLIT samples. IgG1 and IgG4 were depleted from post-SLIT samples to assess its contribution to IgE-cross-blocking.
Results
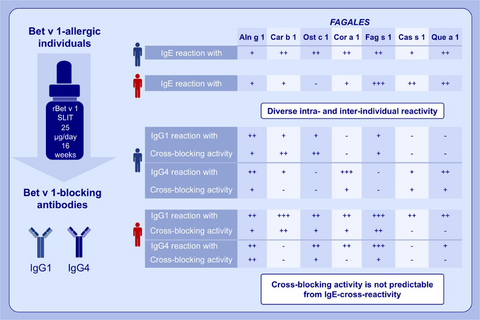
Sublingual immunotherapy with recombinant Bet v 1 boosted cross-reactive IgE antibodies and induced IgG1 and IgG4 antibodies with inter- and intra-individually differing reactivity to the homologs. Highly variable cross-blocking activities of post-SLIT samples to the different allergens were found. IgG1 and IgG4 antibodies displayed cross-blocking activity with individual variance.
Conclusions
Our mechanistic approach suggested that immunotherapy with the reference allergen Bet v 1 induces individual repertoires of cross-reactive IgG1 and IgG4 antibodies. The cross-blocking bioactivity of these antibodies was also highly variable and neither predictable from protein homology nor IgE-cross-reactivity.
Graphical Abstract
Bet v 1-allergic individuals show IgE cross-reactivity with allergens from Fagales. Sublingual immunotherapy with the reference allergen Bet v 1 induces individually diverse repertoires of cross-reactive IgG1 and IgG4 antibodies. The cross-blocking bioactivity of IgG1 and IgG4 antibodies is highly variable and not predictable from IgE-cross-reactivity.
Abbreviations
-
- AIT
-
- allergen-specific immunotherapy
-
- AP
-
- alkaline phosphatase
-
- DU
-
- developmental units
-
- HRP
-
- horseradish peroxidase
-
- O.D.
-
- optical density
-
- PBMC
-
- peripheral blood mononuclear cells
-
- R
-
- correlation coefficient
-
- r
-
- recombinant
-
- RC
-
- relative change
-
- SLIT
-
- sublingual immunotherapy
1 INTRODUCTION
Pollen from birch trees is a prominent elicitor of allergic rhinoconjunctivitis in Europe and North America with an estimated prevalence of 20% in adults.1 Sensitization to the major birch pollen allergen Bet v 1 induces complex patterns of IgE-cross-reactivity with homologous proteins in various foods termed birch pollen-related food allergy.2, 3 Bet v 1-homologs also exist in pollen from trees of the order Fagales, for example Alnus glutinosa (alder), Carpinus betulus (hornbeam), Ostrya carpinifolia (European hop-hornbeam), Corylus avellana (hazel), Fagus sylvatica (beech), Castanea sativa (chestnut) and Quercus alba (oak),4-11 and IgE-cross-reactivity within this group of birch homologous trees prolongs the period of respiratory allergic symptoms for most birch pollen-allergic patients.12, 13
Based on IgE competition experiments, Bet v 1 is considered as the primary sensitizer in allergies to Fagales pollen.5, 8, 13-15 Along these lines, recombinant (r) Bet v 1 in combination with natural birch extract has been shown to identify patients with allergic rhinitis to homologous trees with a sensitivity of 99.2%.16 Moreover, birch allergens have been suggested as exclusive tools for the diagnosis of Fagales pollen allergy also in birch-free areas.17 Beyond diagnostic application, allergen-specific immunotherapy (AIT) with birch pollen has been considered to reduce allergic symptoms to pollen from homologous trees. Indeed, individuals who received AIT with birch pollen showed a significant improvement of symptoms not only during the birch pollen season but also during the pollination of hazel and alder.12, 13, 18 A recent double-blind phase II trial demonstrated that 24 weeks of sublingual immunotherapy (SLIT) with birch pollen reduces allergy to both birch and oak.15 Moreover, birch pollen SLIT induced significant increases of IgG4 antibodies that cross-reacted with allergens from alder, beech, hazel and oak.15
The clinical success of SLIT has been associated with the induction of blocking IgG antibodies, which competitively prevent IgE binding to the administered allergens and thereby inhibit effector cell activation.19, 20 We recently found that sera collected after treatment with a daily sublingual dose of 25 µg rBet v 1 for 16 weeks potently blocked Bet v 1-induced activation of basophils.21 Here, we sought to assess whether this functional IgE-blocking activity can be expanded to the Bet v 1-homologs in alder, beech, chestnut, hazel, hornbeam, hop-hornbeam and oak, respectively. We employed single recombinant allergens to characterize and compare the cross-reactivity and inhibitory activity of rBet v 1-SLIT-induced IgG1 and IgG4 antibodies. For the first time, the IgE-cross-blocking activity of IgG antibodies following therapy with a reference allergen to a panel of homologous allergens was functionally investigated.
2 METHODS
2.1 Allergens
All allergens were recombinant proteins displaying the same IgE-binding capacity as their natural counterparts.22 Bet v 1.0101 was produced in-house as described.21 Car b 1.0109 (hornbeam), Cas s 1.0101 (chestnut), Fag s 1.0101 (beech), Ost c 1.0101 (European hop-hornbeam) and Que a 1.0301 (oak) were produced as previously described.23 Aln g 1.0101 (alder) and Cor a 1.0103 (hazel) were purchased from Biomay, Vienna, Austria.
2.2 Serum and plasma samples
Serum and plasma samples (diluted 1:2 in PBS) were collected at baseline (PRE) and after 16 weeks (POST) of daily SLIT with 25 µg of rBet v 1 (ClinicalTrials.gov number NCT01449786 and EudraCT no 2011–001221–24).24 Ethical clearance for this study has been provided by the local ethics committee (EK228/2011). Sera from six untreated birch pollen-allergic individuals, each containing >100 kUA/l of Bet v 1-specific IgE, were collected and pooled. The pool was characterized by ImmunoCAP (Thermo Fisher, Waltham, Massachusetts, USA) for IgE levels specific for rBet v 1 (224 kUA/L), rBet v 2 (0.44 kUA/L), rBet v 4 (<0.35 kUA/L) and common allergen sources in Austria (see Table S1).
2.3 Analysis of allergen-specific antibodies
Bet v 1-specific IgE and IgG4 levels were quantified by ImmunoCAP (Thermo Fisher). ELISA was applied to quantify Bet v 1-specific IgG1 levels using an in-house standard and for the detection of Fagales-specific antibodies. Microplates (Nunc MaxiSorp, Thermo Scientific) were coated overnight with recombinant allergens (1 µg/ml) and saturated for 6 h with PBS/0.05% Tween 20 supplemented with 1% human serum albumin (HSA). Samples were incubated in duplicates overnight at 4°C. After washing 5 times with PBS/0.05% Tween 20, bound antibodies were detected with the respective enzyme-labelled anti-human subclass antibodies and chromogenic detection reagents. Optical density (O.D.) was recorded. For the detection of IgE, samples were used at a final dilution of 1:4 in PBS/0.05% Tween 20 containing 0.5% HSA. Bound IgE was detected with an alkaline phosphatase (AP)-conjugated mouse anti-human IgE (BD Pharmingen, San Jose, CA, USA). Samples from non-allergic individuals served as negative controls. For the classification of IgE reactivity, the limits of detection (= mean optical density [O.D.] values of four buffer controls plus 10*SD) were subtracted from the mean O.D. values of PRE samples and scored as - for a difference of <0.1, (+) for >0.1–0.2, + for >0.2–0.5, ++ for >0.5–1.0 and +++ for >1.0. For the detection of IgG, samples were used at a final dilution of 1:10. Bound IgG1 was detected with a purified mouse anti-human IgG1 (Invitrogen, Waltham, Massachusetts, USA) followed by a horseradish peroxidase-labelled sheep anti-mouse IgG antibody (Amersham, GE Healthcare, Pittsburgh, Pennsylvania, USA). Bound IgG4 antibodies were detected with an alkaline phosphatase-conjugated anti-human IgG4 antibody (BD Pharmingen). For each individual, the relative change (RC) between mean O.D. values obtained before and after treatment was calculated using the formula: RC = (POST-PRE)/PRE.
2.4 Basophil inhibition tests
Heparinized blood was collected from untreated birch pollen-allergic individuals and allergic individuals without birch pollen allergy with informed consent and ethical clearance by the local ethics committee (EK1344/2018). Basophil inhibition tests were performed with full blood from untreated birch pollen-allergic individuals as described.21 To strip basophils, PBMC (70–150 × 106) from allergic individuals without birch pollen allergy were incubated with 10 ml of a chilled solution of 130 mM NaCl, 5 mM KCl and 10 mM lactic acid (pH 3.9) for 5 min at 4°C.25 Stripping was stopped by addition of 1 ml of 12% unbuffered Tris. After washing twice with PBS, cells were resuspended with the serum pool (10 µl diluted 1:25 in PBS/1 × 106 cells) for 60 min at 37°C. After washing twice with PBS, titrated concentrations of each allergen in the range of 6.66–0.12 ng/ml in 5 µl of HEPES calcium buffer (pH 7.4) supplemented with IL-3 (2 ng/ml) that had been incubated with either 15 µl of PBS or plasma samples for 60 min at 37°C were added. Stimulation with fMLP (Sigma-Aldrich, St. Louis, Missouri, USA) and anti-IgE antibody (SeraCare, Milford, Massachussets, USA) served as positive controls and incubation with HEPES/IL-3 as negative control. The reaction was stopped with HEPES/EDTA (20 mmol/L) after 15 min at 37°C. Cells were stained with anti-CCR3 (APC), anti-CD123 (PerCP) and anti-CD63 (PE) (all from BioLegend, San Diego, California, USA). Basophils were defined as CD123+CCR3+ cells on a FACSCanto II using FACSDiva Software Version 6.1.3 (BD Biosciences, San Jose, CA, USA). Allergen concentrations inducing 30–60% of CD63+ basophils in the presence of PRE samples were normalized to 100%, and the mean percentages of inhibition by POST samples were calculated.
2.5 Depletion of IgG1 and IgG4 antibodies
Plasma samples (2 ml) were incubated with CaptureSelect IgG1 (400 µl) or CaptureSelect IgG4 (200 µl) Human affinity matrix (Thermo Fisher Scientific, Waltham, Massachusetts, USA) for 30 min at RT. After centrifugation, fresh CaptureSelect matrix was added to the supernatant for 30 min at room temperature. Four and two cycles were performed to deplete IgG1 and IgG4, respectively. After centrifugation, the supernatant was assessed for allergen-specific IgG antibodies by ELISA.
2.6 Statistics
Statistical analyses were performed using IBM SPSS 20.0 software (SPSS, Chicago, Illinois, USA). Between-group comparisons were done with the Mann-Whitney test and within-group comparisons with the Wilcoxon signed rank test. Correlations were calculated using Pearson's correlation. All tests were two-tailed, and differences were considered significant if p ≤ .05.
3 RESULTS
3.1 IgE reactivity to Bet v 1-homologs in Fagales before and after rBet v 1-SLIT
Seventeen birch pollen-allergic individuals completed 16 weeks of rBet v 1-SLIT.21 Pre-SLIT samples were analysed for their IgE reactivity to the Bet v 1-homologs Aln g 1, Car b 1, Cas s 1, Cor a 1, Fag s 1, Ost c 1 and Que a 1 by ELISA. All individuals cross-reacted with all homologs except for subjects 5 and 15–17 who were negative with Ost c 1 and Cor a 1, respectively (Table 1).
| Family | Fagales | |||||||
|---|---|---|---|---|---|---|---|---|
| Betulaceae | Fagaceae | |||||||
| Subfamily | Betuloideae | Coryloideae | Fag s 1 | Cas s 1 | Que a 1 | |||
| Allergen | Bet v 1 | Aln g 1 | Car b 1 | Ost c 1 | Cor a 1 | |||
| AA sequence Homology/Identity | 88/81% | 86/75% | 86/74% | 86/73% | 80/66% | 74/59% | 73/59% | |
| Subject | ||||||||
| 1 | 20.3a | +++b | +++ | +++ | +++ | +++ | ++ | +++ |
| 2 | 11.7 | ++ | ++ | ++ | ++ | ++ | + | ++ |
| 3 | 8.0 | + | ++ | ++ | ++ | ++ | + | ++ |
| 4 | 10.1 | ++ | + | + | ++ | ++ | + | ++ |
| 5 | 9.2 | + | + | - | + | +++ | ++ | ++ |
| 6 | 14.5 | + | +++ | + | + | ++ | ++ | ++ |
| 7 | 9.6 | ++ | ++ | ++ | ++ | + | + | ++ |
| 8 | 31.7 | +++ | ++ | + | +++ | +++ | ++ | ++ |
| 9 | 18.1 | + | + | + | + | ++ | + | ++ |
| 10 | 16.7 | ++ | + | + | + | + | + | + |
| 11 | 16.7 | ++ | +++ | ++ | +++ | +++ | + | + |
| 12 | 11.5 | +++ | ++ | ++ | ++ | +++ | + | +++ |
| 13 | 11.2 | + | + | + | + | ++ | + | ++ |
| 14 | 6.5 | + | ++ | + | + | + | + | ++ |
| 15 | 6.3 | + | (+) | - | - | (+) | + | (+) |
| 16 | 2.3 | + | + | (+) | - | (+) | (+) | (+) |
| 17 | 1.5 | + | + | (+) | - | (+) | (+) | (+) |
- Abbreviations: AA, amino acid.
- a kUA/ml.
- b IgE reactivity was scored as negative (-), low (+), moderate +, high ++ and very high +++ as described in Material and Methods.
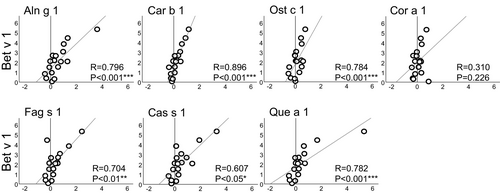
During rBet v 1-SLIT, Bet v 1-specific IgE levels rose significantly (median values pre-SLIT: 11.2 kUA/L and post-SLIT: 36.1 kUA/L, p < .001).21 Except for Cor a 1, the relative change (RC) of Bet v 1-specific IgE levels before and after SLIT correlated significantly with the RC of IgE reactivity to each homolog (R values ranging from .607 to .896, Figure 1). All samples from non-allergic individuals were negative (data not shown).
3.2 IgG-reactivity to Bet v 1-homologs in Fagales following rBet v 1-SLIT
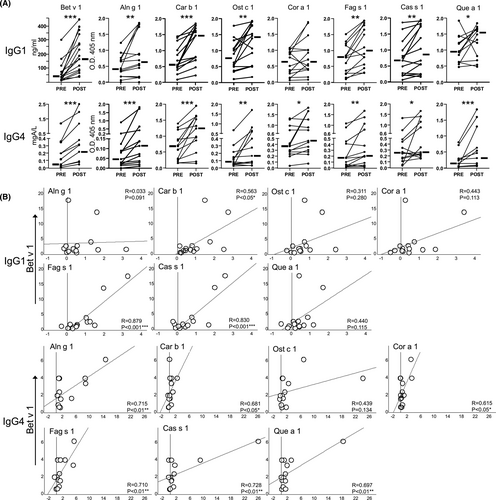
Sublingual immunotherapy enhanced Bet v 1-specific IgG1 levels in 15/17 individuals and Bet v 1-specific IgG4 levels in 14/17 individuals (see Table S2). Figure 2A depicts IgG1 and IgG4 levels to all homologs in pre- and post-SLIT samples from the 15 and 14 responders, respectively. Following therapy, IgG1 responses to all homologs but Cor a 1 were significantly increased. Overall, correlating the RC of IgG1 levels to Bet v 1 and each homolog resulted in coefficient (R) values ranging from .033 to .879 and with significance for Car b 1, Fag s 1 and Cas s 1 (Figure 2B). Otherwise, rBet v 1-SLIT significantly enhanced IgG4 responses to all homologs (Figure 2A). Except for Ost c 1, the RC of the remaining homologs correlated significantly with the RC of Bet v 1 (R values ranging from .615 to .728, Figure 2B). Looking at single subjects, diverse inter- and intra-individual patterns of cross-reactive IgG antibodies became evident (see Table S2). Several individuals developed both IgG1 and IgG4 antibodies to particular homologs, whereas some, for example subjects 7, 8, 10, 11, and 14, developed cross-reacting IgG1 but no IgG4 antibodies. Others, like subjects 4, 5, 12 and 13, showed enhanced IgG4 but not IgG1 levels to particular homologs following rBet v 1-SLIT.
3.3 Cross-blocking activity of rBet v 1-SLIT-induced IgG1 and IgG4 antibodies
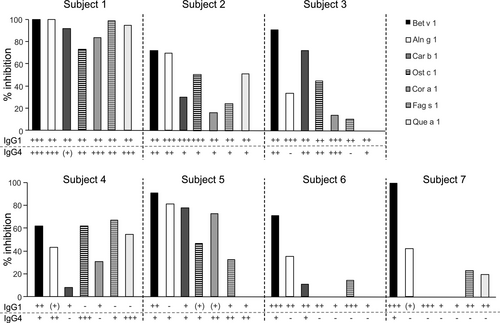
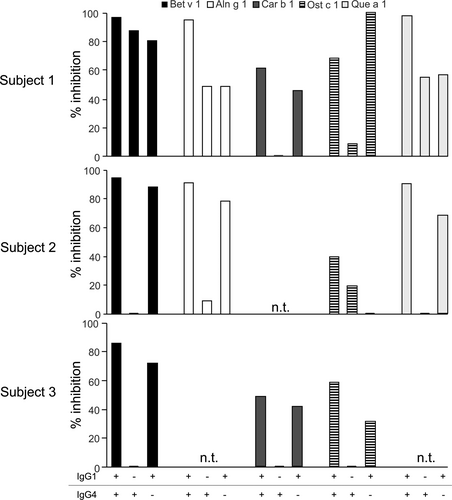
To elucidate the cross-blocking activity of rBet v 1-SLIT-induced IgG antibodies, we chose seven individuals (no. 1–7) whose post-SLIT sera had previously inhibited Bet v 1-induced activation of basophils21 and showed diverse responses of cross-reactive IgG1 and IgG4 antibodies (see Table S2). Their pre- and post-SLIT samples were incubated with Bet v 1-homologs prior to addition to basophils from three untreated birch pollen-allergic individuals. Notably, the same post-SLIT samples showed stark differences of IgE-blocking activity to the same homologs in the three experiments (see Figure S1). To establish a donor-independent basophil activation test, we stripped basophils from non-Bet v 1-sensitized allergic individuals and incubated them with a serum pool containing high levels of Bet v 1-specific IgE antibodies (see Table S1) and cross-reactive with all homologs (data not shown). Re-sensitization of basophils with a 1:25 dilution of the pool was found to be optimal for activation with Bet v 1 (see Figure S2). Then, re-sensitized basophils were stimulated with titrated amounts of all Bet v 1-homologs in two independent experiments (see Figure S3). All recombinant allergens induced reproducible basophil activation. Cas s 1, which was consistently a weak stimulus, was excluded from further testing. Next, re-sensitized basophils were stimulated with the allergens after incubation with either pre- or post-SLIT samples. The percentages of inhibition of basophil activation are depicted in Figure 3. Overall, very distinct cross-blocking activities were observed and reproduced in three independent experiments (see Figure S4). Only subject 1 displayed a strong cross-blocking activity (>80%) to all homologs, whereas the others showed individual cross-blocking activities to the allergens. All individuals showed cross-blocking to Aln g 1 and Fag s 1, though to a highly diverse extent.
3.4 Involvement of IgG1 and IgG4 antibodies in IgE-cross-blocking
To address the relevance of IgG1 and IgG4 for cross-blocking, we depleted these subclasses from post-SLIT samples of subjects 1–3 who had developed both IgG1 and IgG4 responses to several Bet v 1-homologs. Successful removal of allergen-specific IgG1 and IgG4 and preservation of the respective other subclass were confirmed by ELISA (see Figure S5). Thereafter, the blocking activities of depleted and non-depleted samples were compared on re-sensitized basophils employing Bet v 1, Aln g 1, Car b 1, Ost c 1 and Que a 1, respectively (Figure 4). The results further underlined the individual diversity of cross-blocking antibodies. For example, Bet v 1-SLIT-induced specific IgG1 and IgG4 antibodies of subject 1 displayed a comparable blocking activity to Bet v 1, Aln g 1 and Que a 1, while in the same individual IgG1 was the main inhibitor for Car b 1 and Ost c 1. IgG1-depleted post-SLIT samples from subject 2 completely lost IgE-blocking activity to all tested allergens except for Ost c 1. Cross-blocking to this allergen depended on the presence of IgG4. Exclusively, Bet v 1-SLIT-induced specific IgG1 antibodies mediated cross-blocking in subject 3 (Figure 4).
4 DISCUSSION
Due to their structural homology, the cross-reactivity of IgE antibodies among Bet v 1-related proteins in pollen from trees of the order Fagales is very pronounced. Accordingly, the 17 Bet v 1-sensitized, birch pollen-allergic subjects included in this study displayed IgE reactivity with the most abundant isoforms of Aln g 1 (alder), Car b 1 (hornbeam), Cas s 1 (chestnut), Cor a 1 (hazelnut), Fag s 1 (beech), Ost c 1 (hop-hornbeam) and Que a 1 (oak), except for four individuals who were negative to Cor a 1 and/or Ost c 1. We cannot completely exclude that these negative results are due to the limited sensitivity of our ELISA because the respective individuals also displayed low Bet v 1-specific serum IgE levels. However, a recent analysis of more than 500 birch pollen-allergic individuals revealed a stronger IgE-cross-reactivity of birch with alder than with hazel.13 As recently reported for SLIT with birch pollen extract,15 rBet v 1-SLIT boosted cross-reactive IgE antibodies as indicated by the parallel increase of Bet v 1-specific and homolog-specific IgE levels. Also in accordance with that recent trial, we observed correlations between rBet v 1-SLIT-induced IgG4 levels to Bet v 1 and its homologs. The correlations between IgG1 levels were clearly less pronounced.
The rise of allergen-specific IgG antibodies is the most consistent immunological finding in AIT; however, only their functional IgE-blocking activity has been associated with therapeutic efficacy.21, 26 Therefore, this study aimed at the assessment of cross-blocking bioactivity by employing basophil inhibition assays as they in our opinion best mirror prevention of effector cell activation in vivo. Notably, assays with basophils from three birch pollen-allergic individuals who displayed IgE reactivity to all studied allergens resulted in very discrepant blocking activities of the same post-SLIT sample. We referred the observed inhomogeneity to distinct IgE-epitope recognition patterns of the different basophil donors. To establish a more robust, donor-independent assay, we enhanced the IgE repertoire by re-sensitizing stripped basophils with a pool of Bet v 1-specific IgE antibodies. Subsequently, pre- and post-SLIT samples from seven individuals were tested with six Bet v 1-homologs each. The use of the complete Fagales allergen panel disclosed highly diverse inter- and intra-individual cross-blocking activities. Importantly, all individuals showed a marked IgE-blocking activity to Bet v 1 as well as to Aln g 1, which is the homolog most closely related to Bet v 1 among the studied homologs. However, the blocking activity for the homologs from Coryloideae and Fagaceae was very inconsistent among the individuals and not associated with the homology to Bet v 1. Nevertheless, the limited number of tested individuals may contribute to the observed heterogeneity.
Recently, a study employing environmental exposure chambers showed a significant reduction of rhinoconjunctivitis to oak pollen in 46 patients following 24 weeks of SLIT with 12 developmental units (DU) of birch pollen.15 These individuals developed significant levels of Que a-specific IgG4 antibodies. Notably, 47 patients treated in parallel with 7 DU of birch extract developed equal levels of Que a-specific IgG4 antibodies but showed no clinical improvement to oak pollen exposure. This observation corroborates that the blocking bioactivity but not quantity of AIT-induced cross-reactive IgG4 antibodies is decisive for clinical improvement. Unfortunately, the Que a-specific IgE-cross-blocking activity of these groups has not been analysed. Certainly, it remains unknown to which extent in vitro data on IgE-blocking bioactivity can be translated into the reduction of allergic symptoms in vivo. Furthermore, the clinical efficacy of SLIT may involve additional immune mechanisms, for example modulated allergen-specific T cell responses.27, 28 Although we saw an increase of Que a 1-specific IgG1 and/or IgG4 in the tested subjects, only 3 out of 7 individuals (43%) displayed a cross-blocking activity of more than 30% to Que a 1 following 16 weeks of SLIT. Unfortunately, we lack any data on the clinical response to oak and other tree pollen before and after rBet v 1-SLIT. Therefore, no correlation of in vitro and in vivo results is possible. Still, our comprehensive, mechanistic approach provides evidence for highly diverse individual responses regarding cross-blocking IgG induced by treatment with the major birch pollen allergen and it is tempting to speculate that variable immune responses may result in individual clinical responses to therapy.
Our parallel analyses of IgE, IgG1 and IgG4 responses of 17 rBet v 1-SLIT-treated individuals to seven Bet v 1-homologs in Fagales may serve to speculate about the repertoires of disease-eliciting IgE and therapy-induced IgG antibodies. Several subjects displaying IgE antibodies to particular homologs did not develop corresponding IgG antibodies suggesting that rBet v 1-SLIT-induced IgG are no memory response but newly evolved antibodies. Such IgG antibodies may recognize epitopes that are more variable among the different homologs as opposed to more conserved IgE epitopes. Accordingly, we have found that SLIT with rMal d 1, the Bet v 1 homolog in apple, induced a de novo IgG response specific for epitopes exclusive of the apple allergen.21 However, it is conceivable that a broader repertoire of IgG antibodies develops following SLIT for more than 16 weeks. On the other hand, our findings may hint to the existence of IgE epitopes exclusive for the individual Bet v 1-homologs. Such epitopes have been implied by previous studies reporting Fagales pollen allergy in birch-free areas17 and demonstrating that IgE antibodies from allergic individuals in birch-free areas reacted stronger to Car b 1 and Que a 1 than to Bet v 1.23 We also observed that some individuals developed cross-reactive IgG1 but no IgG4 antibodies and vice versa. In view of the model of sequential class switching, enhanced IgG1 levels together with unaltered IgG4 levels after 16 weeks of treatment may be explained by an absence of class switch within this period. Enhanced IgG4 levels in spite of unaltered IgG1 levels may result from either the development of unverifiable IgG1 responses which rapidly switched to IgG4 or point to different antibody repertoires. The depletion of either IgG1 or IgG4 antibodies from post-SLIT samples revealed an intra-individually diverse role for both subclasses as blocking antibodies. Overall, Bet v 1-specific IgG1 antibodies were the more prominent blocking antibodies for most allergens as previously observed for Mal d 1-specific IgG1 antibodies following 16 weeks of SLIT with the apple allergen.21 Nevertheless, the affinity and concomitant IgE-blocking activity of specific IgG4 antibodies might not be fully developed after this short treatment period. We can exclude an involvement of IgA antibodies because post-SLIT samples depleted from total IgG completely lost their IgE-blocking bioactivity (data not shown).
Although based on a limited number of individuals, this mechanistic study demonstrates that therapy with a reference allergen results in individual cross-reactive IgG responses which do not necessarily possess IgE-blocking bioactivity. Functional cross-blocking of a particular homolog can neither be predicted from homology with the reference allergen nor from original IgE-cross-reactivity. Further work is required to understand why sensitization to an allergen induces cross-reactive IgE antibodies of clinical relevance whereas desensitization with the same allergen fails to induce functional cross-blocking IgG antibodies.
ACKNOWLEDGEMENTS
The authors wish to thank Birgit Nagl and Sandra Faustmann for excellent help in blood sample processing who have nothing to disclose. We are grateful for helpful discussions with Karin Hoffmann-Sommergruber and Zsolt Szépfalusi. This study was supported by the Austrian Science Fund (FWF), projects KLI96, W1248, P32953, I4437 and P30936, the Austrian Jubiläumsfond, project 17589, the Medical University of Vienna and the University of Salzburg priority program “Allergy-Cancer-BioNano Research Centre”. Mr. Grilo, Dr. Kitzmüller, Dr. Aglas, Dr. Sánchez Acosta, Mrs. Vollmann, Dr. Ebner, Dr. Horak and Dr. Kinaciyan have nothing to disclose. Dr. Radauer reports grants from Austrian Science Fund (FWF) during the conduct of the study. Dr. Ferreira reports personal fees from HAL Allergy, personal fees from Swiss Institute of Allergy and Asthma Research (SIAF), personal fees from AllergenOnline, outside the submitted work. Dr. Jahn-Schmid has nothing to disclose. Dr. Bohle reports grants from Austrian Science Funds, grants from Austrian Jubiläumsfonds, grants from Medical University of Vienna, during the conduct of the study; personal fees from AllergenOnline, outside the submitted work.
AUTHOR CONTRIBUTIONS
JRG, CK and BB designed the experiments; JRG, CK, GAS and UV performed the experiments; LA and FF provided recombinant allergens; CE, FH, CR and TK provided samples; JRG, BJS and BB analysed the data, and JRG and BB wrote the manuscript.