Efficacy and safety of treatment with omalizumab for chronic spontaneous urticaria: A systematic review for the EAACI Biologicals Guidelines
Ioana Agache, Claudio Rocha are Joint first authorship.
Carlos Canelo-Aybar, Oscar Palomares, and Marek Jutel are Joint last authorship.
[Correction added on 04 October 2021, after first online publication. The affiliations of Marek Jutel have been corrected.].
Abstract
This systematic review evaluates the efficacy and safety of omalizumab for chronic spontaneous urticaria (CSU). PubMed, Embase, and Cochrane Library were searched for RCTs. Critical and important CSU-related outcomes were considered. The risk of bias and the certainty of the evidence were assessed using GRADE. Ten RCTs including 1620 subjects aged 12 to 75 years old treated with omalizumab for 16 to 40 weeks were evaluated. Omalizumab 150 mg does not result in clinically meaningful improvement (high certainty) of the urticaria activity score (UAS)7 (mean difference (MD) −5; 95%CI −7.75 to −2.25), and the itch severity score (ISS)7 (MD −2.15; 95% CI −3.2 to −1.1) does not increase (moderate certainty) quality of life (QoL) (Dermatology Life Quality Index (DLQI); MD −2.01; 95%CI −3.22 to −0.81) and decreases (moderate certainty) rescue medication use (MD −1.68; 95%CI −2.95 to −0.4). Omalizumab 300 mg results in clinically meaningful improvements (moderate certainty) of the UAS7 (MD −11.05; 95%CI −12.87 to −9.24), the ISS7 (MD −4.45; 95%CI −5.39 to −3.51), and QoL (high certainty) (DLQI; MD −4.03; 95% CI −5.56 to −2.5) and decreases (moderate certainty) rescue medication use (MD −2.04; 95%CI −3.19 to −0.88) and drug-related serious AEs (RR 0.77; 95%CI 0.20 to 2.91).
Abbreviations
-
- AE
-
- adverse events
-
- CI
-
- confidence interval
-
- CIU
-
- chronic idiopathic urticaria
-
- CSU
-
- chronic spontaneous urticaria
-
- CU-Q2oL
-
- Chronic Urticaria Quality of Life Questionnaire
-
- DLQI
-
- Dermatology Life Quality Index
-
- EAACI
-
- European Academy of Allergy and Clinical Immunology
-
- EMA
-
- European Medicine Agency
-
- FcεRI
-
- high-affinity receptors for IgE
-
- FDA
-
- Food and Drug administration
-
- GDG
-
- Guideline Development Group
-
- GRADE
-
- Grading of Recommendations Assessment, Development and Evaluation
-
- Ig
-
- immunoglobulin
-
- IL
-
- interleukin
-
- IRR
-
- incidence rate ratios
-
- ISS
-
- itch severity score
-
- MD
-
- mean difference
-
- MID
-
- minimal important difference
-
- PRISMA
-
- Preferred Reporting Items for Systematic Reviews and Meta-Analysis
-
- QoL
-
- quality of life
-
- RCT
-
- randomized controlled trial
-
- ROB
-
- risk of bias
-
- RR
-
- risk ratio
-
- SC
-
- subcutaneous
-
- SR
-
- systematic review
-
- UAS
-
- urticaria activity score
1 INTRODUCTION
Chronic spontaneous urticaria (CSU), formerly also known as chronic idiopathic urticaria (CIU), is a common disease with a prevalence of around 1%. It is a debilitating condition characterized by wheals and itching without or with concomitant angioedema inflicting a substantial burden for patients, their family, and friends, the healthcare system, and society.1-3 Chronic urticaria impacts quality of life (QoL) more than coronary artery disease or respiratory allergy.4, 5
The exact mechanisms leading to the activation of mast cells, the key pathogenic drivers of the development of the signs and symptoms (itch, wheals, and angioedema) in patients with CSU, are not fully characterized. There is, however, strong indication that autoimmunity, either “autoallergic” (type I, with IgE antibodies to self-antigens/allergens) or “autoimmune” (type IIb, with IgG and IgM autoantibodies to IgE or its high-affinity receptor (FcεRI)) is the most frequent cause of CSU.6-9 Many co-factors can be involved in modulating the activation status of mast cells, such as physical agents, pseudoallergens, neuropeptides, or bacterial products and the local inflammatory milieu, with activated eosinophils, basophils, and neutrophils infiltrating the skin.10, 11
The international EAACI/GA2LEN/EDF/WAO urticaria guideline recommends to use a standard-dosed, second-generation H1-antihistamine as the first-line therapy.1 However, H1-antihistamine treatment leads to absence of symptoms in fewer than 50% of patients. Up-dosing of second-generation H1-antihistamines (up to fourfold) as recommended by the EAACI/GA2LEN/EDF/WAO urticaria guideline as second-line therapy can improve response; however, many cases do not respond to antihistamines and need a more effective approach.12, 13 The monoclonal anti-IgE antibody, omalizumab, was the first drug approved for use in patients with CSU who remain symptomatic despite H1-antihistamine treatment. Omalizumab binds to free IgE, which lowers free IgE levels and results in subsequent downregulation of the FcεRI on basophils and mast cells.14 Potential mechanisms of omalizumab in CSU may include reducing the capacity of mast cells to release mediators, reversing basopenia and eosinophilia, improving basophil IgE receptor function, reducing activity of IgG autoantibodies against FcεRI and IgE, reducing activity of IgE autoantibodies against autoantigen, reducing the availability of autoantigens by forming complexes with IgE autoantibodies, reducing the activity of intrinsically “abnormal” IgE, and decreasing coagulation abnormalities associated with disease activity.15-17 As of yet, none of these potential mechanisms alone or in combination has been demonstrated to be the definitive mechanism of action.
The European Medicine Agency (EMA) recommends omalizumab as add-on therapy for the treatment of CSU in adults and adolescents (12 years and above, 300 mg s.c. every 4 weeks) with inadequate response to H1 antihistamine treatment.18 The Food and Drug Administration (FDA) recommends omalizumab for the treatment of CIU (150 or 300 mg s.c. every 4 weeks) in adults and adolescents (12 years of age and older) who remain symptomatic despite H1 antihistamine treatment.19
The European Academy of Allergy and Clinical Immunology (EAACI) is developing clinical practice guidelines for the use of omalizumab in patients with CSU. To inform key clinical recommendations, a systematic review (SR) evaluated the effectiveness and safety of omalizumab in patients with CSU aged 12 years old or older.
2 METHODS
2.1 Guidelines development group
The EAACI CSU Voting Panel and Steering Committee included clinicians and researchers with different backgrounds (the complete list of experts is available on the EAACI website), who voluntarily participate in the development of the EAACI biologicals guideline. They are referred to as the Guidelines Development Group (GDG).
2.2 Structured question and outcome prioritization
The GDG framed the clinical question as “Is the treatment with omalizumab efficacious and safe for patients with CSU?” (Table 1). For the purpose of this SR, the population was defined as patients 12 years or older with a diagnosis of CSU inadequately controlled by H1-antihistamine treatment. The CSU-related outcomes were prioritized by the GDG group using a 1-9 scale (7 to 9 critical; 4 to 6 important; 1 to 3 of limited importance) as suggested by the GRADE approach. The critical outcomes were: urticaria activity score (UAS) 7, the itch severity score 7 (ISS7), and safety (drug-related adverse events and serious adverse events). Important outcomes were as follows: quality of life (QoL), measured with Dermatology Life Quality Index (DLQI), the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL), resource utilization, and rescue medication use (Table 1).
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
|
Patients 12 years or older with a diagnosis of chronic idiopathic or spontaneous urticaria. Patients not adequately controlled on H1-antihistamines. |
Dose of 150 mg or 300 mg Subcutaneous injections every four weeks. | Placebo or usual care/standard of care. |
Critical
Important
|
- Abbreviations: CU-QoL, chronic urticaria quality of life; DLQI = dermatology life quality index
- a Only drug-related adverse and severe adverse events were considered.
2.3 Data source and search methodology
Electronic algorithms in combination with controlled vocabulary and search terms were used to identify relevant randomized controlled trials (RCTs) in (a) MEDLINE (via PubMed, January 2020); (b) Cochrane Controlled Trials Register (via The Cochrane Library, January 2020); and (c) Embase (via Ovid, January 2020). Search algorithms were adapted to the requirements of each database, and validated filters were used to retrieve appropriate designs (table S1). Additional studies provided by the GDG and previous SR were also evaluated.
2.4 Eligibility criteria and selection of studies
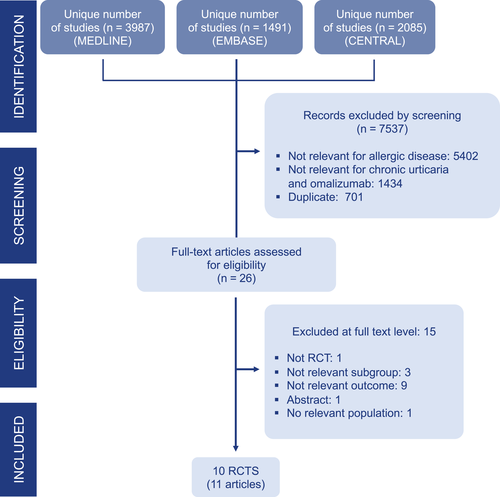
The SR included only RCTs comparing omalizumab versus placebo added to usual care/standard of care in patients with CSU and reporting one of the outcomes of interest as formulated by the GDG (Figure 1). Only studies published in English were included. Abstracts or conference communications not published as full articles in peer-reviewed journals and RCTs using dose or routes not approved by EMA and/or the FDA were excluded. Two reviewers independently screened the references based on the title and abstract followed by eligibility at full-text level. Discrepancies were solved by consensus or with the help of a third reviewer. All citations retrieved were imported into the bibliographic reference software (EndNote X5; Thomson Reuters) to discard duplicates and record screening decisions.
2.5 Data extraction and risk of bias assessment
Details of the study design, patient population, setting, follow-up, and results were extracted by one reviewer and confirmed by a second reviewer. If needed, additional data from the authors of the included studies were requested. The Cochrane Risk of Bias tool for randomized trials was used to assess the risk of bias (ROB).20 The ROB was judged as low, high, or unclear for each domain: random sequence generation, allocation concealment, blinding of participants and personnel, blinding for outcome assessment, incomplete outcome data, and selective reporting.21-23
2.6 Data synthesis and analysis
Main results are described narratively and included in summary of findings tables. For dichotomous data, results are pooled as incidence rate ratios (IRR) and risks ratios (RR). For continuous data, results are reported as mean differences (MD), with 95% confidence intervals (CI). For each outcome, the change from baseline to the end of the treatment was assessed versus placebo. A random-effects model was used to pool data (Review Manager v 5.3 Cochrane Collaboration, Oxford, UK). Where multiple arms were compared to a common placebo arm, standard errors were adjusted to avoid unit of analysis error.24 Statistical heterogeneity between studies was assessed with the Cochrane chi-square test and the magnitude of heterogeneity with the I2 statistic.24, 25 To account for heterogeneity, subgroup analyses were performed for different doses of omalizumab. The median estimate reported in the control arms of the included RCTs was used as baseline risk to estimate absolute effects for each comparison.
2.7 Certainty of the evidence
The certainty (quality) of the evidence of efficacy and safety was rated for each outcome as high, moderate, low, or very low, following the GRADE approach and the standard GRADE domains (risk of bias, imprecision, inconsistency, indirectness, and publication bias).26, 27
For the evaluation of imprecision for each outcome, the following thresholds for the minimal important difference (MID) were considered: 9.5 to 10.0 points for Weekly Urticaria Activity Score,28-33 4.5 to 5 points for Weekly Itchy-Severity Score 30-32 and 2.24 to 3.10 for Dermatology Life Quality Index.34
3 RESULTS
Results are presented following the GRADE informative statements.35
3.1 Search process
The eligibility process is summarized in the PRISMA flow chart (Figure 1). A total of 7,563 unique citations were retrieved from database searches, and 26 were appraised at full-text level. Eleven publications reporting 10 RCTs were included 35-45 (Table 2, Figure 1). Fifteen publications were excluded due to regulatory unapproved dose, differences in population or outcomes of interest, or duplicate data (Table S2).
| Author, Year, trial number, and name | Study design (Number of subjects included) |
Age (years) Placebo vs. Dupilumab |
Population | Intervention | Control | Outcome assessmentb |
|---|---|---|---|---|---|---|
|
Casale 2019 Maurer 2018 NCT02392624 XTEND-CIU |
Multicenter RCT (N = 134; OMA300 = 81; placebo = 53) (First open-label period with omalizumab 300mg every 4 weeks for 24 weeks for all patients and followed by randomized double-blinded period with placebo or omalizumab 300mg for an additional 24 weeks) |
Mean (SD) Placebo = 48.5 (13.2) OMA300 = 43.1 (14.7) |
Patient aged 12-75 years who remain symptomatic despite optimized H1AH treatment. |
Omalizumab 300 mg (Q4W) |
Placebo |
EOT: 24 weeks EFU: 48-60 weeks |
|
Hide 2017, NCT02329223 POLARIS |
Multicenter RCT (N = 218; OMA150 = 71; OMA300 = 73; placebo = 74) |
Mean (SD) Placebo = 42.5 (14.3) OMA300 = 44.6 (14.9) OMA150 = 43.6 (12.2) |
Patient aged 12-75 years who was refractory to conventional H1AH at time of randomization. | Omalizumab 150 mg and 300 mg (Q4W) | Placebo |
EOT: 12 weeks EFU: 24 weeks |
| Jörg 2018 |
Monocentric RCT (post hoc analysis) (N = 30; OMA300 = 20; placebo = 10) |
Mean (SD) Placebo = 42.4 (13.3) OMA300 = 41.8 (15.2) |
Patients aged 18-70 years with CSU diagnosis, refractory to H1AH treatment. | Omalizumab 300 (Q4W) | Placebo |
EOT: 16 weeks EFU: 20 weeks |
|
Kaplan 2013 NCT01264939 GLACIAL |
Multicenter RCT (post hoc analysis) (N = 336; OMA300 = 252; placebo = 84) |
Mean (SD) Placebo = 44.3 (14.7) OMA300 = 42.7 (13.9) |
Patients aged 12-75 years with CU diagnosis refractory to H1AH c H2AH and/or LTRAs. | Omalizumab 300 (Q4W) | Placebo |
EOT: 24 weeksc EFU: 40 weeks |
|
Maurer 2011 NCT00481676 XCUISITE |
Multicenter RCT (N = 49; OMA = 27; placebo = 22) |
Mean (SD) Placebo = 42.3 (15) OMA = 39.1 (9) |
Patients aged 18-70 years with a clinical diagnosis of moderate-to-severe CU. | Omalizumab 75-375mg (Q2W/Q4W) (Doses were derived from the approved asthma dosing table for omalizumab) | Placebo |
EOT: 24 weeks EFU: NA |
|
Maurer 2013 NCT01292473 ASTERIA I |
Multicenter RCT (N = 322; OMA75 = 82; OMA150 = 82; OMA300 = 79 placebo = 79) |
Mean (SD) Placebo = 43.1 (12.5) OMA75 = 39.7 (15) OMA150 = 43 (13.2 OMA300 = 44.3 (13.7) |
Patients aged 12-75 years with moderate-to-sever CU who remained symptomatic despite H1AH treatment. | Omalizumab 75/150/300 mg (Q4W)* | Placebo |
EOT: 12 weeks EFU: 28 weeks |
|
Metz 2017 NCT01599637 MoA |
Multicenter RCT (N = 30; OMA300 = 20 placebo = 10) |
Mean (SD) Placebo = 41.1 (8) OMA300 = 37.5 (11) |
Patients aged 18-75 years with CU refractory to antihistamine treatment | Omalizumab 300 mg (Q4W) | Placebo |
EOT: 12 weeks EFU: 23 weeks |
|
Saini 2011 NCT00130234 MYSTIQUE |
Multicenter RCT (N = 90; OMA75 = 23; OMA300 = 25; OMA600 = 21; placebo = 21) |
Mean (SD) Placebo = 41.2 (16.2) OMA75 = 38.8 (15.5) OMA300 = 42.9 (15.7) OMA600 = 40 (11.1) |
Patients aged 12-75 years with a CU diagnosis that remained symptomatic despite H1AH treatment. | Omalizumab 75/300/600 mg single dosea | Placebo |
EOT: 4 weeks EFU: 16 weeks |
|
Saini 2015 NCT01287117 ASTERIA II |
Multicenter RCT (N = 318; OMA75 = 77; OMA150 = 80; OMA300 = 81; placebo = 80) |
Mean (SD) Placebo = 40.4 (15.6) OMA75 = 40.7 (15.2) OMA150 = 41.1 (14) OMA300 = 42.4 (13.2) |
Patients aged 12-75 years with a CU diagnosis that remained symptomatic despite H1AH treatment. | Omalizumab 75/150/300 mg (Q4W)a | Placebo |
EOT: 24 weeksc EFU: 40 weeks |
|
Staubach 2016 NCT01723072 X-ACT |
Multicenter RCT (N = 91; OMA300 = 44; placebo = 47) |
Median (range) Placebo = 41.1 (20-61) OMA300 = 44.9 (20-73) |
Patients aged 18-75 years with CSU and ≥ 4 episodes of angioedema who were symptomatic despite H1AH treatment. | Omalizumab 300 mg (Q4W) | Placebo |
EOT: 24 weeksc EFU: 28 weeks |
- Abbreviations: CSU, chronic spontaneous urticaria; CU, chronic urticaria; H1AH, H1-antihistamine; H2AH, H2-antihistamine; LTRAs,leukotriene receptor antagonists; N, total number; NCT: clinical trial number; OMA,omalizumab; Q2W, every 2 weeks; Q4W, every four weeks; RCT, randomized control trial; SD, standard deviation.
- a Only data for EMA/FDA approved doses were extracted from these RCTs (omalizumab 150 mg and 300 mg).
- b EOT: end of the treatment, EFU: end of follow-up.
- c These studies also measured itch severity score (Staubach 2016 and Kaplan 2013) and Dermatology life quality index (Saini 2015 and Kaplan 2013) at the middle of the treatment period (12 weeks).
3.2 Characteristics of included studies
The characteristics of studies evaluated are detailed in Table 2. All were randomized control trials, conducted between 2004 and 2017, including patients with CSU receiving omalizumab in addition to standard of care versus placebo. Overall, 1,620 patients aged 12 to 75 years old with CSU/CIU who remained symptomatic despite optimized treatment (H1-antihistamine alone or with addition of an H2-antihistamine and/or leukotriene receptor antagonist) were included. The treatment duration ranged from 4 to 24 weeks, and the extended follow-up without medication ranged from 16 to 40 weeks. Only six studies included patients younger than 18, and among those, the range was between 1.8% to 5.3%.
3.3 Evidence of efficacy
The summary of the results and certainty of evidence per outcome is reported in Table 3 for omalizumab 150 mg every 4 weeks and Table 4 for omalizumab 300 mg every 4 weeks. For efficacy, the metanalysis was performed separately for the 2 doses of omalizumab, grouping the studies in two time periods based on the week the outcomes have been measured (4 to 16 weeks and 24 to 28 weeks).
| Outcomes |
No. of participants (studies) Follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard care | Risk difference with Omalizumab | ||||
|
Patient or population: CSU/CIU 12-75 years old Intervention: Omalizumab 300 mg Comparison: standard care |
|||||
|
Weekly Urticaria Activity Score assessed with: UAS7 Scale from: 0 to 42 |
610 4 to 28 weeks |
⨁⨁⨁◯ MODERATE a,b |
- | Mean UAS7 0 |
MD −11.05 (95% CI −12.87 to −9.24) |
|
Weekly Itch Severity Score Assessed with: ISS7 Scale from: 0 to 21 |
628 4 to 28 weeks |
⨁⨁⨁◯ MODERATE a,c |
- | Mean ISS7 0 |
MD −4.45 (95% CI −5.39 to −3.51) |
|
AE possibly related to study drug Assessed with: Study personnel clinical follow-up |
801 24 to 40 weeks |
⨁⨁◯◯ LOW a,f,g |
RR 1.40 * (0.63 to 3.13) |
85 per 1,000 |
+32 per 1,000 (95% CI −28 to + 156) |
|
Serious AE possibly related to study drug Assessed with: Study personnel clinical follow-up |
335 (1 RCT)45 24 to 40 weeks |
⨁⨁⨁◯ MODERATE f |
- | 36 per 1,000 |
-8 per 1,000 (95% CI −29 to + 69) |
|
Quality of Life Assessed with: DLQI Scale from: 0 to 30 |
732 4 to 28 weeks |
⨁⨁⨁⨁ HIGH a |
- | Mean DLQI 0 |
MD −4.03 (95% CI −5.56 to −2.5) |
|
Rescue medication use Assessed with: number of diphenhydramine tablets/week |
654 4 to 28 weeks |
⨁⨁⨁◯ MODERATE a,e |
- | Mean rescue medication use 0 |
MD −2.04 (95% CI −3.19 to −0.88) |
|
GRADE Working Group grades of evidence High certainty: High confidence that the true effect lies close to that of the estimate of the effect Moderate certainty: Moderately confidence in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Limited confidence in the effect estimate: The true effect may be substantially different from the estimate of the effect Very low certainty: Very limited confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect |
|||||
|
Explanations a. All studies were funded by industry, and all showed positive results in all outcomes. No industry-independent observational or randomized studies to support these results were found. Sponsorship industry bias was assessed as other bias as part of the RoB tool. The panel members considered that there were no major concerns about potential publication/sponsorship bias b. The minimal important difference (MID) for UAS7 is 9.5 to 10.5 points e. The minimal important difference (MID) for ISS7 is 4.5-5 points f. The lower limit of the confidence interval is not clinically relevant (less than 1 tablet) g. The effect may be both harmful or beneficial. h. Substantial heterogeneity (56%). The evidence was downgraded as effects favoured both intervention and placebo. |
|||||
- Abbreviations: CI, Confidence interval; MD, Mean difference; RR, Risk ratio.
- * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
| Outcomes |
No. of participants (studies) Follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard care | Risk difference with Omalizumab | ||||
|
Patient or population: CSU/CIU 12-75 years old Intervention: Omalizumab 300 mg Comparison: standard care |
|||||
|
Weekly Urticaria Activity Score assessed with: UAS7 Scale from: 0 to 42 |
610 4 to 28 weeks |
⨁⨁⨁◯ MODERATE a,b |
- | Mean UAS7 0 |
MD −11.05 (95% CI −12.87 to −9.24) |
|
Weekly Itch Severity Score Assessed with: ISS7 Scale from: 0 to 21 |
628 4 to 28 weeks |
⨁⨁⨁◯ MODERATE a,c |
- | Mean ISS7 0 |
MD −4.45 (95% CI −5.39 to −3.51) |
|
AE possibly related to study drug Assessed with: Study personnel clinical follow-up |
801 24 to 40 weeks |
⨁⨁◯◯ LOW a,f,g |
RR 1.37 (0.67 to 2.82)* |
85 per 1,000 |
+32 per 1,000 (95% CI −28 to + 156) |
|
Serious AE possibly related to study drug Assessed with: Study personnel clinical follow-up |
335 (1 RCT)45 24 to 40 weeks |
⨁⨁⨁◯ MODERATE f |
RR 0.77 (0.20 to 2.91)* |
36 per 1,000 |
-8 per 1,000 (95% CI −29 to + 69) |
|
Quality of Life Assessed with: DLQI Scale from: 0 to 30 |
732 4 to 28 weeks |
⨁⨁⨁⨁ HIGH a |
- | Mean DLQI 0 |
MD −4.03 (95% CI −5.56 to −2.5) |
|
Rescue medication use Assessed with: number of diphenhydramine tablets/week |
654 4 to 28 weeks |
⨁⨁⨁◯ MODERATE a,e |
- | Mean rescue medication use 0 |
MD −2.04 (95% CI −3.19 to −0.88) |
|
GRADE Working Group grades of evidence High certainty: High confidence that the true effect lies close to that of the estimate of the effect Moderate certainty: Moderately confidence in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Limited confidence in the effect estimate: The true effect may be substantially different from the estimate of the effect Very low certainty: Very limited confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect |
|||||
|
Explanations a. All studies were funded by industry, and all showed positive results in all outcomes. No industry-independent observational or randomized studies to support these results were found. Sponsorship industry bias was assessed as other bias as part of the RoB tool. The panel members considered that there were no major concerns about potential publication/sponsorship bias b. The minimal important difference (MID) for UAS7 is 9.5 to 10.5 points e. The minimal important difference (MID) for ISS7 is 4.5-5 points f. The lower limit of the confidence interval is not clinically relevant (less than 1 tablet) g. The effect may be both harmful or beneficial. h. Substantial heterogeneity (56%). The evidence was downgraded as effects favoured both intervention and placebo. |
|||||
- Abbreviations: CI, Confidence interval; MD, Mean difference; RR, Risk ratio.
- * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
3.3.1 Weekly Urticaria Activity Score (UAS7)
Three RCTs assessed disease activity by use of the UAS7 for omalizumab 150 mg 36, 39, 41 and six for omalizumab 300 mg36-40, 42 given every 4 weeks for up to 24 weeks. Overall, compared to standard of care, treatment with omalizumab 150 mg every 4 weeks did not result in a clinically meaningful reduction of disease activity as assessed by use of the UAS7 (MD −5; 95% CI −7.75 to −2.25; high certainty of evidence). In contrast, omalizumab 300 mg every 4 weeks led to a clinically meaningful decrease in UAS7 with moderate certainty of evidence (MD −11.05; 95% CI −12.87 to −9.24).
The number of complete responders (UAS7 = 0) was also evaluated for both doses. Three and five RCTs assessed complete responders for omalizumab 150 mg 35, 39, 42 and 300 mg,35, 39, 40, 42, 43 respectively, given up to 24 weeks. Both doses increased the likelihood of achieving complete response compared to standard of care: RR 2.26 (95%CI 1.13 to 4.51) for omalizumab 150 mg (30 out of 157 achieved complete response in the intervention group and 14 out of 176 in the standard of care group) and RR 4.12 (95% CI 3.05 to 7.37) for omalizumab 300 mg (96 out of 222 achieved complete response in the intervention group and 20 out of 231 in the standard of care group).
One RCT 44 assessed worsening in UAS7 (≥12 points for ≥ 2 consecutive weeks) for omalizumab 300 mg after stopping the treatment. Patients in the placebo group were at higher risk for UAS7 worsening as compared with the omalizumab 300 mg group (RR 2.88; 95%CI 1.79 to 4.63).
3.3.2 Weekly Itch Severity Score (ISS7)
Four RCTs assessed the effects on itch, by use of the ISS7, for omalizumab 150 mg 35, 37, 38, 42 and eight for omalizumab 300 mg 35-40, 42, 45 used for up to week 24. Compared to standard of care, omalizumab 150 mg did not result in clinically meaningful reductions of ISS7 values (MD −2.15; 95%CI −3.20 to −1.10, high certainty of evidence), whereas omalizumab 300 mg did so, with moderate certainty of evidence (MD −4.65; 95%CI −5.41 to −3.89).
The number of complete responders (ISS7 = 0) was evaluated by one RCT41 at week 20 for omalizumab 300 mg, showing an increased likelihood to achieve complete response compared to standard of care (RR 1.67; 95%CI 0.21 to 12.97), although results are inconclusive due to the low RR and the wide CI.
Two studies evaluated ISS7 responses at 24 weeks of treatment for both doses of omalizumab in patients 17 years old or younger.38, 45 Overall, omalizumab decreased ISS7 values in the pediatric population compared with standard of care (MD −1.44; 95%CI −7.06 to 4.78), although below the MID.
3.3.3 Dermatology life quality index (DLQI)
Three RCTs reported on the effects on Qol impairment as assessed by use of the DLQI at week 12 for omalizumab 150 mg 36, 38, 42 and six for omalizumab 300 mg at 24 weeks.36, 38, 40, 42, 45, 46 Compared to standard of care, omalizumab 150 mg did not meaningfully improve QoL and reduce DLQI values (MD −1.95; 95%CI −3.06 to −0.83; moderate certainty of evidence). Omalizumab 300 mg reduced QoL impairment and DLQI values (MD −4.01; 95%CI −4.94 to −3.08), with an improvement in QoL above the MID with high certainty of evidence.
One RCT assessed DLQI worsening (≥3 points increase) after stopping the treatment.44 Patients in the placebo group had a higher likelihood of DLQI worsening as compared to the omalizumab 300 mg group (RR 3.34;95% CI 2.07 to 5.40).
Three RCTs assessed the effects of omalizumab 300 mg at 12 weeks on disease-specific QoL impairment by use of the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL).38, 40, 45 These studies demonstrated a significant improvement of disease-specific QoL impairment compared to standard of care (MD −15.34; 95%CI −24.84 to −5.84).
One RCT assessed work productivity and activity impairment (WPAI), reporting two sub-scales separately, work impairment score (WIS) and activity impairment score (AIS).44 Compared to standard of care, omalizumab 300 mg did improve WIS (MD −24.24; 95% CI −35.74 to −12.74) and AIS (−26.59; 95% CI −37.36 to −15.72) at 24 weeks.
3.3.4 Rescue medication use
Five RCTs assessed rescue medication use (number of tablets of diphenhydramine per week) at week 12, two for omalizumab 150 mg 36, 38 and three for omalizumab 300 mg.36, 38, 45 Compared to standard of care, both doses decreased the use of rescue medication with moderate certainty of evidence: MD −1.68 (95%CI −2.95 to −0.40) for omalizumab 150 mg and MD −2.04 (95%CI −3.19 to −0.88) for omalizumab 300 mg.
3.4 Evidence for safety
Drug-related adverse events (AE) were reported in three and four RCTs with omalizumab 150 mg and 300 mg, respectively.36, 38, 42, 45 All RCTs assessed this end-point at the end of follow-up (24-40 weeks). Both doses may increase the risk of drug-related AE with low certainty of the evidence: RR 1.40 (95%CI 0.63 to 3.13) for omalizumab 150 mg and RR 1.37 (95%CI 0.67 to 2.82) for omalizumab 300 mg. Drug-related serious AE were assessed for omalizumab 300 mg,33 showing a decrease with moderate certainty of the evidence compared to placebo (RR 0.77; 95% CI 0.20 to 2.91), although results are inconclusive due to the small RR. One study43 reported a single anaphylactic episode during the open-label phase of the study.
4 DISCUSSION
4.1 Main findings
The current systematic review shows that omalizumab 300 mg as add-on treatment significantly reduces the signs and symptoms and burden of CSU, with moderate certainty of evidence. High certainty of evidence for efficacy outcomes was not achieved mainly due to imprecision. For the 150 mg dose, the MID is not reached for any of the observed end-points. For both doses, there is an increased likelihood of achieving complete response and a lower risk of experiencing UAS7 worsening after stopping treatment.
Overall, omalizumab improved QoL, but only omalizumab 300 mg achieved clinical significance (above the MID) with high certainty of evidence. Additionally, omalizumab 300 mg showed a significant improvement in QoL when assessed with CU-QoL and a lower risk of experiencing DLQI worsening after stopping treatment.
Both doses probably reduce rescue medication use and may increases drug-related AE; however, with inconclusive results. Omalizumab 300 mg might decrease serious drug-related AE.
All studies were funded by two pharmaceutical companies and reported positive effect results, which might raise a concern of a potential sponsorship bias.
4.2 Results in the context of previous research
In alignment with the results reported by this SR, all previous systematic reviews assessing omalizumab efficacy and safety in adolescent and adults with CSU/CIU report an improvement in symptoms score assessed and highlight the better efficacy of omalizumab 300 mg over the 150 mg dose.46-51 In the most recent meta-analysis published by Jia et al, however, there was similar efficacy for 150mg, and 300 mg omalizumab, respectively.52 Only three SR reported similar results for the impact on quality of life, rescue medication, and safety.46, 49, 52 In contrast with the current results, three SR46, 49, 52 report a high certainty of evidence for omalizumab 300 mg safety. A possible explanation for this difference is that the current SR for safety the assessment was limited to drug-related AE.
An important difference with previous SRs is that most of them included nonapproved doses of omalizumab and other treatments for CSU/CIU as part of the SR, while the current SR focused only on the licensed doses of omalizumab, making the results more informative to develop recommendations and applicable to daily clinical practice. Another important difference is the assessment of the certainty of evidence using the GRADE approach. With the exception of Urgert et. al., all previous SRs limited their evaluation to the risk of bias. The current SR considered all relevant aspects related with the certainty of evidence like heterogeneity, indirectness, or imprecision of the results. For instance, the use of MID in the assessment of imprecision enabled the evaluation of the clinical relevance of addition of omalizumab for each outcome.
4.3 Limitations and strengths
The current systematic review has a number of strengths. First, an extended systematic search from three main databases was conducted, for both safety and efficacy. Second, rigorous methods, including the use of the GRADE approach to rate the certainty of the evidence, were followed. The outcomes evaluated were prioritized a priori, and the minimal important difference was included, when available. An optimal presentation of results in a friendly user format improves communication of key messages to patients, clinicians, and other stakeholders.
The current SR also has limitations. Only studies published in English were included. However, the studies included in previous systematic reviews were thoroughly evaluated and additional studies suggested through the GDG were considered, which mitigates the risk of missing studies. No observational studies which could inform on outcomes with low quality of evidence (adverse events, rescue medication use) were included; however, they will be considered in formulating recommendations for clinical practice.
4.4 Implications for practice and research
Only omalizumab 300 mg showed an improvement in chronic spontaneous urticaria related critical outcomes, with moderate certainty. There is however a significant improvement in quality of life, with high certainty of evidence.
Short-term safety data are reassuring. More detailed reporting from RCTs is warranted in combination with long-term safety evaluation including observational studies and registries.
The available data supporting the efficacy and safety in the pediatric population are very scarce, which highlights the need for rigorous trials in this population.
CONFLICT OF INTERESTS
IA serves as associate editor of Allergy. YS, MP, PA-C, CR, IS, JB, and CCA declare funding from EAACI. CA reports grants from Allergopharma, Idorsia, Swiss National Science Foundation, Christine Kühne-Center for Allergy Research and Education, European Commission Horizon 2020 Framework Programme, Cure, Novartis Research Institutes, Astra Zeneca, Scibase, and is on the Sanofi/Regeneron advisory board. MA declares grants from Allergopharma, Idorsia, Swiss National Science Foundation, Christine Kühne-Center for Allergy Research and Education, European Commission's Horizon's 2020 Framework Programme, Cure, Novartis Research Institutes, AstraZeneca, Scibase, and other from Sanofi/Regeneron. KB has received personal fees from Novartis. SG reports personal fees from AstraZeneca, GSK and Novartis. TE has received grants or other from DBV, Innovation Fund Denmark, Regeneron, the Allergy and Anaphylaxis Program SickKids; serves as associate editor for Allergy and in the local advisory board of ALK. KE reports grants and/or personal fees from AbbVie, BMS, Boehringer Ingelheim, Lilly, LEO, Janssen, grants from Galapagos, UCB, Novartis, and Sanofi. AG-A declares grants and/or personal fees from Novartis, Uriach Pharma, GSK, Regeneron Sanofi, Instituto Carlos III FEDER, Leo Pharma, Almirall, Amgen, Menarini, MSD. JG declares grants and/or personal fees from Sanofi-Regeneron, Novartis, AbbVie, Janssen, LEO Pharma, L’Òreal, Mylan, and has been issued a patent. MM reports grants and/or personal fees from Sanofi/Regeneron, Allakos, Alnylam, Amgen, Aralez, ArgenX, AstraZeneca, BioCryst, Blueprint, Celldex, Centogene, CSL Behring, Dyax, FAES, Genentech, GINNOVATION, Innate Pharma, Kalvista, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Pharming, Pharvaris, Roche, Shire/Takeda, Third HarmonicBio, UCB, and Uriach. GO reports personal fees, grants and/or nonfinancial support from the University of Oxford, Sanofi, Celgene, Novartis, Janssen, Orbit, UCB, AnaptysBio, Eli Lilly, and Orbit Discovery. O. Peck reports grants and others from Regeneron, Pfizer, AbbVie, and Incyte. LOM has received grants from GSK and personal fees from AHL. O. Palomares received research grants from Inmunotek SL and Novartis; received fees for giving scientific lectures from: Allergy Therapeutics, Amgen, AstraZeneca, Diater, GSK, Inmunotek S.L, Novartis, Sanofi-Genzyme and Stallergenes; participated in advisory boards from Novartis and Sanofi-Genzyme. MJ reports personal fees from ALK-Abello, Allergopharma, Stallergenes, Anergis, Allergy Therapeutics, Circassia, Leti, Biomay, HAL, AstraZeneca, GSK, Novartis, Teva, Vectura, UCB, Takeda, Roche, Janssen, MedImmune, and Chiesi. All other authors have no conflict of interest within the scope of the submitted work.




