Estrogen receptor-α signaling increases allergen-induced IL-33 release and airway inflammation
Jacqueline-Yvonne Cephus
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorVivek D. Gandhi
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorRuchi Shah
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorJordan Brooke Davis
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorHubaida Fuseini
Department of Pathology, Microbiology, and Immunology, Vanderbilt University, Nashville, Tennessee, USA
Search for more papers by this authorJeffrey A. Yung
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorJian Zhang
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorHirohito Kita
Allergic Diseases Research Laboratory, Mayo Clinic, Phoenix, Arizona, USA
Search for more papers by this authorVasiliy V. Polosukhin
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorWeisong Zhou
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorCorresponding Author
Dawn C. Newcomb
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Department of Pathology, Microbiology, and Immunology, Vanderbilt University, Nashville, Tennessee, USA
Correspondence
Dawn C. Newcomb, Vanderbilt University Medical Center, 1161 21st Avenue, T-1218 MCN, 1161 21st Avenue, T-1218 MCN, Nashville, TN 37232.
Email: [email protected]
Search for more papers by this authorJacqueline-Yvonne Cephus
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorVivek D. Gandhi
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorRuchi Shah
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorJordan Brooke Davis
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorHubaida Fuseini
Department of Pathology, Microbiology, and Immunology, Vanderbilt University, Nashville, Tennessee, USA
Search for more papers by this authorJeffrey A. Yung
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorJian Zhang
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorHirohito Kita
Allergic Diseases Research Laboratory, Mayo Clinic, Phoenix, Arizona, USA
Search for more papers by this authorVasiliy V. Polosukhin
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorWeisong Zhou
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorCorresponding Author
Dawn C. Newcomb
Department of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Department of Pathology, Microbiology, and Immunology, Vanderbilt University, Nashville, Tennessee, USA
Correspondence
Dawn C. Newcomb, Vanderbilt University Medical Center, 1161 21st Avenue, T-1218 MCN, 1161 21st Avenue, T-1218 MCN, Nashville, TN 37232.
Email: [email protected]
Search for more papers by this authorAbstract
Background
Group 2 innate lymphoid cells (ILC2) are stimulated by IL-33 to increase IL-5 and IL-13 production and airway inflammation. While sex hormones regulate airway inflammation, it remained unclear whether estrogen signaling through estrogen receptor-α (ER-α, Esr1) or ER-β (Esr2) increased ILC2-mediated airway inflammation. We hypothesize that estrogen signaling increases allergen-induced IL-33 release, ILC2 cytokine production, and airway inflammation.
Methods
Female Esr1-/-, Esr2-/-, wild-type (WT), and IL33fl/fleGFP mice were challenged with Alternaria extract (Alt Ext) or vehicle for 4 days. In select experiments, mice were administered tamoxifen or vehicle pellets for 21 days prior to challenge. Lung ILC2, IL-5 and IL-13 production, and BAL inflammatory cells were measured on day 5 of Alt Ext challenge model. Bone marrow from WT and Esr1-/- female mice was transferred (1:1 ratio) into WT female recipients for 6 weeks followed by Alt Ext challenge. hBE33 cells and normal human bronchial epithelial cells (NHBE) were pretreated with 17β-estradiol (E2), propyl-pyrazole-triol (PPT, ER-α agonist), or diarylpropionitrile (DPN, ER-β agonist) before allergen challenge to determine IL-33 gene expression and release, extracellular ATP release, DUOX-1 production, and necrosis.
Results
Alt Ext challenged Esr1-/-, but not Esr2-/-, mice had decreased IL-5 and IL-13 production, BAL eosinophils, and IL-33 release compared to WT mice. Tamoxifen decreased IL-5 and IL-13 production and BAL eosinophils. IL-33eGFP + epithelial cells were decreased in Alt Ext challenged Esr1-/- mice compared to WT mice. 17β-E2 or PPT, but not DPN, increased IL-33 gene expression, release, and DUOX-1 production in hBE33 or NHBE cells.
Conclusion
Estrogen receptor -α signaling increased IL-33 release and ILC2-mediated airway inflammation.
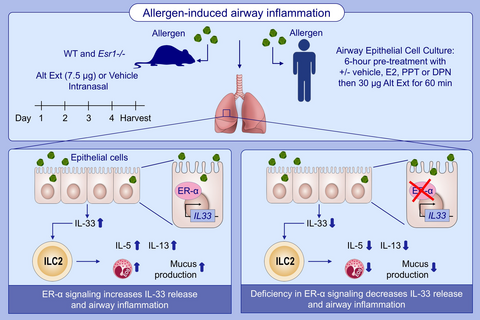
Graphical Abstract
Allergen-induced airway inflammation is increased by estrogen signaling. Compared to WT mice, Alt Ext challenged Esr1 −/− mice have decreased IL-5 and IL-13 production, eosinophils number, and IL-33 release. Estrogen receptor-α signaling has no direct effect on ILC2 proliferation or cytokine expression. Abbreviations: Alt Ext, Alternaria extract; DPN, diarylpropionitrile; E2, 17β-estradiol; Eos, eosinophils; Esr1 −/−, estrogen receptor alpha deficient mice; ER-α, estrogen receptor α; ILC2, group 2 innate lymphoid cells; PPT, propyl-pyrazole-triol; WT, wild-type
CONFLICTS OF INTEREST
The authors have no conflict of interest in relation to this work.
Supporting Information
| Filename | Description |
|---|---|
| all14491-sup-0001-FigS1-S5.pdfPDF document, 986.9 KB | Figures S1-S5 |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1 Center for Disease Control USD of H and HS. National health interview survey. Published Online First: 2015. https://www.cdc.gov/nchs/fastats/asthma.htm. Accessed November 15, 2019.
- 2 American Lung A, Statistics U, Health Education D, A AL, Division ALA and SU and HE, ALA. Trends in Asthma Morbidity and Mortality. 2012. http://www.lung.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf. Accessed November 20, 2019.
- 3Shames RS, Heilbron DC, Janson SL, Kishiyama JL, Au DS, Adelman DC. Clinical differences among women with and without self-reported perimenstrual asthma. Ann Allergy, Asthma Immunol. 1998; 81: 65-72.
- 4Rao CK, Moore CG, Bleecker E, et al. Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the severe asthma research program. Chest. 2013; 143: 984-992.
- 5Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003; 112: 283-288.
- 6Gomez Real F, Svanes C, Bjornsson EH, et al. Hormone replacement therapy, body mass index and asthma in perimenopausal women: a cross sectional survey. Thorax. 2006; 61: 34-40.
- 7Real FG, Svanes C, Omenaas ER, et al. Lung function, respiratory symptoms, and the menopausal transition. J Allergy Clin Immunol. 2008; 121(1): 72.
- 8Troisi RJ, Speizer FE, Willett WC, Trichopoulos D, Rosner B. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. AmJRespirCrit Care Med. 1995; 152: 1183-1188.
- 9Belanger K, Hellenbrand ME, Holford TR, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obs Gynecol. 2010; 115: 559-567.
- 10Boonpiyathad T, Sözener ZC, Satitsuksanoa P, Akdis CA. Immunologic mechanisms in asthma. Semin Immunol. 2019; 46: 101333.
- 11Hoyler T, Klose CS, Souabni A, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012; 37: 634-648.
- 12Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011; 12: 1055-1062.
- 13Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014; 40: 425-435.
- 14Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 2014; 134(3): 671.
- 15Liu T, Wu J, Zhao J, et al. Type 2 innate lymphoid cells: a novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir Med. 2015; 109: 1391-1396.
- 16Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2015; 137(1): 75.
- 17Cephus J-Y, Stier MT, Fuseini H, et al. Testosterone attenuates Group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017; 21: 2487-2499.
- 18Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010; 464: 1367-1370.
- 19Li Y, Wang W, Lv Z, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol. 2018; 200: 2253-2262.
- 20Bonnelykke K, Sleiman P, Nielsen K, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014; 46: 51-55.
- 21Mathias RA, Grant AV, Rafaels N, et al. A genome-wide association study on African-ancestry populations for asthma. J Allergy Clin Immunol. 2010; 125:336.
- 22Savenije OE, Mahachie John JM, Granell R, et al. Association of IL33-IL-1 receptor-like 1 (IL1RL1) pathway polymorphisms with wheezing phenotypes and asthma in childhood. J Allergy Clin Immunol. 2014; 134: 170-177.
- 23Torgerson DG, Ampleford EJ, Chiu GY, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011; 43: 887-892.
- 24Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016; 16: 676-689.
- 25Hardman CS, Panova V, McKenzie AN. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur J Immunol. 2013; 43: 488-498.
- 26Barlow JL, Peel S, Fox J, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013; 132: 933-941.
- 27Zhou W, Toki S, Zhang J, et al. Prostaglandin I2 signaling and inhibition of Group 2 innate lymphoid cell responses. Am J Respir Crit Care Med. 2016; 193: 31-42.
- 28Hristova M, Habibovic A, Veith C, et al. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J Allergy Clin Immunol. 2016; 137(5): 1545.
- 29Drake LY, Kita H. IL-33: biological properties, functions, and roles in airway disease. Immunol Rev. 2017; 278: 173-184.
- 30Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011; 7: 321-329.
- 31Gerriets VA, Kishton RJ, Johnson MO, et al. Foxp3 and Toll-like receptor signaling balance T reg cell anabolic metabolism for suppression. Nat Immunol. 2016; 17: 1459-1466.
- 32Fuseini H, Yung JA, Cephus JY, et al. Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. J Immunol. 2018; 201: 1843-1854.
- 33Laffont S, Blanquart E, Savignac M, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017; 214: 1581-1592.
- 34Kadel S, Ainsua-Enrich E, Hatipoglu I, et al. A major population of functional KLRG1(-) ILC2s in female lungs contributes to a sex bias in ILC2 numbers. Immunohorizons. 2018; 2: 74-86.
- 35Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006; 116: 561-570.
- 36Yung JA, Fuseini H, Newcomb DC. Hormones, sex, and asthma. Ann Allergy Asthma Immunol. 2018; 120: 488-494.
- 37Bartemes K, Chen CC, Iijima K, Drake L, Kita H. IL-33-responsive group 2 innate lymphoid cells are regulated by female sex hormones in the uterus. J Immunol. 2018; 200: 229-236.
- 38Uchida M, Anderson EL, Squillace DL, et al. Oxidative stress serves as a key checkpoint for IL-33 release by airway epithelium. Allergy. 2017; 72: 1521-1531.
- 39Lopez M, Salvaggio JE. Mold-sensitive asthma. Clin Rev Allergy. 1985; 3: 183-196.
- 40Neukirch C, Henry C, Leynaert B, Liard R, Bousquet J, Neukirch F. Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol. 1999; 103: 709-711.
- 41Downs SH, Mitakakis TZ, Marks GB, et al. Clinical importance of alternaria exposure in children. Am J Respir Crit Care Med. 2001; 164: 455-459.
- 42Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004; 113: 227-234.
- 43Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 2007; 120: 610-617.
- 44Laffont S, Seillet C, Guery JC. Estrogen receptor-dependent regulation of dendritic cell development and function. Front Immunol. 2017; 8: 108.
- 45Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of Interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013; 49: 741-750.
- 46Halim TY, McKenzie AN. New kids on the block: group 2 innate lymphoid cells and type 2 inflammation in the lung. Chest. 2013; 144: 1681-1686.
- 47Van Dyken SJ, Nussbaum JC, Lee J, et al. A tissue checkpoint regulates type 2 immunity. Nat Immunol. 2016; 17: 1381-1387.
- 48Warren KJ, Sweeter JM, Pavlik JA, et al. Sex differences in activation of lung-related type 2 innate lymphoid cells in experimental asthma. Ann Allergy Asthma Immunol. 2017; 118: 233-234.
- 49Carey MA, Card JW, Bradbury JA, et al. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. AmJRespirCrit Care Med. 2007; 175: 126-135.
- 50Kabata H, Moro K, Fukunaga K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013; 4: 2675.
- 51Babina M, Kirn F, Hoser D, et al. Tamoxifen counteracts the allergic immune response and improves allergen-induced dermatitis in mice. Clin Exp Allergy. 2010; 40: 1256-1265.
- 52Sarmiento J, Perez B, Morales N, et al. Apoptotic effects of tamoxifen on leukocytes from horse peripheral blood and bronchoalveolar lavage fluid. Vet Res Commun. 2013; 37: 333-338.
- 53Choi HJ, Chung YS, Kim HJ, et al. Signal pathway of 17β-estradiol-induced MUC5B expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2009; 40: 168-178.
- 54Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. Estradiol increases mucus synthesis in bronchial epithelial cells. PLoS One. 2014; 9:e100633.
- 55Jain R, Ray JM, Pan JH, Brody SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol. 2012; 46: 446-453.
- 56Watanabe Y, Tajiki-Nishino R, Tajima H, Fukuyama T. Role of estrogen receptors α and β in the development of allergic airway inflammation in mice: a possible involvement of interleukin 33 and eosinophils. Toxicology. 2019; 411: 93-100.
- 57Boots AW, Hristova M, Kasahara DI, Haenen GRMM, Bast A, van der Vliet A. ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J Biol Chem. 2009; 284: 17858-17867.
- 58Shah R, Newcomb DC. Sex bias in asthma prevalence and pathogenesis. Front Immunol. 2018; 9: 2997-2998.
- 59Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008; 181: 2790-2798.
- 60Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005; 174: 8183-8190.
- 61Shikotra A, Choy DF, Ohri CM, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012; 129: 104-109.