Clinical factors associated with peanut allergy in a high-risk infant cohort
Funding information
NIH-NIAID U19AI066738 and U01AI066560. The project was also supported by Grant Numbers UL1 RR-025780 (National Jewish), UL1 TR-000067 (Mount Sinai), UL1 TR-000039 (Arkansas), UL1 TR-000083 (U North Carolina), and UL1 TR-000424 (Johns Hopkins) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Abstract
Background
Prognostication of peanut allergy (PNA) is relevant for early interventions. We aimed to determine baseline parameters associated with the development of PNA in 3- to 15-month-olds with likely egg and/or milk allergy, and/or moderate to severe atopic dermatitis (AD) and a positive egg/milk skin prick test (SPT), but no known PNA.
Methods
The primary endpoint was PNA [confirmed/convincing diagnosis or last classified as serologic PNA (<2 years, ≥5 kUA/L, otherwise ≥14 kUA/L, peanut IgE)] among 511 participants (median follow-up, 7.3 years). Associations were explored with univariate logistic regression; factors with P < 0.15 were analyzed by stepwise multiple logistic regression, using data stratified by PNA status and randomly assigned to development and validation datasets.
Results
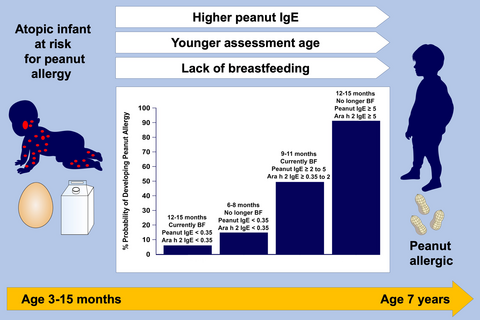
205/511 (40.1%) had PNA. Univariate factors associated with PNA (P < 0.01) included increased AD severity, larger egg and peanut SPT, greater egg, milk, peanut, Ara h1-h3 IgE, higher peanut IgG and IgG4, and increased pregnancy peanut consumption. P-values were between 0.01 and 0.05 for younger age, non-white race, lack of breastfeeding, and increased lactation peanut consumption. Using a development dataset, the multivariate model identified younger age at enrollment, greater peanut and Ara h2 IgE, and lack of breastfeeding as prognosticators. The final model predicted 79% in the development and 75% in the validation dataset (AUC = 0.83 for both). Models using stricter or less strict PNA criteria both found Ara h2 as predictive.
Conclusions
Key factors associated with PNA in this high-risk population included lack of breastfeeding, age, and greater Ara h2 and peanut-specific IgE, which can be used to prognosticate outcomes.
Graphical Abstract
In high-risk infants (eg, moderate to severe eczema and egg/milk sensitizationor likely egg/milk allergy), parameters associated with the development of peanut allergy were identified. Multivariate models identified younger age at initial assessment, higher peanut IgE and Ara h2 IgE levels, and lack of breastfeeding (BF) as significant associated factors. Models were constructed to predict risk of peanut allergy based on these variables.
CONFLICT OF INTEREST
Dr. Wood reports grants from Astellas, grants from DBV, grants from Aimmune, grants from Regeneron, grants from NIAID, outside the submitted work. Dr. Dawson reports grants from DAIT/NIAID/NIH during the conduct of the study. Ms. Henning reports grants from DAIT/NIAID/NIH, during the conduct of the study. Dr. Jones reports grants from NIH-NIAID, during the conduct of the study; personal fees from FARE-Food Allergy Research and Education, personal fees from Aimmune herapeutics, grants from Aimmune Therapeutics, grants from DBV Technologies, grants from Astellas, Inc., grants from FARE, grants from NIHNIAID, personal fees from EMMES Corporation, grants from Sanofi, Inc., grants from Regeneron, Inc., outside the submitted work. Dr. Lindblad reports grants from DAIT/NIAID/NIH during the conduct of the study. Dr. Burks reports personal fees from Aimmune Therapeutics, Inc., personal fees from Astella Pharma Global Development, personal fees from Consortia TX, Inc., personal fees from DBV Technologies, personal fees from Intrommune Therapeutics , personal fees from Prota Therapeutics, personal fees from N-Fold, LLC, personal fees from Aravax, personal fees from Genetech, personal fees from Hycor Biomedical, grants from NIH, grants from Johns Hopkins/NIH, grants from NIH, grants from NIH, grants from FARE, grants from Wallace Research Foundation, grants from NIH, grants from NIH, grants from PRA Health Sciences, Inc., other from Allertein stock, other from Mastcell Pharmaceuticals, other from UpToDate royalties, outside the submitted work; In addition, Dr. Burks has a patent US #7879977 with royalties paid, a patent US #6835824 with royalties paid, a patent US #6486311 with royalties paid, a patent US #6441142 with royalties paid, a patent US #5973121 with royalties paid, and a patent US #5558869 with royalties paid.