Thinking bigger: How early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease
Abstract
Imbalance, or dysbiosis, of the gut microbiome of infants has been linked to an increased risk of asthma and allergic diseases. Most studies to date have provided a wealth of data showing correlations between early-life risk factors for disease and changes in the structure of the gut microbiome that disrupt normal immunoregulation. These studies have typically focused on one specific risk factor, such as mode of delivery or early-life antibiotic use. Such “micro-level” exposures have a considerable impact on affected individuals but not necessarily the whole population. In this review, we place these mechanisms under a larger lens that takes into account the influence of upstream “macro-level” environmental factors such as air pollution and the built environment. While these exposures likely have a smaller impact on the microbiome at an individual level, their ubiquitous nature confers them with a large influence at the population level. We focus on features of the indoor and outdoor human-made environment, their microbiomes and the research challenges inherent in integrating the built environment microbiomes with the early-life gut microbiome. We argue that an exposome perspective integrating internal and external microbiomes with macro-level environmental factors can provide a more comprehensive framework to define how environmental exposures can shape the gut microbiome and influence the development of allergic disease.
1 INTRODUCTION
The developmental origins of health and disease (DOHaD) hypothesis suggest that a child's environment from conception to 1000 days greatly influences the child's risk for chronic diseases.1 This hypothesis is becoming increasingly adopted for immune-mediated diseases, including asthma and allergies. In the context of allergic diseases, the complex community of microorganisms that reside in the human gut is emerging as a critical early-life factor that is capable of influencing long-term disease outcomes.2 This narrative review focuses on early-life environmental exposures as primary drivers of disease development. Genetic factors alone cannot explain the rapid rise in childhood allergies seen in most Westernized nations in a single generation, nor the more recent increases witnessed by other increasingly affluent countries such as India and China.3
Reduced early-life microbial exposure and loss of microbial biodiversity in the urban environment have led to changes in the type, degree, and timing of microbial stimulation in early-life which is now being linked to an increased predisposition to chronic allergic conditions.4 Known as the “microflora hypothesis,” researchers have suggested an important role for a diverse gut microbiota in shaping host immune development, and that disruption or dysbiosis of the “normal” gut microbiota contributes to the development of immune disorders such as asthma and allergies.5, 6 Today over half the world's population lives in an urban setting and the United Nation projects that 66% of us will live in a built city environment within two decades.7 Therefore, it is imperative that we begin to understand the association(s) between the host, the microbes, and our environment. This important new knowledge will empower us to take evidence-based steps toward reducing the health burden of allergic diseases and optimizing human health.
2 TERMINOLOGY USED IN DESCRIBING THE HUMAN MICROBIOME
Recent advances in next-generation sequencing technology have rapidly expanded our understanding of the range of microbes that live in and on us. In response, some have suggested that humans should be considered as holobionts—a biomolecular assembly of the human host plus its associated microbes that together form an ecological unit.8 Indeed the complex community of bacteria, fungi, archaea, viruses, and protozoans that live with us contributes a nearly equal number of cells to the human holobiont as there are human somatic cells.2 We note here that an increasing number of studies have begun to highlight the importance of low-abundance microbes comprising approximately 0.1% of cells9, 10 within the microbiota that appear to influence host health. Known as the “rare biosphere”,11 this population of microbes includes archaea and micro-eukaryotes such as protozoa and fungi.12 Members of the “rare biosphere” can often be keystone taxa with disproportionate ecological and immunological impacts.13, 14
In this review, we will use the terminology of the Human Microbiome Project.15 Specifically, the human microbiota refers to the microorganisms that live inside and on humans. While the gut is the most densely populated area of our bodies, the microbiota of other niches (eg, skin and airways) are also important for health outcomes.15 The microbiome refers to the genomes of these microbial symbionts which provide traits that humans did not need to evolve on their own. As discussed by the joint initiative of both American and European societies of asthma, allergy, and immunology (PRACTALL), human microbiomes can be viewed as a one organ interacting with host mucosal surfaces.16 Indeed, recent studies have started to explore this holistic approach with evidence indicating that lung and gut share common function in modulating immune responses.17 While the integration of various niches of microbiota within the human body is an active area of research, to date the role of the gut microbial communities has received most attention in relation to the immunological mechanisms implicated in allergic diseases.18
3 THE INFANT GUT MICROBIOME AND EARLY-LIFE IMMUNOMODULATION
The neonatal period of life is one of remarkable developmental changes, including maturation of the gut microbiota and the establishment of a self-tolerant and functional immune system.19 Birth exposes the newborn to broad range of microbes which then seed the infant gut with a dynamic but highly unstable and impressionable community of organisms.20 The cross-talk between this developing microbiota and immune system is subsequently shaped by environmental exposures and local factors within the gut until it matures to a steadier state by age three years.21-26
In support of the microflora hypothesis, several epidemiological studies have found differences in the composition and diversity of the early-life gut microbiota of allergic/asthmatic children and healthy children.27-33 Altered gut microbiota communities (“dysbiosis”) within allergy-prone infants, however, often do not persist past the first 100 days of life,33 indicating that this period represents a “critical window” of development during which the host is most susceptible to microbiota-dependent immunomodulation.34 Evidence from mouse studies has further supported the existence of a “critical window” during which time microbial exposures via the gut microbiota act to limit the development of pro-inflammatory immune cell populations and promote the development of anti-inflammatory regulatory immune cell populations.5, 35-40 Moreover, microbiota-modifying early-life exposures and experiences,20, 41-44 including delivery by cesarean section,45, 46 hospital delivery,32 lack of furry pets in the home,47, 48 antibiotic exposure,49-51 reduced maternal,52 and infant farm exposure,48, 53, 54 and having fewer siblings55 all have been shown to increase a child's risk of developing asthma and other atopic diseases later in life (see Figure 1). The mycobiome has also been linked to other atopic conditions, such as atopic dermatitis.56, 57 A recent study showed that compared to infants at low risk of allergy, the early-life gut microbiota of infants at high risk of allergy contained higher levels of fungi and skewed immune development of germ-free mice toward a Th17 signature.58 Together these studies highlight the importance of the early-life window during which fungal exposures may skew immune development relevant to allergic disease. More recently, human and mouse studies have begun to elucidate the specific host immune cell and microbial mechanisms involved in early-life microbiota-mediated immunomodulation and the dysbiosis-asthma paradigm.34, 59 Despite only just beginning to glean insights into the mechanisms by which the gut microbiota influences immune responses, studies have shown that members of the microbiota can influence host immune pathways through signals initiated by both direct contact with microbial surface antigens and by indirect effects of microbe-derived metabolites. While a complete discussion of the mechanisms by which the microbiota has been shown to modulate the host immune system is beyond the scope of this review, we will summarize the most well-studied mechanisms herein (and for more detailed information see60-64).

One mechanism though which microbes within the gut microbiota modulate host immune responsiveness and development is via direct interactions with host receptors that recognize conserved microbial surface structures, known as microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs; MAMPs is the preferred term since all microbes, and not solely pathogens, express these molecular structures). MAMPs are recognized by host pattern recognition receptors triggering molecular cascades that lead to immune cell recruitment and responses that differ according to the identity of the molecular trigger and context within which the signal is initiated. As an example, all gram-negative bacteria—a group of microbes that make up a significant portion of the gut microbiota—express the MAMP lipopolysaccharide (LPS) in their outer cell membrane, and perinatal LPS exposure has been shown to be protective against Th2-type airway inflammation and airways hyperresponsiveness in murine models of allergic asthma52, 65, 66. Other microbe-specific MAMPs, such as polysaccharide A found in the cell capsule of Bacteroides fragilis, have also been shown to have beneficial immunomodulatory properties67, 68
In addition to having direct effects on the immune system, gut microbiota communities indirectly modulate host immune health through the production of metabolites and other molecules that both influence the colonization of other microbes and affect host physiology. Arguably one of the most important groups of metabolites derived from the bacterial microbiota are short-chain fatty acids (SCFAs). SCFAs are generated via bacterial degradation of dietary fiber and not only provide energy to epithelial cells, but have also been implicated in protection against a vast array of human immune and metabolic diseases.69 Acetate, propionate, and butyrate are the most abundant SCFA molecules within the gut, and acetate in particular has been shown in mouse and human studies to protect against allergic asthma. Indeed, exposure to a high fiber diet improves allergic airway diseases in both clinical and mouse asthma studies by promoting the development of anti-inflammatory regulatory T (Treg) cells and other mechanisms.70-74 Beyond dietary fiber and SCFAs, the Learning Early About Peanut Allergy (LEAP) study powerfully reinforces the importance of early-life diet in modulating immune responses and preventing later food allergy development.
As research in this area continues and the mechanisms by which additional microbial structures and metabolites interact with the host to modulate immune responses are elucidated, the possibility of rationally designing a microbiota-derived pre-/pro-biotic for the prevention of allergic disease is becoming a tantalizingly possibility.18, 75 Indeed, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, the World Allergy Organization (WAO) guideline panel determined that there is a likely net benefit from using probiotics primarily related to the prevention of eczema. Although it should be acknowledged that this recommendation was tempered with the statement that “All recommendations are conditional and supported by very low quality evidence”.76 While the role probiotics deserves more high-quality studies; in the meantime, however, research into other environmental influences on the gut microbiota suggests that simple behavioral modifications (eg, minimizing antibiotic exposure) may be equally as effective at promoting the establishment of a healthy gut microbiota in .
4 THE NEED TO THINK OUTSIDE: MULTISCALE EFFECTS OF ENVIRONMENTAL EXPOSURES ON GUT MICROBIOTA STRUCTURE
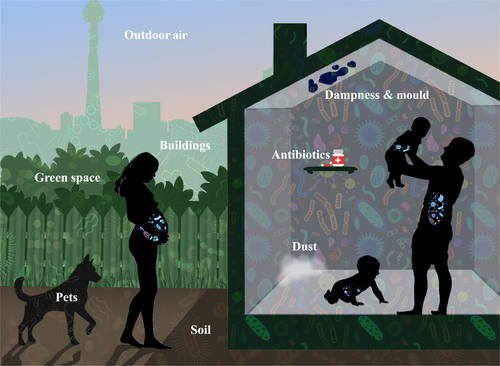
The role of the gut microbiome in ensuring healthy immune function and preventing atopic disorders is complex. This complexity is driven by both the dynamic nature of the microbiota and the range of factors that have the potential to modify this microbial community. When considering environmental factors that may shape the gut microbiota, we can appreciate that these factors act at very different scales; for example, the direct, large-scale impact of a course of oral antibiotics compared to the strong yet smaller-scale impact of living in a rural environment. This highlights the importance of adopting an integrated multiscale approach when quantifying the impact of environmental factors that shape the development and integrity of the gut microbiome (illustrated in Figure 2). Here we review a number of key environmental exposures that should be considered when exploring links between the gut microbiota and allergic disease.
4.1 Antibiotics
It is now well-established that the use of antibiotics in early-life correlates with the risk of developing allergic diseases, including asthma and atopic dermatitis.77-79 In a meta-analysis of both prospective and retrospective cohort studies, the relative risk of polled studies showed an estimated 200% increase in risk of developing asthma for children who are prescribed an antibiotic in the first year of life.51, 77 Antibiotics also affect the developing gut microbiota. For example, by comparing the bacterial diversity of newborns followed up during the first few weeks of life, Tanaka et al80 demonstrated that newborns who received antibiotics in the first week of life had less Bifidobacterium, as well as unusual presence of Enterococcus in stool samples collected at one week as compared with untreated newborns. At one month of age, these antibiotic-treated infants also had an overgrowth of Enterobacteriaceae and Enterococcus.21 The authors further noted that children born by cesarean section to mothers who received intravenous antibiotics had the same, yet less abundant, microbiota development.21 In sum, early use of antibiotics disturbs the fragile infant gut microbiota either transiently or permanently, depending on timing,81 by shifting the composition of the gut microbiota, decreasing the overall diversity of the infant's microbiota, and potentially selecting for drug-resistant bacteria.82
4.2 Air pollution and the air we breathe
Urban air pollution, mainly driven by energy and transportation demands, has become a major public health threat due to rapid urbanization trends.83 Increased ambient levels of air pollutants (more specifically traffic-related toxicants) have been extensively and consistently shown to be associated with the rise in morbidity associated with allergic respiratory diseases.83, 84 The most abundant and well-studied components of air pollution in urban areas are nitrogen dioxide, ozone, and particulate matter. Coalescing evidence from laboratory and epidemiological studies implicates potential mechanistic pathways such as oxidative stress, inflammatory pathways and immunological responses, as well as adjuvant effects to respiratory sensitization to inhalable antigens.83-86 Studies of natural experiments in human populations, such as the East-West German comparison before and after reunification, provide hints into the role air pollution has on atopic disorders and its potential effect on the microbiome. Following the reunification of Germany, it was possible to compare genetically similar populations with strikingly different prevalence in both allergic disorders and environmental exposures, including diet and indoor/outdoor air pollution. The latter was quantified and shown to significantly correlate with the difference in allergic disorders trends (hay fever, atopic dermatitis). Western Germany, with much higher rates of allergic disorders, had higher NO2 levels, driven by traffic exhaust, whereas Eastern Germany had much lower rates of allergic disorders and relatively high environmental SO2 levels, driven by coal combustion.87
Along the same vein, a study of children living in Finnish and Russian Karelia (an area of Northern Europe that is socioeconomically distinct but geoclimatically similar) has suggested the importance of environmental exposures early in life, especially airborne microbial exposures, in determining allergic disease risk. These regions are geographically close and have populations from the same genetic pool, but of markedly different socioeconomic status (SES). Although levels of sensitization to pollens and pets were similar during the 1950s, allergies were more common among Finnish children a mere 30 years later, an increase that has been linked to differences in bacteria found in inhalable environmental dust.88 The association between environmental biodiversity found in soil carried in house dust and sensitization in the Finnish group supports the hypothesis of strong environmental effect on the commensal microbiota. From the “Karelia” studies, it appears that beyond avoiding established risk factors for allergic disorders, such as antibiotic use, the mere contact with a diverse natural environment including the air we breathe may increase immune tolerance and protect from becoming sensitized to allergens. While a more comprehensive assessment of the external (ie, built environment) and internal microbiomes (eg, gut or lung microbiota) in these populations is warranted, these natural experiment studies provide a strong foundational rationale for considering the role ubiquitous exposures such as particles and microorganisms in the air we breathe in microbe-mediated immunomodulation relevant to the onset of allergic disorders. In expanding on these exposures (antibiotics and air pollution) with varying risks at the individual versus population level, we underscore the importance of an integrated view of modifiable environmental factors to help tailor interventions with personalized and societal impacts that would increase the biodiversity of the human-built environment.
5 BUILT ENVIRONMENT MICROBIOMES
The infant gut microbiota accumulates bacterial diversity over the first few months and years of life.25 While the key drivers of this bacterial diversity are just beginning to be investigated, the biodiversity hypothesis provides strong support that our built environment, in particular the indoor environment where we spent the vast majority of our time89 may shape to the development of the gut microbiome.
The built environment includes all of the physical components of where we live and work (eg, homes, buildings, streets, open spaces, and infrastructure). As such, it can be viewed as the human-made environment as opposed to natural urban forests, for instance. Studying the built environment is key not only for urban planning and policy implications but also in understanding how environmental microbiomes affect the diversity of human gut microbiome. Understanding of the microbial diversity afforded by selecting shrubs, trees or other plants, or modified by redesigning a neighborhood, its homes, schools, and buildings can impact human health differently from that provided by exposure to nonhuman-made features of the built environment—a concept has been evocatively termed “microbiome rewilding”.90
5.1 Human-made environment and allergy
A rich literature has investigated how features of the built environment—its biodiversity, allergens, and pollution—contribute to the development of atopic dermatitis, rhinitis, and asthma in children (key elements are summarized in Table 1).91 Environmental biodiversity can be driven by nature, as shown by Ruokolainen et al92, who demonstrated that living near forests and farmland versus industrialized areas in Finland and Estonia offered protection against allergic sensitization in children 6 years of age and older. However, as emphasized earlier, indoor exposures have a strong plausibility in driving the human early-life gut microbiome diversity. Indoor exposures characterized in the El Paso Children's Health Study enumerate many components of the built environment—pests, pets, cooking, and heating gases—that strongly associate with allergy and asthma.93 The timing of exposure to various features of built environment also impacts allergic outcomes as seen in the Cohort for Childhood Origin of Asthma and Allergic Disease where Lee et al (2018) identify prenatal, and not postnatal, mold abundances—not diversity—as predictors of atopic dermatitis.94
| Exposure categories | Place | Childhood immune-mediated outcomes related to selected built environment features |
|---|---|---|
|
External microbial exposome in the home: microbial load/ diversity allergens house dust mite |
Spain, Germany111; Sweden139; USA139-142; Europe141; Japan143; UK, Taiwan, Colombia, Australia, Canada, China139; Portugal144, 145; Austria, Netherlands, Switzerland, and children from European descent,133 global120, 146, 147 |
Protective effects ↑ microbial load ►↓ asthma and allergic rhinitis later in childhood111 ↑ levels of allergens, Firmicutes and Bacteroidetes (within first year) ► ↓ atopy or wheeze47 and ↓ allergic sensitization and wheezing outcomes at age 3 years141 Increased risks Bacterial groups (Firmicutes and Bacteroidetes; especially Listeria monocytogenes, Bacillus spp, Corynebacterium spp) ►↑ asthma and allergy133 ↑ total fungal concentration and community composition (eg, Alternaria, Candida albicans, Cladosporium cladosporioides, Epicoccum nigrum, Penicillium brevicompactum), and ↑ bacterial richness ►↑ asthma severity142 ↑ lipopolysaccharides in primary school classrooms ► ↑ prevalence of allergic sensitization children145 No Associations No difference between bacterial concentrations in home of asthmatics vs control homes144 |
| Dampness or mold in the home | Westernized countries148; Europe149; Asia150, 151; Germany152 |
Increased risks ↑ exposure to mold/dampness ► ↑ risk of asthma development, eczema, allergic rhinitis.98, 141, 148, 149 Water damage in home ► ↑ risk of allergic rhinitis149, 150, 152 |
| Exposure to air pollutants, Microbial Volatile Organic Compounds (MVOCs) and other chemicals | USA,122, 124, 153, 154 Europe and North America,120, 141 Taiwan,155 Hong Kong, China and Japan 143, 151, 156 |
Increased risks ↑ Traffic air pollution (TRAP) ► ↑ risk of asthma, sensitization to aero- and food allergens, eczema, and hayfever83-85, 120 ↑ Second hand smoke ► ↑ risk of asthma, allergic sensitization, and eczema120, 121, 151 |
|
No Associations No difference in total VOC concentrations in homes of children with allergic disease and healthy children144 No association MVOC concentrations and atopic dermatitis156 |
||
| Land use around home and urban vs. rural differences | Europe 111, 157, 158; Germany159; North America124, 141, 160; China150 |
Decreased risks Home on or near forest/agricultural land ► ↓ risk of allergic disease92 Greenness around home ►↓ risk of allergic disease development92 Family-based farms ►↓ risk of allergies, asthma, and early-virus triggered wheeze66, 102, 157, 160 Highway or commercial district within 200 m of residence ►↑ risk of allergic rhinitis111, 161 |
|
No/inconclusive Associations No association between soil composition/acidity around home and asthma, hay fever, eczema, or allergic SPT outcome162 Inconclusive evidence for association between greenness and asthma or allergic disease110, 111, 158, 161 |
A key limitation shared by many of the studies that explore links between the human-made built environment and allergic disease is the difficulty in assessing concomitant exposures (ie, a unified measure of global exposure) that may contribute to the overall causal biology that drives atopic disorders. Coined by Christopher Wild in 2005,95 the concept of the exposome represents a paradigm shift where biological responses are examined as a function of cumulative environmental co-occurring exposures starting from the time of conception.96 Defining the exposome complements genomic data, and applying this knowledge to human health research necessitates defining the exposome in terms of measurable components,97 namely the external exposome which includes extrinsic environmental factors such as the built environment, diet, smoking, and the internal exposome which encompasses all “omics" measures including the microbiome. Recent advances in sequencing technologies are beginning to address this limitation by defining the microbiomes associated with the built environment. This is allowing researchers to elucidate the potential interactions between human health, features of the built environment, and their respective microbiomes.
5.2 Human-made environment and microbes
In this section, we review the current understanding of microbial exposure in the human-made environment (as illustrated in Figure 2).
5.2.1 Indoor microbes
In comparison with the extensive evidence on the impact of fungi in the built environment to human health,98 airborne bacteria in built environments have not been as exhaustively researched. A comprehensive review by Leung and Lee99 has identified the following key points: (a) that the built environment microbiome is dependent on housing and lifestyle characteristics. Specifically, the quantity and diversity of microbial exposure depends not only on the engineering factors of the building (design, material, ventilation, and type of dwelling) but also on the geographical location—a crucial finding that emphasizes the importance of characterizing the proximal landforms and uses; and (b) that microbial communities of the built environment are largely dependent on their occupants (humans and animals) who shed their microorganisms on floor, surfaces and/or in the ambient air. Indeed, humans are estimated to shed a staggering 15 million bacterial cells per person per hour100!
The study of household dust is often used to characterize the indoor microbial communities. In general, house dust from urban environments is poor in microbial components and has distinct immunomodulatory capacities (eg, maintaining immunologic tolerance) as compared with dust from farming environments.101 Particularly influential in this domain are both large European studies contrasting farm versus urban environments, as well as the studies in North America comparing environmental exposures and allergic sensitization among Amish and Hutterite communities. From Europe, the Prevention of Allergy-Risk Factors for Sensitization in Children Related to Farming and Anthroposophic Lifestyle (PARSIFAL) and the Multidisciplinary Study to Identify the Genetic and Environmental Causes of Asthma in the European Community (GABRIEL) studies, established that children living on farms were exposed to a wider range of microbes and that this exposure explains much of the inverse relation between asthma and hay fever and growing up on a farm.53 It is known, however, that not all farming environments are protective.102 To address this discrepancy, Stein and colleagues4 studied the Amish and Hutterite populations whose cultural heritage and lifestyles are remarkably similar other than farming practices: Amish follow traditional farming practices, Hutterites use industrialized farming techniques. Notably, the prevalence of asthma in Amish versus Hutterite schoolchildren is 5.2% versus 21.3% and the prevalence of allergic sensitization is 7.2% versus 33.3%.103, 104 The major findings of this important work were that endotoxin levels in Amish house dust were 6.8 times as higher than Hutterites, and that the Amish dust activated the innate immune system to reduce immune reactivity. This work has implications that extend well beyond the farm, as defining the specific protective molecules may empower new approaches for the prevention of asthma and allergic sensitization.
While the bulk of the evidence on the effect of environmental microbes on the inception of allergic diseases comes from farm studies, recent birth cohort studies have also shed light on the role of urban environmental microbes. Of particular interest is the nested case-control investigation in the Urban Environment and Childhood Asthma (URECA) study where Lynch and colleagues examined inner-city homes with low neighborhood SES across different cities and assessed the house dust content.47 The microbial richness in the samples was inversely related to the risk of atopy. These authors showed that higher exposure to specific Firmicutes and Bacteroidetes together with high levels of pests and pets allergens conferred protection against the development of allergies. The interaction between allergen levels and bacterial microbiome is a novel addition to what we have learnt through birth cohort studies on the protective effect of pets early in life against wheezing, asthma, and allergies.105 Identifying such interactions can help detangle whether richness or total microbial biomass, or animal-derived allergens or organisms106 from other environmental sources interact and lead to impaired immunoregulation.
5.2.2 Microbes in the human-made outdoors
Compared to natural surroundings which are considered important in increasing the resilience of the immune system,107 human-made green urban areas can also be potential sources of harmful allergen exposures.108 Birth cohort studies conducted in several geographical areas in the Northern Hemisphere identified associations between allergic health, including rhinitis and sensitization, and the allergenicity of features of green urban spaces.109-111 Current management practices of the man-made outdoors—for example maintenance of turf grass lawns, tree and shrub pruning, pesticide and herbicide applications—threaten this aspect of the built environment biodiversity.112 In addition, human or anthropogenic activities related to accelerated urbanization have directly impacted our outdoors and thereby the diversity of certain plant pollens.113 These effects have modified the pollen microbiome and increased their allergenicity as shown in studies demonstrating that cumulative pollen exposure during the first 3 months of life is significantly associated with increased risk of hay fever at age 6/7 years.113, 114
In response to these trends that are gradually reducing our environmental microbial biodiversity, research has investigated the possibility of restoring a biodiverse habitat in urban green spaces and whether it can “rewild” the environmental microbiome to a state that would halt the immune dysregulation of urban populations. This terminology was suggested by Mills and colleagues90 who supported it through a case study of active restoration which within 10 years restored a natural environmental microbiome with potential for increased human health benefits.
When considering both culture-dependent and high-throughput culture-independent molecular techniques to analyze microbial communities at the micro and macro-level, there has been a recognition of the need to establish a unified knowledge base as demonstrated by the initiative launched by the US National Academies of Sciences, Engineering, and Medicine.115 Quantifying our extrinsic exposome (ie, the combined outdoor and indoor environmental allergens, microbes, pollutants, and household chemicals) would be a first step toward this call. Systems biology approaches can be expanded to assess its relevance to our intrinsic exposome (ie, microbiome, epigenome, transcriptome) for ensuring the best preventative measures that would benefit the preservation of ecosystem biodiversity and human health.107 Perhaps, these gaps and future challenges are not as daunting as they seem when we consider the consensus that microbial exposure in urban settings is generally largely dominated by human occupants and to a lesser extent by pets, and even less so by outdoor sources.116
6 INTEGRATING MICROBES AND FEATURES OF THE BUILT ENVIRONMENT WITH THE HUMAN MICROBIOME
In order to understand the interplay between the human microbiota, the built environment, and immune health, studies are beginning to validate and explore underlying mechanisms using animal models informed by epidemiologic observations. Cohort studies that encompass both urban and rural environment exposures clearly indicate an interplay between the different microbiomes and have provided strong rationale for validation studies. For instance, from the observed differences between the Amish and Hutterite populations described above, a follow-up animal validation study showed that administering Amish house dust to ovalbumin-sensitized mice reduced immune responsiveness, whereas the Hutterite house dust led to the opposite effect in the same mouse model,4 thereby highlighting the critical role of innate immunity in providing environmentally outsourced protection. Similarly, leveraging the findings by Lynch and colleagues on the interaction between pets allergen and indoor bacteria,47 a murine model showed that mice exposed to dust from homes with dogs are protected against airway inflammatory responses to allergens and have less airway pathology after inoculation with respiratory syncytial virus (RSV).117 This study further identified L johnsonii as a pivotal species that would protect against the asthmagenic pathogen RSV.
Understanding allergic disease requires understanding all the components of environmental factors: from host microbiome, to the various built environment microbiomes, and their effect on metabolic and inflammatory processes. An additional layer of complexity to acknowledge is the need to consider allergic diseases in the context of developmental trajectory. To harness this complexity (ie, assessing the multiple routes of exposures while also incorporating the various scales by which the built environment may affect allergic outcomes), the exposome paradigm offers a dynamic framework for research where efforts to characterize it by specific time period provides the opportunity to discover actionable interventions. Further, the adoption of the exposome framework necessarily removes discipline-associated silos and is best operationalized by the adoption of a systems biology approach to address the complexity of interactions between systems.118 These include interactions among microbiota and between microbiota and the host, as well as interaction with other omics data (eg, metagenomics, transcriptomics, and metabolomics).16 While systems biology, via tools such as machine learning, networks analysis, and artificial intelligence algorithms, can aid in developing networks to understand causal relationships, this approach needs to be complemented by a reductionist approach that deciphers mechanisms and allergic endotypes that can be treated.119
6.1 Prenatal exposome
In utero exposure to environmental stressors can disrupt developmental processes and alter body metabolism and physiology, thereby leading to chronic pathologies in later life—this is a core concept of the DOHaD hypothesis. The past 5-10 years have seen several epidemiological studies in Europe, North America, and Japan that have adopted the exposome concept.97, 120-122 There is now good evidence for the effect of prenatal exposure to environmental contaminants including air pollution on the developing immunologic and respiratory system.120, 123 In parallel, several microbiome surveys have shown that maternal microbial exposures during pregnancy can beneficially affect the immune function of the newborn.124 Recent studies have focused on the role of the placenta and, although still somewhat controversial, some have shown the presence of bacterial genetic material in that organ.125-127 These data refute the “sterile womb” dogma and support the possibility of transplacental protection against allergic phenotypes.
An important factor that should be highlighted when considering the prenatal exposome is the contribution of socioeconomic position of the environment into which the infant is born. Robinson et al pooled environmental data (socioeconomic indicators, built environment, etc) in nine cities and urban areas from across Europe and assessed the urban exposome of pregnant women.128 The exposomes of pregnant women displayed considerable variability and challenged the traditional view of environmental justice and health inequities. These authors demonstrated that while in some cities women in lower socioeconomic positions were exposed to higher levels of environmental hazards that can affect allergy development, others are not subject to similar environmental inequalities, thereby supporting the need for including indicators of socioeconomic position in future studies.
6.2 Postnatal exposome
Birthmarks the point in development where the child's exposome receives a large and diverse microbial bolus from the mother and the built environment, initiating gut microbiome establishment and adding to the complexity of fully assessing the postnatal exposome. Within the first year of life, an estimated 1013-1014 microbes, comprising up to 1,000 species colonize the gastrointestinal tract.129 While the parent-child vertical transmission ensures the colonization of early gut microbes, the environment ensures a horizontal transmission that affects the assembly of the gut microbiota.130
Precise assessment of the external exposome after birth is crucial as demonstrated by North and colleagues.121 Leveraging data collected in a small yet diverse birth cohort, these authors found that SES and urbanicity, factors that capture aspects of the external exposome, were important modifiers of the host-specific characteristics, or the specific external exposome (eg, gestational age). Interestingly, this investigation of pre- and postnatal exposomes highlighted how mold and dampness in the homes after birth was among the strongest risk factors for wheeze and other features suggestive of asthma. The addition of microbial exposure, often viewed as the internal exposome, adds another layer of complexity to the definition of the exposome, yet is necessary to provide a plausible mechanistic understanding of the interplay between built environment microbes-host microbes that ultimately may drive allergic phenotypes.
7 IDENTIFIED GAPS AND FUTURE RESEARCH AGENDA
Built environment microbiomes are dynamic and interact with the occupants’ gut microbiomes in ways that we are only now starting to unravel. Characterizing interrelationships between these communities can help define how to build our homes and shape our neighborhoods to offer an optimal “built environment” from a microbe composition viewpoint. Embracing this concept, we are seeing the emergence of studies that borrow from the beneficial effects of farming exposures to influence human health outcomes. Just as the “rewilding hypothesis” drove the 10-year effort to restore a natural environmental biodiversity that was predicted to benefit the human microbiome, an ongoing study in Finland is testing whether the introduction of microorganisms associated with forest soil can influence human allergic disease.131 There is clearly an exciting potential for translational impact by engineering the built environment microbiome to influence health outcomes.131-133
Assessing the influences of the built environment and indoor microbial exposures on not only the composition and function of the gut microbiome, but also on human functional responses, requires moving from correlational studies to longitudinal studies coupled with animal model validation studies to test for causation. Thus, while the wealth of new data has added tremendous complexity, powerful tools and methods to quantify the exposome are now being developed that will help us to understand how environmental and human microbiomes (and their interactions) contribute to the immune dysregulation that drives the development of allergic disease.129
8 FUTURE RESEARCH PERSPECTIVES
- Lung-Gut cross-talk: The advent of sophisticated multiomics techniques has enabled the characterization of microbiota in different human body sites.129 The interplay between the microbiomes of the lung and gut represent a promising area of research given their importance in the pathogenesis of lung disease.163 Next steps should take these microbiome analyses across larger, diverse study populations to not only characterize microbial communities’ structure, but also define their function and metabolic products to link them with immune-mediated health outcomes.
- Polymicrobial communities: While most microbiome research has focused on the bacterial communities, the role of fungi in the human microbiome has been eliciting increased interest. In light of recent clinical and experimental studies, accounting for inter-kingdom interactions164 in microbiome studies is potentially as important as accounting for known and widely accepted risk factors such as age.
- Environmental microbiota and polymicrobial communities: Our health is predominantly determined by where we live. We need to consider the role of the environment on human polymicrobial communities and immunoregulation. A first promising research direction is the unification and standardization of methods to collect, store, and analyze environmental microbial communities. Two platforms are available for microbiome-environment studies: Qiita and SourceTracker.165 Clinicians, ecologists, microbiologists, and data scientists need to come together as a community and start adopting such tools as the field is in great need of data integration and harmonization to help realize the therapeutic potential of human and environmental microbiomes.
9 MAJOR MILESTONE DISCOVERIES
- Early-life gut microbiota communities differ among allergic/asthmatic and healthy children.
- Early-life factors that modify microbial exposures also influence an infant's risk of developing atopic diseases in childhood.
- Gut microbial dysbiosis associated with asthma and allergies occurs during a “critical window” in early-life.
- Early-life antibiotic exposure disrupts the early-life gut microbiota and increases a child's risk of developing atopic diseases.
- Allergic diseases are more prevalent in geographical regions where air pollution levels are higher. Animal models support a plausible mechanistic effect on gut microbial dysbiosis.
- Microbially rich environmental and house dust exposures are protective against allergic disease development.
- Living on farms and near forests offers protection against developing allergic diseases in childhood. The biodiverse nature of this “farming environment” likely confers protection. This was demonstrated in experimental studies of mouse models of allergic respiratory diseases where some organisms found in cowsheds show promise for prevention,166 including Acinetobacter lwoffii F78, Lactococcus lactis G121, and Staphylococcus sciuri W620.
CONFLICT OF INTEREST
All co-authors have no conflict of interest.
ACKNOWLEDGMENTS
Work relevant to this review in Turvey and Finlay laboratories is funded by the Canadian Institutes Health Research, AllerGen NCE, Genome BC, Genome Canada (274CHI) and the Michael Smith Foundation for Health Research.




