ARIA pharmacy 2018 “Allergic rhinitis care pathways for community pharmacy”
AIRWAYS ICPs initiative (European Innovation Partnership on Active and Healthy Ageing, DG CONNECT and DG Santé) POLLAR (Impact of Air POLLution on Asthma and Rhinitis)GARD Demonstration project
Funding information
European Union Development and Structural Funds, Fondation FMC VIA-LR.
Abstract
Pharmacists are trusted health care professionals. Many patients use over-the-counter (OTC) medications and are seen by pharmacists who are the initial point of contact for allergic rhinitis management in most countries. The role of pharmacists in integrated care pathways (ICPs) for allergic diseases is important. This paper builds on existing studies and provides tools intended to help pharmacists provide optimal advice/interventions/strategies to patients with rhinitis. The Allergic Rhinitis and its Impact on Asthma (ARIA)-pharmacy ICP includes a diagnostic questionnaire specifically focusing attention on key symptoms and markers of the disease, a systematic Diagnosis Guide (including differential diagnoses), and a simple flowchart with proposed treatment for rhinitis and asthma multimorbidity. Key prompts for referral within the ICP are included. The use of technology is critical to enhance the management of allergic rhinitis. However, the ARIA-pharmacy ICP should be adapted to local healthcare environments/situations as regional (national) differences exist in pharmacy care.
Abbreviations
-
- AHA
-
- active and healthy aging
-
- AIRWAYS ICPs
-
- integrated care pathways for airway diseases
-
- AR
-
- allergic rhinitis
-
- ARIA
-
- allergic rhinitis and its impact on asthma
-
- CARAT
-
- control of allergic rhinitis and asthma test
-
- CDSS
-
- clinical decision support system
-
- CRD
-
- chronic respiratory diseases
-
- DG CONNECT
-
- directorate-general for communications networks, content and technology
-
- DG Santé
-
- directorate-general for health and food safety
-
- DG
-
- directorate-general
-
- EFA
-
- European Federation of allergy and airways diseases patients’ associations
-
- EIP on AHA
-
- European innovation partnership on AHA
-
- EIP
-
- European innovation partnership
-
- GARD
-
- WHO global alliance against chronic respiratory diseases
-
- HCP
-
- healthcare professional
-
- ICP
-
- integrated care pathway
-
- JA-CHRODIS
-
- joint action on chronic diseases and promoting healthy ageing across the life cycle
-
- MACVIA
-
- contre les MAladies Chroniques pour un VIeillissement Actif (Fighting chronic diseases for AHA)
-
- MASK
-
- mobile airways sentinel network
-
- MeDALL
-
- mechanisms of the Development of ALLergy (FP7)
-
- mHealth
-
- mobile health
-
- NCD
-
- noncommunicable disease
-
- OTC
-
- over-the-counter
-
- POLLAR
-
- impact of air POLLution on Asthma and Rhinitis
-
- QOL
-
- quality of life
-
- SCUAD
-
- severe chronic upper airway disease
-
- VAS
-
- visual analog scale
-
- WHO
-
- World Health Organization
-
- WPAI-AS
-
- work productivity and activity questionnaire
1 INTRODUCTION
Allergic diseases such as rhinitis and asthma are common, complex conditions, associated with allergen-specific IgE and nonallergic mechanisms.1, 2 These diseases represent an enormous burden associated with personal, medical, and social costs as well as impairment in work productivity,3-6 impacting health and social inequalities in all age-groups.7
Allergic rhinitis (AR) is a highly diverse chronic disease spanning from mild-intermittent rhinitis to severe chronic upper airway disease (SCUAD).8 There is evidence that the condition is suboptimally managed with several unmet needs which include:
- Understanding the different endotypes (rhinitis and asthma)9 and the presence of different phenotypes of AR and multimorbidities which can impact AR and asthma control,
- Improving AR diagnosis using modern technology,10
- Delivering management strategies and interventions which address suboptimal rhinitis and asthma control arising not only from suboptimal medical management/treatment, but as a result of a range of cultural and/or social barriers,8, 11
- Assessing risk factors such as allergens and pollutants to incorporate them into management strategies, using technologies that promote sentinel networks within multidisciplinary care pathways (ie, integrated care pathways [ICPs]),12
- Stratifying patients, based on their needs, in order to optimize the use and effectiveness of ICPs,13 and
- Promoting multidisciplinary teams within integrated ICPs, endorsing innovation in clinical trials, and encouraging patient empowerment.
Integrated care pathways are structured multidisciplinary care plans which detail essential steps in the care of patients. They promote the translation of guidelines into local protocols and their subsequent application to clinical practice. They empower patients and their carers (health and social). ICPs differ from practice guidelines as they are utilized by a multidisciplinary team and have a focus on the quality and coordination of care. Pharmacists are at the forefront of ICPs for AR. An ICP is intended to act as a guide to treatment.
This paper builds on existing studies and provides tools intended to help pharmacists provide optimal advice/interventions/strategies to patients with AR. The Allergic Rhinitis and its Impact on Asthma (ARIA)-pharmacy ICP includes a diagnostic questionnaire specifically focusing attention on key symptoms and markers of the disease, a systematic Diagnosis Guide (including a differential diagnosis), and a simple flowchart with proposed treatment for rhinitis and asthma multimorbidity. Key prompts for referral within the ICP are included. The use of technology is critical to enhance the management of AR. However, the ARIA-pharmacy ICP should be adapted to local healthcare environments/situations.
2 PHARMACIST CHALLENGE IN ALLERGIC RHINITIS
Considering the challenges associated with AR and the identified needs, it is clear that the pharmacist has a role for AR management in practice, through a guided change management process. This is both within the scope of pharmacy practice and in line with future models of integrated care. Pharmacists are ideally placed to manage this extremely important link in care pathways.
Worldwide, pharmacists receive advanced training in basic and clinical sciences. Given the importance of self-medication in many allergic diseases and in iatrogenic disease, pharmacist interventions are well placed to maximize the benefits and minimize the adverse events associated with pharmacotherapy. This is particularly important in AR and asthma multimorbidity14 as well as in the elderly patients, a large number of whom present with allergic diseases in combination with other chronic diseases.15 Moreover, most AR medications are available over-the-counter (OTC).16-19 The impact of the switch from prescription to OTC medications has been profound in AR20 with a significant impact on cost and health utilization reduction.21 Therefore, as trusted healthcare professionals in the community, pharmacists are well placed to play a critical role identifying the symptoms of AR, recommending appropriate OTC treatment,22-24 and integrating ICPs into healthcare teams.
Pharmacists (along with patients, clinicians, and other healthcare professionals [HCPs]) are faced with the relative merits and downsides of the various treatment options. Clinical practice guidelines for AR management developed over the past 20 years have improved the care of AR patients25 and provide a critical framework for AR management.25-27 These guidelines are becoming particularly important for HCPs in primary care since, in most countries, few AR patients consult a specialist physician. In fact, many patients with AR under-recognize their condition4 and, as such, do not even consult a physician.28 A large proportion of AR patients actually self-manage their condition,5, 6 and the pharmacist is often the first HCP to whom a person with nasal symptoms presents.29, 30 This further strengthens the important role of pharmacists within multidisciplinary healthcare teams, acting at different steps of the ARIA-pharmacy ICPs.23, 31-33
The specific role of pharmacists within ICPs can be evidenced through several strategies that have been initiated34 or completed in the AR management in the pharmacy, and in studies confirming the important impact of pharmacist interventions on AR outcomes.18, 35-43 The specific areas of pharmacist impact in AR are summarized in Table 1.
|
3 ARIA
Allergic Rhinitis and its Impact on Asthma commenced during a World Health Organization (WHO) workshop in 199944 and was further developed by the WHO Collaborating Center for Rhinitis and Asthma (2002-2013). This work was undertaken in four phases, which are briefly summarized below:
- Phase 1: The initial goals were (a) to propose a new AR classification, (b) to promote the concept of multimorbidity in asthma and rhinitis, and (c) to develop guidelines with all stakeholders that could be used globally for all countries and populations. ARIA has been disseminated and implemented in over 70 countries globally,7, 45-54 and was revised and updated in 2008.7
- Phase 2: Focused on the transparent reporting of guidelines to facilitate understanding and acceptance using the GRADE approach.25, 26
- Phase 3: MASK (Mobile Airways Sentinel NetworK), an ARIA initiative, is focusing on (a) the implementation of multi-sectoral ICPs (b) using emerging technologies (c) with real-world data (d) for individualized and predictive medicine (e) in rhinitis and asthma multimorbidity, (f) by a multidisciplinary group or by patients themselves (self-care) using the AIRWAYS ICPs algorithm (Figure 2) (g) across the life cycle.12, 55
- Phase 4 concerns change management strategies.56 ARIA-pharmacy ICPs represent one of the change management steps.
Allergic Rhinitis and its Impact on Asthma in the pharmacy23 and its pocket guide were published in 2004 to help pharmacists with the management of AR symptoms and their impact on asthma. Most recommendations proposed in 2004 are still valid but should be updated due to the larger number of medications now available OTC. In the initial approach, ICPs were not considered and the new information and communication technology (ICT) was not available.
4 INTEGRATED CARE PATHWAYS FOR ALLERGIC RHINITIS
4.1 AIRWAYS ICPs
The B3 Action Plan of the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA, DG Santé, and DG CONNECT)57 promotes integrated care models for chronic diseases, including the use of remote monitoring. Based on this initiative and its aims to produce evidence-based translational outcomes, an ICP for airways disease (AIRWAYS ICPs) was developed. Its aim was to launch a collaboration to develop practical multi-sectoral ICPs (a) to reduce chronic respiratory disease (CRD) burden, mortality, and multimorbidity; (b) to improve the education of all stakeholders; (c) to improve work productivity; (d) to promote AHA; and (e) to reduce inequities in all populations globally.58
AIRWAYS ICPs consider a multidisciplinary approach to AR and asthma multimorbidity management, with the pharmacist at the forefront of the algorithm (Figure 1). A very large number of AR patients use OTC drugs23 and are treated in community pharmacies, while the vast majority of patients who visit primary care physicians or specialists have moderate/severe rhinitis.59-63
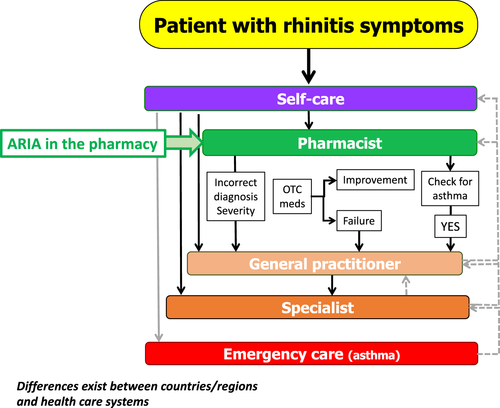
However, pharmacy practice varies widely across countries and ICPs should be tailored to local needs taking into account cultural barriers, socio-economic considerations, healthcare practices, and available OTC medications.
4.2 mHealth in the management of AR incorporating technology for better AR management
mHealth, including apps running on consumer smart devices (ie, smartphones and tablets), has the potential to profoundly impact health care.64 An evidence-based app for use in AR management is available. MASK (Mobile Airways Sentinel NetworK) (ARIA Phase 3) is an app that is an information and communications technology (ICT) system centered around the patient.12, 55, 65 It is an implementation tool of the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA57, 66). A mobile phone app (available for Android and iOS), the Allergy Diary, launched in 23 countries and 17 languages,55, 67 is associated with an interoperable tablet for physicians and other HCPs,65 using visual analogue scales (VAS), the simple common language to manage AR.68, 69
The Allergy Diary also encompasses questions relating to the patient's experience of AR symptoms, an additional quality of life questionnaire (EQ-5D [EuroQol]),70-72 and a validated tool for simultaneously assessing AR and asthma control (Control of Allergic Rhinitis and Asthma Test [CARAT]).73-76 These can be used for self-monitoring or for inclusion into clinical trials. The Allergy Diary is an integrated approach and can be used by patients in the management of their AR, by HCPs in assisting and guiding management and by researchers to collect large-scale population data on AR status and management globally.
4.3 Clinical decision support system: additional resource for pharmacists
Clinical decision support systems (CDSS) are software algorithms that advise HCPs on the diagnosis and management of patients based on the interaction of patient data and medical information, such as prescribed drugs. CDSS should be based on the best evidence to aid patients and HCPs in shared decision making. In a prospective intervention study, a computerized pharmacy CDSS for the counseling of patients with AR was tested. The results showed that pharmacists omitted many questions mandatory to assessing whether self-medication is appropriate, showing the importance of the CDSS.77
An AR CDSS (MASK CDSS) is available for pharmacists as a companion to the Allergy Diary (ARIA Allergy Diary Companion).12, 55 The ARIA Allergy Diary Companion is based on an algorithm to assist in the selection of pharmacotherapy for patients with AR and to stratify their disease severity.55 It uses a simple step-up/step-down individualized approach to AR pharmacotherapy and may hold the potential for optimal control of symptoms, while minimizing side effects and costs. Its use is encouraged for pharmacists; however, its application may vary depending on the availability of medications in the different countries and on resources.
5 MANAGEMENT OF ALLERGIC RHINITIS IN THE PHARMACY: ARIA-PHARMACY ICP
Based on the ICP concept, on mHealth, and on the CDSS, ARIA Phase 4 has developed an ICP for the Community Pharmacy, to assist pharmacists in the management of AR within the AIRWAYS ICPs framework (Figure 1). In summary, this frames the role of the pharmacist in AR across 4 domains:
- Recognition and classification of allergic rhinitis symptoms: As pharmacists are often the healthcare location at which people with AR either initially present or most frequently present, recognizing and classifying AR becomes critical.
- Identification of AR-related ocular symptoms and asthma: The pharmacist's role includes not only assisting in confirming that AR is present but will allow for the identification of possible other conditions and patients at risk, requiring immediate referral to a general practitioner.
- Allergic rhinitis treatment: initiating/recommending optimal treatment, as medication management has been proven to be the most effective way of managing AR.25, 26
- Patient support and AR monitoring over time: supporting the patient with appropriate evidence-based education, self-management support, and long-term monitoring over time.
These 4 domains are discussed below:
5.1 Recognition and classification of allergic rhinitis symptoms
Although AR diagnosis requires tests to confirm the allergic sensitization, most patients self-diagnose their AR, consulting pharmacists without a doctor diagnosis of AR. Several questionnaires are available for the screening of allergic diseases.78, 79 Most AR patients have multiple nasal symptoms (rhinorrhea, sneezing, nasal pruritus, and obstruction), a large percentage have ocular symptoms (ocular tearing, redness, and pruritus), and many have asthma. In order for pharmacists to evaluate the possibility of screening for AR, a series of questions should be asked to patients with nasal symptoms. The questionnaire in Table 2, while it will not be able to lead to a definitive diagnosis of AR, may enable pharmacists to determine whether a diagnosis of AR should be further investigated and can assist in identifying warning symptoms that need further medical investigation.
| Symptoms | Cold | Allergic rhinitis |
|---|---|---|
| Duration | 5-10 days | Variable but can last months or present recurrent episodes |
| Season | Most often in winter but may be possible at any time | Any time of the year, more common during pollen seasons which can occur in winter |
| Course of the disease | Symptoms usually take a few hours or days to be severe | Symptoms can be severe within minutes |
| Nasal and ocular symptoms |
|
|
| Sore throat | Common | Sometimes |
| Cough | Common | Sometimes |
| Chest discomfort |
|
Rare, except for those with allergic asthma |
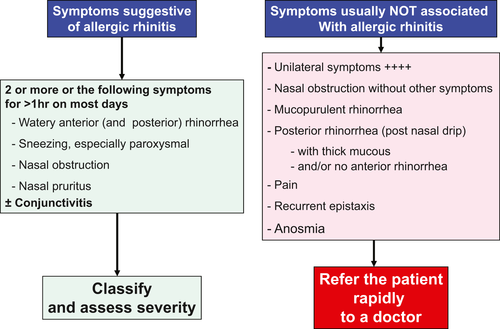
Figure 2 summarizes the list of symptoms, which, if they occur in isolation, may be suggestive of a condition other than AR. The key feature of AR to remember is that AR symptoms are never unilateral and minimal bleeding may occur during long-term intranasal therapy. Furthermore, although nasal obstruction, loss of smell,80 facial pain, or post–nasal drip may all be AR symptoms, when they occur as single symptoms, they are unlikely to be of allergic origin. Purulent discharge, especially if accompanied by fever, is suggestive of an infection (Figure 2). AR may present with symptoms similar to those of a number of other conditions induced by nonallergic triggers including viral infections such as the common cold. Table 2 may help the pharmacist to differentiate allergy from other causes including infection.
The ARIA guideline proposes a classification of AR based on symptom control, QOL, and daily impact as well as duration.7, 44 Disease control associated with several health outcomes, including QOL59-63 and some mHealth end points,72, 81, 67, 82, 83 should be considered in ICPs. Although the duration of rhinitis is an important indicator of asthma multimorbidity,84 and duration and efficacy of treatment in AR,61 AR control should be considered as the most important end point for the pharmacist.
Allergic rhinitis “control” in AR patients is the main goal of treatment.85 Control is the degree to which therapy goals are currently met such as glycemic control in diabetes. However, measures of AR control are somewhat different to many other chronic diseases as while they include symptom scores, alternative scales/scores, which are reflective of the impact of AR on day-to-day living, have been shown to be validated as measure of AR control. For example, visual analogue scales (VAS) assessing impact of AR,69, 86 quality of life (QOL) measures, and scores with several items which cover symptoms and impact on daily living87, 88 are commonly used. In deciding which measure of AR control is most appropriate, it is important for a score to be simple and responsive to change over time, as AR control changes; hence, VAS scores, which can be used in all age-groups,89, 90 are available in a wide variety of languages,91, 92 and have been shown to be valid for assessing AR control, are useful instruments for assessing AR control (Figure 3).

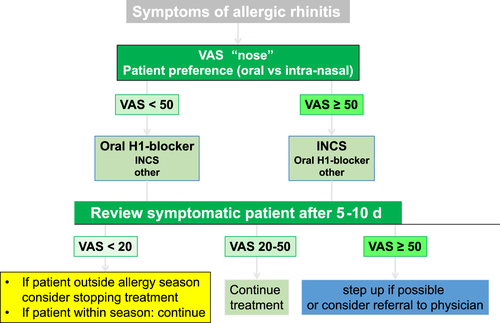
A VAS (range: 0-20) can be used to assess control before and some days after treatment (Figure 6). If the scale is <20/100, the patient has controlled AR; if the scale is from 20 to 50/100, the patient has partly controlled disease; and for ≥50/100, the patient has uncontrolled disease (Figure 6). Using VAS can help the pharmacist to assess response to OTC treatment. VAS can be used to assess overall AR control (VAS global measure) as it relates to nasal symptoms and eye symptoms93 (Figures 3,6). There is a high degree of correlation between VAS global measured (“Overall how much are your allergic symptoms bothering you today?”) and VAS for nose symptoms.81, 83 An electronic form of the VAS exists in the Allergy Diary81, 83 and has been validated against several end points.
5.2 Identification of AR-related ocular symptoms and asthma
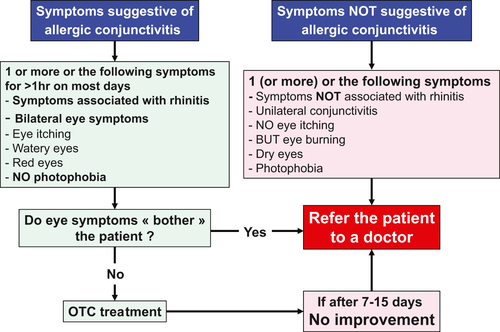
Ocular symptoms are commonly associated with nasal symptoms in AR, and they can be diagnosed using simple questions. However, some forms of conjunctivitis require referral to a physician (Figure 4). A VAS for ocular symptoms may also be used and is included in the Allergy Diary (Figure 3).
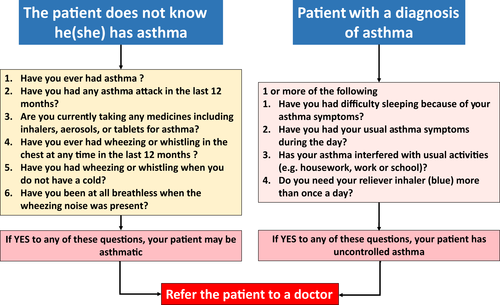
Asthma is a common multimorbidity of AR and should be checked. A proposal has been made in ARIA in the pharmacy23 (Figure 5). All patients with rhinitis should be evaluated for asthma, particularly if they have persistent and/or moderate-severe rhinitis.
5.3 AR Treatment
Goals for the treatment of rhinitis should be determined after an accurate diagnosis of AR and a validated assessment of control, including evaluation of coexisting asthma. AR treatment goals include the following:
- Normal sleep.
- Ability to undertake normal daily activities, including work and school attendance, without limitation or impairment, and the ability to participate fully in sport and leisure activities.
- No troublesome symptoms.
- No or minimal side effects of rhinitis treatment. It should be recognized that many OTC drugs for the treatment of AR can induce sedation and should be avoided.
In all guidelines, it has been considered that many medications may be used at several steps of severity and duration,44 including antihistamines (oral and intranasal), intranasal corticosteroids (INCS), leukotriene receptor antagonists, and chromones (intranasal and eye drops). Anti-cholinergics and decongestants (intranasal and oral) are sometimes noted in the treatment of AR on a short-term basis; however, there is little good quality evidence for their effectiveness45 and prolonged use of decongestants (>10 days) may lead to rebound swelling of the nasal mucosa, drug-induced rhinitis (also known as rhinitis medicamentosa), and tachyphylaxis.7 Table 3 summarizes the specific pharmacological effects of these different agents on specific AR symptoms.94
| Sneezing | Rhinorrhea | Nasal obstruction | Nasal itch | Eye symptoms | |
|---|---|---|---|---|---|
| H1-antihistamines | |||||
| Oral | ++ | ++ | + | +++ | ++ |
| Intranasal | ++ | ++ | + | ++ | 0 |
| Eye drops | 0 | 0 | 0 | 0 | +++ |
| Corticosteroids | |||||
| Intranasal | +++ | +++ | ++ | ++ | ++ |
| Chromones | |||||
| Intranasal | + | + | + | + | 0 |
| Eye drops | 0 | 0 | 0 | 0 | + |
| Decongestants | |||||
| Intranasal | 0 | 0 | ++++ | 0 | 0 |
| Oral | 0 | 0 | + | 0 | 0 |
| Anti-cholinergics | 0 | 0 | 0 | ||
| Anti-leukotrienes | 0 | 0 | ++ | ||
| Intranasal steroids and intranasal antihistamine 1 | +++ | +++ | +++ | +++ | +++ |
In consideration of the specific role of the pharmacist, however, it is important to focus on pharmacotherapy that is available OTC in pharmacies, that is, antihistamines and, in some countries, intranasal corticosteroids. Considering OTC medications, intranasal corticosteroids (INCS) are more effective than oral or intranasal H1-antihistamines27, 44 but many patients prefer oral drugs. Other medications include leukotriene antagonists, nasal washing, vasoconstrictors, and cromoglycate. In some countries (currently in New Zealand), the combination of azelastine and fluticasone propionate is OTC. This medication may be preferred if the patient wants a rapid onset of action of the treatment.26
Figure 6 provides a clinical treatment pathway to help pharmacists choose the appropriate OTC medications, based on assessment of AR control using VAS. The patient's preference should always be considered. OTC medication availability differs between countries.
5.3.1 Immunotherapy and AR treatment
The application of both subcutaneous immunotherapy and sublingual immunotherapy (for treatment of allergies to pollens, ragweed, and house dust mite) is reserved for patients with severe AR. They have been shown to improve the quality of life of people with AR and reduce the need for other pharmacological therapy.95-97 Currently in most countries, initiation of immunotherapy is both administered and monitored by specialist physicians. The role of pharmacy within the context of this treatment has not as yet been defined.
5.3.2 Nonpharmacological AR treatment
In addition to pharmacological treatment, patients with AR have historically been recommended nonpharmacological strategies such as allergen avoidance or minimization and nasal washing. When it comes to seasonal AR, it is evident that avoidance of seasonal allergens is effective. That is, patients with seasonal AR do not experience symptoms outside of season. However, while the level of evidence is low, during pollen season, patients can be advised to close windows at night, drive with closed windows, and wear wrap-around glasses when outdoors, to prevent exacerbation of symptoms; and wear sunglasses98 and nasal filters,99 and apply balms and ointments to the nose94 during pollen season to reduce symptoms. For patients with occupational AR, avoiding exposure to the occupational agent trigger AR is recommended.100
There is limited evidence that saline washings, irrigation, or sprays are effective in reducing AR symptoms and potentially reducing the amount of pharmacotherapy needed7, 101; however, they are well tolerated, safe, and inexpensive102; hence for patients who would like to try them, they can be safely recommended.
5.4 Patient Support and AR monitoring over time
In considering the way in which pharmacists can support the patient with AR, it is important to build on the process of recognition, classification, and treatment recommendations with appropriate education, self-management support, and both short- and long-term monitoring.
Education around the condition of AR is fundamental; however, education about the way in which treatment works, the need to be both adherent and use intranasal devices correctly, is also critical. In particular, it is well recognized that nonadherence to AR treatment adds to the burden of disease. It is difficult to accurately determine nonadherence to AR treatment, as some medications are taken seasonally (as needed) and many are available over-the-counter in the pharmacy. It is, however, estimated that for people using intranasal corticosteroids to treat their AR, adherence is approximately 35%.103 Supporting the patient to better understand the way in which medication works and the need to take it regularly is critical. It has been shown that when pharmacists work with patients to assist them in setting their goals for AR management, better long-term AR outcomes are achieved.36, 37
Further to this, patients must be shown how to use their intranasal sprays correctly. Little is known about the proportion of patients who use their intranasal medication correctly; however, the way in which the patient uses their intranasal spray does impact on the way in which the spray distributes in the nasal cavity104 and the occurrence of local side effects.105 Therefore, as with asthma devices, it is recommended that pharmacists train the patient how to use their intranasal device with a placebo device.
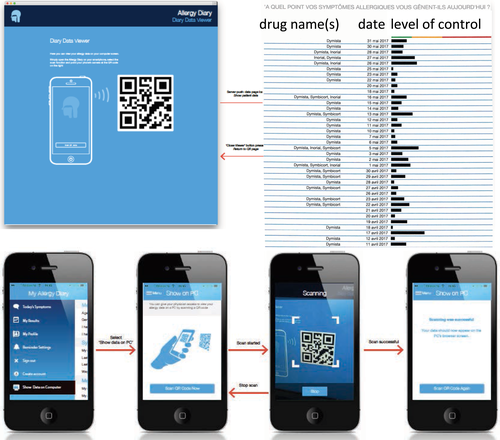
Monitoring adherence with AR treatment is a challenge. This is where the use of technology can assist. There are several mHealth tools for AR follow-up, but only the Allergy Diary has been validated. The Allergy Diary (MASK-rhinitis) has been implemented in 23 countries and 16 languages. Over 23 000 users have been recorded.55, 81, 67, 106, 83, 107-109 This tool appears to be appropriate for pharmacists, in particular since it is extremely simple, does not require the pharmacist to undertake any particular education on its use, is user-friendly for the patient, and can be used for follow-up over time.
For confidentiality reasons, patients cannot give access of the electronic data that they record in the Allergy Diary (both symptom VAS and medication use) to HCPs. However, they can print their daily AR control VAS responses and medication use, as summarized in Figure 7. Comparison of control at first dispensing of an OTC medication with the evolution of control during treatment will guide pharmacists to stop or increase the treatment with OTC medications or suggest referral to a physician. Moreover, this control chart will help the physician to optimize the treatment.
6 CONCLUSION
The present paper has summarized the need for community pharmacists to play an important role in ICPs for AR. Although each recommendation varies between countries due to the available OTC medications, legislation, and cultural differences, ARIA pharmacy may be used as a model for the implementation of ICPs in different countries.
CONFLICTS OF INTEREST
J Bousquet reports personal fees and others from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Sanofi-Aventis, Takeda, Teva, and Uriach; and others from Kyomed, outside the submitted work. S Bosnic-Anticevich reports grants from TEVA and personal fees from TEVA, Boehringer Ingelheim, Sanofi, GSK, and AstraZeneca, outside the submitted work. E Correia-de-Sousa reports other from Boehringer Ingelheim and Novartis, and grants from AstraZeneca, during the conduct of the study. Dr. Cruz reports personal fees and nonfinancial support from Boehringer Ingelheim and AstraZeneca, grants and personal fees from GSK, others from MSD and Sanofi, and personal fees from Novartis, CHIESI, Eurofarma, and Boston Scientific, outside the submitted work. P Devillier reports personal fees from Astra Zeneca, GlaxoSmithKline, Meda Pharma, Sanofi, Novartis, and Chiesi, outside the submitted work. T Haahtela reports personal fees from Mundipharma, Novartis, and Orion Pharma, outside the submitted work. JC Ivancevich reports personal fees from Faes Farma and Eurofarma Argentina, and others from Sanofi Argentina and Lab. Casasco, outside the submitted work. J Just reports grants and personal fees from Novartis, ALK, and AstraZeneca; and personal fees from Thermo Fisher and Zambon, outside the submitted work. P Kuna reports personal fees from Adamed, Boehringer Ingelheim, AstraZeneca, Chiesi, FAES, Berlin-Chemie, Novartis, Polpharma, and Allergopharma, outside the submitted work. Dr. Kritikos reports personal fees from AstraZeneca, GlaxoSmithKline, and Pfizer, outside the submitted work. V Kvedariene has received payment for consultancy from GSK and for lectures from Stallergenes Greer and Berlin-Chemie, outside the submitted work. DE Larenas-Linnemann reports personal fees from GSK, AstraZeneca, Meda, Boehringer Ingelheim, Novartis, Grunenthal, UCB, Armstrong, Siegfried, DBV Technologies, MSD, and Pfizer; and grants from Sanofi, AstraZeneca, Novartis, UCB, GSK, TEVA, Chiesi, and Boehringer Ingelheim, outside the submitted work. R Mösges reports personal fees from ALK, Allergopharma, Allergy Therapeutics, Friulchem, Hexal, Servier, Klosterfrau, Bayer, FAES, GSK, MSD, Johnson&Johnson, Meda, Stada, UCB, and Nuvo; grants from ASIT biotech, Leti, Optima, Bitop AG, Hulka, and URSAPHARM; grants and personal fees from Bencard and Stallergenes; personal fees and nonfinancial support from Lofarma and Novartis; and nonfinancial support from Atmos, Roxall, Bionorica, Otonomy, and Ferrero, outside the submitted work. Dr. Mullol reports personal fees from Sanofi-Genzyme-Regeneron, ALK-Abelló A/S, Menarini Group, MSD, GlaxoSmithKline, Novartis, UCB Pharma, and GENENTECH—Roche; and grants and personal fees from Mylan-Meda Pharma and URIACH Group, outside the submitted work. R Naclerio associated with the advisory boards of Sanofi, Novartis, ActoBio, Revance, and Biomedical. Y Okamoto reports personal fees from Shionogi Co. Ltd., Torii Co. Ltd., GSK, MSD, Kyowa Co. Ltd., and Eizai Co. Ltd.; grants and personal fees from Kyorin Co. Ltd. and Tiho Co. Ltd.; and grants from Yakuruto Co. Ltd. and Yamada Bee Farm, outside the submitted work. N Papadopoulos reports personal fees from Novartis, Faes Farma, Biomay, HAL, Nutricia Research, Menarini, Novartis, Meda, AbbVie, Novartis, MSD, Omega Pharma, and Danone; and grants from Menarini, outside the submitted work. O Pfaar reports grants and personal fees from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, Biotech Tools S.A, LETI/LETI Pharma, and Anergis S.A.; grants from Biomay, Nuvo, Circassia, and GlaxoSmithKline; personal fees from Novartis Pharma, Meda Pharma, Mobile Chamber Experts (a GA2LEN Partner), Pohl-Boskamp, and Indoor Biotechnologies; outside the submitted work. D Plavec reports grants and personal fees from GlaxoSmithKline; personal fees from Menarini, Pliva, AbbVie, Novartis, MSD, Chiesi, and Revenio; personal fees and nonfinancial support from Boehringer Ingelheim; and nonfinancial support from and Philips, outside the submitted work. A Todo-Bom reports grants and personal fees from Novartis, Boehringer Ingelheim, Mundipharma, GSK (GlaxoSmithKline), Teva Pharma, and AstraZeneca; and grants from Leti, outside the submitted work. M Wagenmann reports personal fees from AstraZeneca, HAL Allergy, Meda Pharma, Stallergenes, ALK-Abelló, and Teva; grants and personal fees from Allergopharma and Sanofi-Aventis; and grants from Allakos, F. Hoffmann-La Roche, GlaxoSmithKline, Otonomy, and Strekin, outside the submitted work. S Waserman reports personal fees from Merck, GSK, Novartis, Behring, Shire, Sanofi, Barid Aralez, Mylan Meda, and Pediapharm, outside the submitted work. T Zuberbier reports Organizational affiliations: Committee member: WHO Initiative “Allergic Rhinitis and Its Impact on Asthma” (ARIA); Member of the Board: German Society for Allergy and Clinical Immunology (DGAKI); Head: European Centre for Allergy Research Foundation (ECARF); Secretary General: Global Allergy and Asthma European Network (GA2LEN); and Member: Committee on Allergy Diagnosis and Molecular Allergology, World Allergy Organization (WAO).
AUTHOR CONTRIBUTION
S Bosnic-Anticevich (Woolcock Institute of Medical Research, University of Sydney and Sydney Local Health District, Glebe, NSW, Australia) and J Bousquet planned the project and wrote the first draft of the paper. E Costa (UCIBIO, REQUIMTE, Faculty of Pharmacy and Competence Center on Active and Healthy Ageing of University of Porto (AgeUPNetWork), University of Porto, Portugal), E Menditto (CIRFF, Federico II University, Naples, Italy), O Lourenço (Faculty of Health Sciences and CICS – UBI, Health Sciences Research Centre, University of Beira Interior, Covilhã, Portugal), E Novellino (Department of Pharmacy of University of Naples Federico II, Naples, Italy), S Bialek (Department of Biochemistry and Clinical Chemistry, Faculty of Pharmacy with the Division of Laboratory Medicine, Warsaw Medical University, Warsaw, Poland), V Briedis (Head of Department of Clinical Pharmacy of Lithuanian University of Health Sciences, Kaunas, Lithuania), R Buonaiuto (Pharmacist, Municipality Pharmacy, Sarno, Italy), H Chrystyn (RiRL, 5a Coles Lane, Oakington, Cambridge, UK), B Cvetkosvki (Woolcock Institute of Medical Research, University of Sydney and Sydney Local Health District, Glebe, NSW, Australia), S Di Capua (Farmacie Dei Golfi Group, Massa Lubrense, Italy), V Kritikos (Woolcock Institute of Medical Research, University of Sydney and Sydney Local Health District, Glebe, NSW, Australia), A Mair (DG for Health and Social Care, Scottish Government, Edinburgh, UK), V Orlando (CIRFF, Federico II University, Naples, Italy), E Paulino (Farmacias Holon, Lisbon, Portugal), J Salimäki (Association of Finnish Pharmacies), R Söderlund (Department of Nephrology and Endocrinology, Karolinska University Hospital, Stockholm, Sweden), R Tan (Woolcock Institute of Medical Research, University of Sydney and Sydney Local Health District, Glebe, NSW, Australia), DM Williams (Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC, USA), and P Wroczynski (Department of Biochemistry and Clinical Chemistry, Faculty of Pharmacy with the Division of Laboratory Medicine, Warsaw Medical University, Warsaw, Poland) were pharmacists who reviewed the paper and made some additive concepts. I Agache (Transylvania University Brasov, Brasov, Romania), IJ Ansotegui (Department of Allergy and Immunology, Hospital Quirón Bizkaia, Erandio, Spain), JM Anto (ISGlobAL, Centre for Research in Environmental Epidemiology (CREAL), Barcelona, Spain; IMIM (Hospital del Mar Research Institute), Barcelona, Spain; CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain; Universitat Pompeu Fabra (UPF), Barcelona, Spain), A Bedbrook (MACVIA-France, Fondation partenariale FMC VIA-LR, Montpellier, France), C Bachert (Upper Airways Research Laboratory, ENT Dept, Ghent University Hospital, Ghent, Belgium), M Bewick (iQ4U Consultants Ltd, London, UK), C Bindslev-Jensen (Department of Dermatology and Allergy Centre, Odense University Hospital, Odense Research Center for Anaphylaxis (ORCA), Odense, Denmark), J Brozek (Department of Health Research Methods, Evidence, and Impact, Division of Immunology and Allergy, Department of Medicine, McMaster University, Hamilton, ON, Canada), GW Canonica (Personalized Medicine Clinic Asthma & Allergy, Humanitas University, Humanitas Research Hospital, Rozzano, Milan, Italy), V Cardona (Allergy Section, Department of Internal Medicine, Hospital Vall ‘dHebron & ARADyAL research network, Barcelona, Spain), W Carr (Allergy and Asthma Associates of Southern California, Mission Viejo, CA, USA), T Casale (Division of Allergy/Immunology, University of South Florida, Tampa, Fla), NH Chavannes (Department of Public Health and Primary Care, Leiden University Medical Center, Leiden, The Netherlands), J Correia de Sousa (Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal; ICVS/3B's, PT Government Associate Laboratory, Braga/Guimarães, Portugal), AA Cruz (ProAR – Nucleo de Excelencia em Asma, Federal University of Bahia, Brazil and WHO GARD Planning Group, Brazil), W Czarlewski (Medical Consulting Czarlewski, Levallois, France), G De Carlo (EFA European Federation of Allergy and Airways Diseases Patients’ Associations, Brussels, Belgium), P Demoly (Department of Respiratory Diseases, Montpellier University Hospital, France; Epidemiology of Allergic and Respiratory Diseases, Department Institute Pierre Louis of Epidemiology and Public Health, INSERM and UPMC Sorbonne Université, Medical School Saint Antoine, Paris, France), P Devillier (Laboratoire de Pharmacologie Respiratoire UPRES EA220, Hôpital Foch, Suresnes Université Versailles Saint-Quentin, Université Paris Saclay, France), MS Dykewicz (Section of Allergy and Immunology, Saint Louis University School of Medicine, Saint Louis, Missouri, USA), M Gaga (ERS President 2017-2018, Athens Chest Hospital, 7th Resp Med Dept and Asthma Center, Athens, Greece), Y El-Gamal (Pediatric Allergy and Immunology Unit, Children's hospital, Ain Shams University, Cairo, Egypt), J Fonseca (CINTESIS, Center for Research in Health Technologies and Information Systems, Faculdade de Medicina da Universidade do Porto, Porto, Portugal and MEDIDA, Lda, Porto, Portugal), WJ Fokkens (Department of Otorhinolaryngology, Academic Medical Centre, Amsterdam, the Netherlands), MA Guzmán (Immunology and Allergy Division, Clinical Hospital, University of Chile, Santiago, Chile), T Haahtela (Skin and Allergy Hospital, Helsinki University Hospital, Helsinki, and University of Helsinki, Finland), PW Hellings (Laboratory of Clinical Immunology, Department of Microbiology and Immunology, KU Leuven, Leuven, Belgium), M Illario (Division for Health Innovation, Campania Region and Federico II University Hospital Naples (R&D and DISMET) Naples, Italy), JC Ivancevich (Servicio de Alergia e Immunologia, Clinica Santa Isabel, Buenos Aires, Argentina), J Just (Allergology department, Centre de l'Asthme et des Allergies Hôpital d'Enfants Armand-Trousseau (APHP); Sorbonne Universités, UPMC Univ Paris 06, UMR_S 1136, Institut Pierre Louis d'Epidémiologie et de Santé Publique, Equipe EPAR, Paris, France), I Kaidashev (Ukrainian Medical Stomatological Academy, Poltava, Ukraine), M Khaitov (National Research Center, Institute of Immunology, Federal Medicobiological Agency, Laboratory of Molecular immunology, Moscow, Russian Federation), N Khaltaev (GARD Chairman, Geneva, Switzerland), T Keil (Institute of Social Medicine, Epidemiology and Health Economics, Charité - Universitätsmedizin Berlin, Berlin, and Institute for Clinical Epidemiology and Biometry, University of Wuerzburg, Germany), L Klimek (Center for Rhinology and Allergology, Wiesbaden, Germany), ML Kowalski (Department of Immunology and Allergy, Healthy Ageing Research Center, Medical University of Lodz, Poland), P Kuna (Division of Internal Medicine, Asthma and Allergy, Barlicki University Hospital, Medical University of Lodz, Poland), V Kvedariene (Faculty of Medicine, Vilnius University, Vilnius, Lithuania), DE Larenas-Linnemann (Center of Excellence in Asthma and Allergy, Médica Sur Clinical Foundation and Hospital, México City, Mexico), D Laune (KYomed INNOV, Montpellier, France), LTT Le (University of Medicine and Pharmacy, Hochiminh City, Vietnam), KC Lodrup Carlsen (Oslo University Hospital, Department of Paediatrics, Oslo, and University of Oslo, Faculty of Medicine, Institute of Clinical Medicine, Oslo, Norway), B Mahboub (Department of Pulmonary Medicine, Rashid Hospital, Dubai, UAE), D Maier (Biomax Informatics AG, Munich, Germany), J Malva (Coimbra Institute for Clinical and Biomedical Research (iCBR), Faculty of Medicine, University of Coimbra, Portugal; Ageing@Coimbra EIP-AHA Reference Site, Coimbra, Portugal), P Manning (Department of Medicine (RCSI), Bon Secours Hospital, Glasnevin, Dublin, Ireland), M Morais-Almeida (Allergy Center, CUF Descobertas Hospital, Lisbon, Portugal), R Mösges (Institute of Medical Statistics, and Computational Biology, Medical Faculty, University of Cologne, Germany and CRI-Clinical Research International-Ltd, Hamburg, Germany), J Mullol (Rhinology Unit & Smell Clinic, ENT Department, Hospital Clínic; Clinical & Experimental Respiratory Immunoallergy, IDIBAPS, CIBERES, University of Barcelona, Spain), L Münter (Danish Committee for Health Education, Copenhagen East, Denmark), R Murray (MedScript Ltd, Dundalk, Co Louth, Ireland), R Naclerio (Johns Hopkins School of Medicine, Baltimore, Maryland, USA), L Namazova-Baranova (Scientific Centre of Children's Health under the MoH, Russia, Russian National Research Medical University named Pirogov, Moscow, Russia), K Nekam (Hospital of the Hospitaller Brothers in Buda, Budapest, Hungary), TD Nyembue (ENT Department, University Hospital of Kinshasa, Kinshasa, Congo), K Okubo (Dept of Otolaryngology, Nippon Medical School, Tokyo, Japan), RE O'Hehir (Department of Allergy, Immunology and Respiratory Medicine, Alfred Hospital and Central Clinical School, Monash University, Melbourne, Victoria, Australia; Department of Immunology, Monash University, Melbourne, Victoria, Australia), K Ohta (National Hospital Organization Tokyo National Hospital, Tokyo, Japan), Y Okamoto (Dept of Otorhinolaryngology, Chiba University Hospital, Chiba, Japan), GL Onorato (MACVIA-France, Fondation partenariale FMC VIA-LR, Montpellier, France), S Palkonen (EFA European Federation of Allergy and Airways Diseases Patients’ Associations, Brussels, Belgium), P Panzner (Department of Immunology and Allergology, Faculty of Medicine and Faculty Hospital in Pilsen, Charles University in Prague, Pilsen, Czech Republic), NG Papadopoulos (Division of Infection, Immunity & Respiratory Medicine, Royal Manchester Children's Hospital, University of Manchester, Manchester, UK, and Allergy Department, 2nd Pediatric Clinic, Athens General Children's Hospital “P&A Kyriakou,” University of Athens, Greece), HS Park (Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, South Korea), R Pawankar (Department of Pediatrics, Nippon Medical School, Tokyo, Japan), O Pfaar (Department of Otorhinolaryngology, Head and Neck Surgery, Section for Rhinology and Allergy, University Hospital Marburg, Philipps-Universität Marburg, Germany), J Phillips (Centre for empowering patients and communities, Faulkland, Somerset, UK), D Plavec (Children's Hospital Srebrnjak, Zagreb, School of Medicine, University J.J. Strossmayer, Osijek, Croatia), TA Popov (University Hospital ‘Sv Ivan Rilski’“, Sofia, Bulgaria), P Potter (Allergy Diagnostic and Clinical Research Unit, University of Cape Town Lung Institute, Cape Town, South Africa), EP Prokopakis (Department of Otorhinolaryngology University of Crete School of Medicine, Heraklion, Greece), RE Roller-Wirnsberger (Medical University of Graz, Department of Internal Medicine, Graz, Austria), M Rottem (Division of Allergy Asthma and Clinical Immunology, Emek Medical Center, Afula, Israel), D Ryan (Honorary Clinical Research Fellow, Allergy and Respiratory Research Group, The University of Edinburgh, Past President SLAAI, FACAAI, Edinburgh, UK), B Samolinski (Department of Prevention of Environmental Hazards and Allergology, Medical University of Warsaw, Poland), M Sanchez-Borges (Allergy and Clinical Immunology Department, Centro Médico-Docente la, Trinidad and Clínica El Avila, Altamira, Caracas, Venezuela), HJ Schunemann (Department of Health Research Methods, Evidence, and Impact, Division of Immunology and Allergy, Department of Medicine, McMaster University, Hamilton, ON, Canada), A Sheikh (The Usher Institute of Population Health Sciences and Informatics, The University of Edinburgh, Edinburgh, UK), JC Sisul (Sociedad Paraguaya de Alergia Asma e Inmunologı′a, Paraguay), D Somekh (European Health Futures Forum (EHFF), isle of Wright, UK), C Stellato (Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, University of Salerno, Salerno, Italy), T To (The Hospital for Sick Children, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada), A Todo-Bom (Imunoalergologia, Centro Hospitalar Universitário de Coimbra and Faculty of Medicine, University of Coimbra, Portugal), PV Tomazic (Department of ENT, Medical University of Graz, Austria), S Toppila-Salmi (Skin and Allergy Hospital, Helsinki University Hospital, Helsinki, and University of Helsinki, Finland), A Valero (Pneumology and Allergy Department CIBERES and Clinical & Experimental Respiratory Immunoallergy, IDIBAPS, University of Barcelona, Spain), A Valiulis (Vilnius University Institute of Clinical Medicine, Clinic of Children's Diseases, and Institute of Health Sciences, Department of Public Health, Vilnius, Lithuania; European Academy of Paediatrics (EAP/UEMS-SP), Brussels, Belgium), E Valovirta (Department of Lung Diseases and Clinical Immunology, University of Turku and Terveystalo allergy clinic, Turku, Finland), MT Ventura (University of Bari Medical School, Unit of Geriatric Immunoallergology, Bari, Italy), M Wagenmann (Dept of Otorhinolaryngology, Universitätsklinikum Düsseldorf, Germany), D Wallace (Nova Southeastern University, Fort Lauderdale, Florida, USA), S Waserman (Department of Medicine, Clinical Immunology and Allergy, McMaster University, Hamilton, Ontario, Canada), M Wickman (Centre for Clinical Research Sörmland, Uppsala University, Eskilstuna, Sweden), PK Yiallouros (Cyprus International Institute for Environmental & Public Health in Association with Harvard School of Public Health, Cyprus University of Technology, Limassol, Cyprus; Department of Pediatrics, Hospital “Archbishop Makarios III”, Nicosia, Cyprus), A Yorgancioglu (Celal Bayar University Department of Pulmonology, Manisa, Turkey), OM Yusuf (The Allergy and Asthma Institute, Pakistan), HJ Zar (Department of Paediatrics and Child Health, Red Cross Children's, Hospital, and MRC Unit on Child & Adolescent Health, University of Cape Town, Cape Town, South Africa), ME Zernotti (Universidad Católica de Córdoba, Córdoba, Argentina), L Zhang (Department of Otolaryngology Head and Neck Surgery, Beijing TongRen Hospital and Beijing Institute of Otolaryngology, Beijing, China), M Zidarn (University Clinic of Respiratory and Allergic Diseases, Golnik, Slovenia), and T Zuberbier (Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Comprehensive Allergy Center, Department of Dermatology and Allergy, a member of GA2LEN, Berlin, Germany) are members of the ARIA-MASK group. They all participated (i) in the concept of the paper, (ii) the revision of the draft, and (iii) will deploy ARIA in the pharmacy in their own country. ARIA in the pharmacist will be used as a multistakeholder care pathway, and a multidisciplinary group is needed. All authors approved the paper.




