Allergen manufacturing and quality aspects for allergen immunotherapy in Europe and the United States: An analysis from the EAACI AIT Guidelines Project
Abstract
Adequate quality is essential for any medicinal product to be eligible for marketing. Quality includes verification of the identity, content and purity of a medicinal product in combination with a specified production process and its control. Allergen products derived from natural sources require particular considerations to ensure adequate quality. Here, we describe key aspects of the documentation on manufacturing and quality aspects for allergen immunotherapy products in the European Union and the United States. In some key parts, requirements in these areas are harmonized while other fields are regulated separately between both regions. Essential differences are found in the use of Reference Preparations, or the requirement to apply standardized assays for potency determination. As the types of products available are different in specific regions, regulatory guidance for such products may also be available in one specific region only, such as for allergoids in the European Union. Region-specific issues and priorities are a result of this. As allergen products derived from natural sources are inherently variable in their qualitative and quantitative composition, these products present special challenges to balance the variability and ensuring batch-to-batch consistency. Advancements in scientific knowledge on specific allergens and their role in allergic disease will consequentially find representation in future regulatory guidelines.
Abstract

1 INTRODUCTION
Allergens used in the diagnosis and treatment (allergen immunotherapy [AIT]) of type I allergies are biological medicinal products. An essential prerequisite for any detailed consideration of a specific allergen product with regard to safety and efficacy is that these are of sufficient quality, that is with respect to identity, content and purity. For biological products, such quality is highly dependent on the manufacturing process and the capability of this process to produce a consistent product and control its quality accordingly. For the European Union (EU), Directive 2001/83/EC defines a biological substance as a substance that is produced by or extracted from a biological source and that needs for its characterization and the determination of its quality a combination of physicochemical-biological testing, together with the production process and its control.1 In line with this, the manufacturing process is considered to be one of the defining characteristics for a product. Consequently, two products produced from the same source material, but using differing manufacturing processes should be considered to be different products, unless their comparability with regard to their qualitative and quantitative composition has actually been demonstrated. Even potentially small differences in the manufacturing, for example, of the extraction process2-4 may result in considerable differences between products. Sufficient quality of allergen products and consistency of batches are considered to be prerequisites for successful application of these products in clinical practice.5-7 Only when consistency of quality within clinical batches and in comparison with batches to be marketed is assured, can efficacy and safety (to be proven by respective clinical documentation) be expected to be similar. To comply with these requirements, numerous regulations need to be considered and followed by allergen manufacturers. The main aspects addressed in these guidance documents are appropriate control of the manufacturing process, the intermediates and products produced, and appropriate batch-control testing.8 Based on the range of clinical trials performed,9, 10 Europe and the United States (US) may arguably be the most important regions for the gathering of clinical evidence and the subsequent marketing authorization application of candidate AIT products. Here, we provide an overview of how AIT products are regulated with respect to their manufacturing and quality aspects in Europe and the United States. As in both regions there are currently only AIT products available that are derived from natural extracts, we focus on this type of product. While an overview on the regulation of AIT products in different parts of the world11 as well as some key aspects concerning the regulation and standardization of AIT extracts in the EU and United States12 have been previously published, an actual comparison of manufacturing and quality aspects between Europe and the United States has not been described and discussed so far for allergen products.
In recent decades, multiple areas have experienced harmonization between the United States and the EU. For instance, marketing authorization applications are compiled according to a shared format—the Common Technical Document (CTD). An overview on the most important segments with respect to the quality documentation is summarized in Table 1. The respective requirements on the content of these dossiers are mostly harmonized within Europe and guidance developed by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) is applied widely, including in Europe, the United States and Japan. ICH guidelines already cover a wide range of issues, among others stability issues, changes in the manufacturing process, validation of manufacturing processes and test methods, and quality systems (eg, Ref. 13-20). Current good manufacturing practice (cGMP) regulations apply to the manufacturing of drug products and biological products in both the EU and the United States, assuring the identity, strength, quality and purity of drug products by requiring that manufacturers adequately control manufacturing operations. This includes establishing strong quality control management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories.21 However, when it comes to the requirements for the quality documentation of an allergen product to be provided by allergen manufacturers to obtain marketing authorization, there are also diverging approaches between the EU and the United States.
| Data on the manufacturing of the active substance | Data on the manufacturing of the finished product |
|---|---|
| General Information on the active substance | Description and composition of the medicinal product |
| Description of Pharmaceutical Development typically addressed in finished product section |
Pharmaceutical development
|
|
Manufacturing process of the active substance
|
Manufacturing process of the finished product
|
| Control of excipients typically not applicable for the active substance | Control of Excipients |
|
Control of the active substance
|
Control of the finished product
|
| Reference standards or materials | Reference standards or materials |
| Stability of the active substance | Stability of the finished product |
This analysis has been prepared by the European Academy of Allergy and Clinical Immunology's (EAACI) Taskforce on Regulatory Aspects of Allergen Immunotherapy (AIT) and is part of the EAACI AIT Guidelines. Pertaining to these guidelines, we have previously described the general regulatory approaches for AIT products in selected parts of the world.11
2 LEGAL REQUIREMENTS AND GUIDANCE ON MANUFACTURING AND QUALITY ASPECTS IN EUROPE
In Europe, Article 8(3) in accordance with Annex I of the Directive 2001/83/EC represents the basis for the request for quality documentation to be provided for marketing authorization of a medicinal product. As also referenced here, the European Pharmacopoeia (Ph. Eur.), which is a collection of pharmaceutical rules developed by the European Directorate for the Quality of Medicines & Healthcare (EDQM), are applicable and thus mandatory to be followed by manufacturers. The Ph. Eur. includes monographs on specific medicinal products (such as the monograph on allergen products22 or the monograph on parenteralia23) as well as on pharmaceutical test methods. The monograph on allergen products applies to allergen products manufactured from natural sources, thereby excluding allergens derived by recombinant DNA technology and epicutaneous test allergens of chemical origin such as fragrances. It describes acceptance criteria for the source materials, manufacturing requirements and testing of the final product. It states requirements for the purity, identity and potency of allergen products, but also includes specific requests for subtypes of allergen products, for example mandatory potency testing of therapeutic allergens and the requirement of sterility testing for skin prick tests. In addition, the monograph also defines standards for the establishment of an in-house reference preparation (IHRP). In the 1980s, several international collaborative studies developed reference preparations for allergens that have become acknowledged by the World Health Organization (WHO) as International Standards for Allergens. However, these standards inherited a number of shortcomings, for example incomplete information on their preparation and dependence on individual protocols resulting in lack of comparableness of assigned units.24 They are therefore rarely used in practice. As a result, and as long as no internationally harmonized and agreed standards are available allowing comparison of products from different manufacturers, each manufacturer establishes its own IHRP for each respective product, consequentially resulting in labelling of arbitrary in-house units. This IHRP is then used as a standard to compare batches produced for the market (eg, for identity verification or potency testing). As a result of this modus operandi, there are substantial differences in the individual allergen contents of the marketed products.25-28 Due to the different and nonstandardized assays applied, these characteristics are not directly comparable and critically these differences are not evident to the physician or the patient. Although there are only few data available on the effect of differing contents of major allergen on clinical efficacy,29-31 better comparability of these products would be highly desirable.5, 32 The currently ongoing BSP090 project of the EDQM aims at developing standards for the quantification of certain allergens and has already developed European Pharmacopeia reference standards for the major grass allergen Phl p 5 and the major birch allergen Bet v 133, 34 as well as validated methods for the quantification of Bet v 1.35 In addition to the aforementioned monograph on allergen products, recently, several monographs on allergen source materials have been included in the Ph. Eur., each covering a separate group of allergens.36-40 It should be noted that, although defining a common set of standards for the source materials, these monographs allow much flexibility in the choice of methods used for characterization. It therefore remains within the responsibility of the manufacturers to choose and justify those methods most suited to reliably characterize respective source materials.
Furthermore, guidelines developed by different parties, such as working groups of the European Medicines Agency (EMA), the WHO and ICH, form an extensive collection of documents giving guidance on various aspects of the manufacturing of medicinal products, including allergen products. Concerning the quality and manufacturing issues of allergen products, the “Guideline on Allergen Products: Production and Quality Issues”41 developed by EMA's Committee for Medicinal Products for Human Use (CHMP) and the associated Biologics Working Party (BWP) is the most important allergen-specific guidance available. Drafted in 2008, it gives the most up-to-date overview of the requirements to be considered for allergen manufacturing and controls thereof. In contrast to the aforementioned monograph on allergen products, it covers the manufacturing of allergens from both natural as well as biotechnological sources. The guideline details aspects of manufacturing and process controls for various stages of the manufacturing process, including specific considerations on the source materials, the drug substance and the drug product. One crucial aspect presented in this guideline is the concept of homologous groups. According to this concept, allergens from related species can be integrated in a homologous group.42 Overall, the guideline proposes six different homologous groups (for trees [Fagales group, Oleaceae group, Cupressaceae group], grass and cereal pollen, weeds and mites). These groups may be extended and new groups may be formed if certain criteria are fulfilled (Table 2). Within a homologous group, extrapolation of data on clinical efficacy and safety as well as limited data on quality aspects (such as process validation and stability studies) from a representative species to other species of the same group is accepted by regulatory bodies.
| Criteria for grouping as homologous groups | Data extrapolation allowed | Homologous groups as accepted in Ref. (41) |
|---|---|---|
| Comparable physicochemical and biological properties of the source material | Stability data | Birch group (Alder, Hornbeam, Hazel, Oak, Sweet Chestnut, Beech) |
| Cross-reactivity/Structural homology of the allergens | Manufacturing process validation | Oleaceae group (Olive, Ash, Privet, Lilac) |
| Identical formulation of the finished product | Efficacy | Cupressaceae group (Cedar, Cypress) |
| Identical production process of the allergen extract and of the finished product | Safety | Sweet grasses (Sweet vernal grass, Oat, Cocksfoot, Meadow fescue, Velvet grass, Barley, Perennial ryegrass, Timothy grass, Kentucky bluegrass, Cultivated rye, Cultivated wheat) |
| Weed pollen (Ragweed, Mugwort, Pellitory) | ||
| House dust mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae) |
In addition, the guideline specifies requirements for different types of allergen products produced, for example considerations on the demonstration of stability as well as laying out a framework for characterization of allergoids.
2.1 Characterization of allergoids in the EU
Allergoids are allergens that are chemically modified (eg, by treatment with glutaraldehyde or carbamylation) to reduce IgE activity43 and are commonly available on the European market. Many of these are additionally adsorbed to carriers such as aluminium hydroxide. The characterization of this group of products is especially challenging as, due to the resulting cross-linking, various critical tests cannot be performed after the chemical modification. Therefore, specific tests such as determination of total allergenic activity are not performed at the drug product stage but earlier in the manufacturing process, for example at the stage of the native allergen extract. As a result, the manufacturer must take additional measures (eg, by setting appropriate in-process limits) to ensure that the potency of the final drug product still remains within a controlled range even though it cannot be further determined after modification. For certain modifications, the identity of the drug product cannot be confirmed by common test methods (eg, protein profile by SDS-PAGE) that are typically applied to native extracts. Nevertheless, manufacturers must monitor several quality attributes of these products, including the efficacy and stability of the adsorption, for example by determining the total soluble protein and/or the presence of IgE-binding components in the supernatant. With the latest version of the EMA Guideline on the quality of allergen products,41 additional requirements were introduced to allow further in-depth characterization. Test methods have to be established to identify the relevant allergens in the modified form. Furthermore, potency tests are required that allow discrimination between native and modified molecules. These tests should permit a quantitative conclusion on the content of the active substance after modification and should be specific to confirm the identity of the modified drug substance. An in-depth review on qualitative aspects concerning allergoids can be found in a recent review by Zimmer et al.44
2.2 Legal requirements and guidance on manufacturing and quality aspects in the United States
In the United States, the basis for manufacturing and quality requirements for allergen products are US laws (Acts) and Federal Regulations. Allergen products are regulated by the US Food and Drug Administration (FDA) as biological products (biological medicinal products) under the Public Health Service Act (PHS Act) and related amendments; and as drug products under the Federal Food, Drug and Cosmetics Act (FD&C Act) and related amendments. Additional laws may contain important provisions for regulation of biological products and drug products, but the PHS Act and FD&C Act and their related amendments are the primary laws under which biological products are regulated. The US Congress has the sole authority to enact federal laws in the US. Laws authorize or require that federal agencies develop regulations through a public rule-making process that include details necessary for implementation. Federal Regulations, which have the force of law, specify manufacturing and quality requirements for drug products, biological products, medical devices and foods. Federal regulations for FDA-regulated products are located in Title 21 of the Code of Federal Regulations (21 CFR). US laws and regulations provide the critical legal framework for regulation of allergen products and are designed to ensure that allergen products meet manufacturing and quality standards and are safe, pure and potent. Additionally, the FDA has developed guidance documents that describe FDA's current thinking on a range of regulatory topics including manufacturing and product quality. FDA guidance documents are not legally binding on FDA or the public and allow for use of alternative approaches provided they satisfy the requirements of the applicable laws and regulations. Finally, as stated above, several ICH guidance documents are also applicable to allergen products and provide a harmonized approach on certain manufacturing and quality-related issues.
With some exceptions, manufacturing and quality requirements for allergen products are similar to those for other biological products or drugs. Generally, manufacturing and quality standards do not differ between allergen products approved for diagnosis or therapy. Food and Drug Administration's main regulatory standard for ensuring product quality is the cGMP regulations for finished pharmaceuticals, located at 21 CFR parts 210 and 211. The regulations contain the minimum cGMP standards for methods to be used in, and the facilities or controls to be used for, the manufacture, processing, packing or holding of a drug. Application of cGMPs is intended to assure that a drug meets the requirements of the FD&C Act as to safety, and the drug has the identity and strength and meets the quality and purity characteristics that it purports or is represented to possess. Additional cGMP requirements for biologics are located at 21 CFR parts 600-680, and specific requirements for allergenic products are located at 21 CFR part 680. Although the cGMP regulations at 21 CFR parts 210 and 211 specifically refer to finished pharmaceuticals, compliance with general principles of cGMPs is also required under the FD&C Act. FDA guidance documents inform applicants on approaches to complying with cGMPs. Examples of guidance document topics include process validation principles and practices,45 sterile drug products produced by aseptic processing,46 analytical procedures and methods validation for drugs and biologics,47 and quality systems approach to cGMP regulations.48 Harmonization has been achieved in several areas relevant to cGMP and quality systems that are applicable to allergen products, including ICH guidance documents for stability studies,14 methods validation16 and quality systems.17-20
As indicated above, cGMP requirements for allergen source materials and allergen products are located at 21 CFR part 680. Allergen source material regulations include testing and quality requirements for pollen, mould, mammalian and avian source materials (21 CFR 680.1). Basic requirements for allergen product manufacturing include limits on extraneous allergenic substances during the manufacturing process and in culture media used in production (21 CFR 680.1). Specific testing requirements include identity and potency (21 CFR 680.3). The potency test regulation (21 CFR 680.3[e]) is specifically related to sterile injectable allergen extracts derived from natural biological source materials that are currently licensed for diagnosis and treatment of type I allergies. Currently licensed allergen extracts are identified as standardized or nonstandardized. The potency test regulation requires that the potency of each lot of allergenic extract be determined according to a potency test method that measures the allergenic activity of the product. Historically, in addition to development of a suitable potency test, FDA develops and maintains US reference standards and serum pools used by licensed manufacturers for final drug product release testing of each lot of standardized extract and for routine stability studies. Standardization ensures that standardized allergen extracts have a consistent measure of potency across manufacturers. The potency test regulation further specifies that licensed manufacturers may continue to use nonstandardized units until notified by FDA of the existence of a potency test. Accordingly, nonstandardized extracts are labelled in units of weight/volume (w/v) or protein nitrogen units (PNUs) and are exempt from stability testing (21 CFR 211.166[d]) and identity testing (21 CFR 680.3[a]). The approach to standardization and designation of allergenic extracts as standardized or nonstandardized allergenic extracts is applicable to currently licensed extracts. This approach may not apply to future extracts or new types of products such as peptides or recombinant products. FDA assesses each product during the development and licensure phase to determine the applicable potency unitage.
Additional regulations under the General Biological Products Standards (21 CFR Part 610) also apply to allergenic extracts. These regulations include requirements for lot release, sterility testing, constituent materials (ingredients and preservative content) and cultures used in manufacturing. In addition to specific regulatory requirements, FDA's Guidance for Submission of Chemistry, Manufacturing and Controls Information for Allergenic Products49 details additional considerations for source materials and manufacturing and process controls for the drug substance and the drug product.
2.3 Differences in regulatory approaches between the EU and the United States
The EMA Guideline on Allergen Products: Production and Quality Issues41 delineates specific approaches to the use of IHRPs and homologous groups that are different from US approaches. For US standardized allergen extracts, a national reference standardized to maintain consistent potency among US manufacturers. FDA permits the use of IHRPs properly calibrated to US reference standards for standardized extracts although this approach is rarely used. For nonstandardized extracts, IHRPs are not used because:
- As nonstandardized extracts are explicitly exempted by regulation from potency testing and stability studies, there is no inherently obvious role for use of IHRPs, and
- The proprietary biological unitage associated with IHRP use would raise significant concerns in the United States about potentially increasing the variability that already exists among nonstandardized extracts.
With respect to homologous groups, the EMA guideline formalizes an approach to homologous groups of allergen species, based on manufacturing similarities and cross-reactivity among allergen species from a taxonomic group. Proposed groups (see above) are based on existing data, although manufacturers may propose additional groupings from new data. According to EMA guidance,41, 50 data may be extrapolated from one member of a homologous group to others in support of product quality and even clinical claims. Although FDA guidance documents have not delineated similar regulatory treatment of allergen extracts with either cross-reactive or otherwise similar source materials, the FDA has applied comparable logic in its analyses of nonstandardized allergen extracts,51 and in a recent Biologics License Application (BLA) approval for a sublingual tablet formulation for which the data supported a broader indication for the product.
The EMA guideline also provides specific requirements for distinct types of allergen products such as recombinant allergens or synthetic peptides, or novel classes of active substances such as allergoids or conjugates. These types of allergen products are not currently licensed in the United States. Regardless of allergen product type, FDA reviewers work with Investigational New Drug (IND) Sponsors and BLA Applicants to establish the safety and efficacy of the product, and to ensure that the manufacturing process is properly designed and validated; quality is built into the process; test methods are suitable for their intended use and provide adequate control of the manufacturing process and the product; conformance to cGMPs is established; and consistency with clinical lots is demonstrated.
In the United States, allergen products in transdermal patch delivery systems and prefilled syringes are considered combination products under the FD&C Act and the regulations at 21 CFR Part 3.52 Applicants seeking licensure for such products must meet applicable regulatory requirements for combination products during the clinical development and licensing phases as well as the postapproval phase. Certain FDA guidance documents for combination products and transdermal drug delivery systems may apply to allergen products.53, 54 An overview on key similarities and differences in the regulation of allergen manufacturing and quality control between the EU and the United States is depicted in Table 3.
| Key similarities | Key differences |
|---|---|
|
|
|
- Approaches may differ to some extent for different types of products (eg, subcutaneous vs sublingual products).
2.4 Variability in the product characteristics of AIT products
Production and control of allergen products derived from natural extracts can prove to be a considerable challenge. The complexity of the source material and the final product is high, as it is composed not only of a mixture of allergenic proteins, but also of nonallergenic proteins as well as other nonallergenic compounds. There is high variability in the source material itself resulting in final products that mirror this variability. This is also reflected by the comparably wide acceptance criteria allowed by the European Pharmacopoeia for the testing of allergen products.22
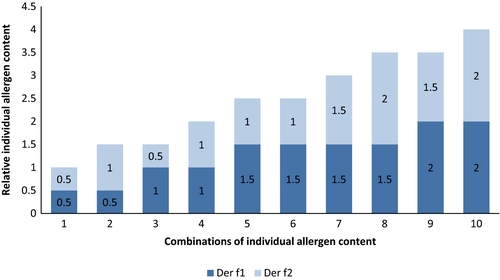
For example, the potency as determined by the total allergenic activity may vary between 50% and 150% of a stated amount.22 This wider limit is required given the variability between batches of starting material but also to accommodate for the inherent variability of the (mostly immunochemical) potency assays. Accordingly, a batch produced at a total allergenic activity of 50% would be in line with a given specification and, presuming that all other specifications are fulfilled as well, could be marketed. The same would be true for a batch at the higher end of the specification (eg, at 150% of a stated amount). In consequence, substantial variation with regard to the potency of an allergen product is possible where a new batch is being applied to a patient. However, it should be noted that a product must comply with such specifications over its whole shelf life, which implies that given some expected decrease over time, the levels at release should not be at the lowest specification level. Similar to the determination of total allergenic activity, such circumstances are observed for additional parameters such as the determination of the total protein content (for therapy allergens, a range of 50%-150% is allowed) and the determination of individual allergens, where a range of 50%-200% is allowed. Figure 1 illustrates the variation that would be possible for a hypothetical product in regard to the content of individual allergens. However, applicants must justify a chosen specification and it may be required to tighten such specification beyond the limits requested by the Ph. Eur.22 Monographs are considered to demand minimum requirements. Furthermore, there are also specific in-process controls (IPCs; eg, protein content and allergenic activity) that are typically applied and monitored during manufacturing. Trend analysis of such IPCs in the manufacturing process is applied to prevent that the variability in the starting material leads to extensive batch-to-batch variation in potency. It therefore is a balancing act of allowing some variation to account for this while restricting variation to an acceptable degree to secure batch-to-batch consistency.
Even with such comparably wide ranges allowed, the natural origin of the source materials of AIT products can result in considerable difficulties. As it is essential that a medicinal product can be produced consistently and its qualitative and quantitative composition remains comparable and consistent throughout its life cycle, a manufacturer must ensure that the manufacturing process is flexible enough to balance variances and trends in the composition of the source material and that such trends are recognized as early as possible. For example, it has been reported that the allergenic composition of pollen is changing as a long-term trend, possibly due to climate change.55 As it is not possible to simply change the strength or composition of a product due to such observations, for example with respect to the overall allergenic activity, the process must assure that the product can still be produced according to the given quality characteristics and in assurance of batch-to-batch consistency.
2.5 Future perspectives
Despite much progress achieved in harmonization, there remain considerable differences in the in-depth quality and manufacturing requirements between the United States and the EU. Region-specific issues and priorities are a result of this. There appears to be consensus in Europe that updating of existing regulations may be needed here to account for the changes in the landscape of available allergen products observed in this region. Scientific guidance and legislation may need to differentiate more between certain types of allergen products. For example, in the EU, current guidance applicable to allergen products only scarcely considers the impact of allergy prevalence. In the Guideline on Allergen Products: Production and Quality Issues,41 some initial considerations are presented for products where certain tests may not be applicable (eg, “because a sufficient number of patients is not available to create an appropriate sera pool”). Staggered requirements for quality and/or clinical data in dependence of the prevalence of a specific allergy may be a path forward to support the market availability of a wider array of products while, at the same time, securing the quality, efficacy and safety of these products as far as possible.
Finally, a comprehensive characterization of AIT products with regard to quality aspects remains essential. The knowledge on which the relevant allergens for specific types of allergies are is rapidly increasing, as has been shown for Bet v 1 in birch pollen allergic patients.56, 57 Accordingly, current guidelines already implemented the request on the definition and verification of the presence of relevant allergens for each product.22, 41 Another example concerns hymenoptera AIT where it has been suggested that minor allergens are critically important in the probability of success of the treatment for some patients.58 Accordingly, choosing products that reliably contain such critical compounds for specific patients may become essential. As analytical procedures evolve and allow more precise characterizations of allergen products, it is safe to predict that the regulatory framework will follow these developments with some follow-up time. In line with this and considering that allergens are the defining elements for the efficacy of these products, demands on their characterization and verification with regard to identity and quantity on a routine basis in these products will likely intensify in the future.
DISCLAIMER
The views expressed in this review are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the respective national competent authorities, the European Medicines Agency or one of its committees or working parties.
AUTHOR CONTRIBUTIONS
This document was drafted by Bonertz A, Slater J, Bridgewater J, Rabin R, Hoefnagel M, Timon M, Pini C and Vieths S. It was revised following critical review by Roberts G, Pfaar O, Sheikh A, Ryan D and then by all the co-authors. The EAACI task force developing the manuscript was chaired by Vieths S. Coordination of authors’ contributions was done by Bonertz A. This study is part of the EAACI AIT guidelines project, chaired by Muraro A and coordinated by Roberts G.
CONFLICTS OF INTEREST
G Roberts reports a patent for the use of sublingual immunotherapy to prevent the development of allergy in at risk infants issued. His university has received payments for giving expert advice to ALK, presenting at company symposia for ALK, Allergen Therapeutics and Meda plus as a member of an Independent Data Monitoring Committee for Merck. C Pini reports being married to a person employed at Merck Serono Italy (pharmaceutical company not involved in the production of allergens but belonging to the same group holding Allergopharma). O Pfaar reports grants and personal fees from ALK-Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL Allergy Holding B.V./HAL Allergie GmbH, grants and personal fees from Bencard Allergie GmbH/Allergy Therapeutics, grants and personal fees from Lofarma, grants from Biomay, grants from Nuvo, grants from Circassia, grants and personal fees from Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, personal fees from Novartis Pharma, personal fees from MEDA Pharma, grants and personal fees from Anergis S.A., personal fees from Sanofi US Services, personal fees from Mobile Chamber Experts (a GA2LEN Partner), personal fees from Pohl-Boskamp, personal fees from Indoor Biotechnologies, outside the submitted work. C Akdis reports grants from Actellion, grants from EU FP 7 Projects Medall and Predicta, grants from Allergopharma, grants from Swiss National Science Foundation, grants from Christine Kühne Center for Allergy Research and Education, outside the submitted work; L Poulsen reports being Vice President Congresses and Executive Committee Member of European Academy of Allergy and Clinical Immunology. R van Ree reports consultancy and speaker fees for HAL Allergy BV, consultancy for Citeq BV and speaker fees for ThermoFisher Scientific. Funding from EU FP7, Dutch Science Foundation and HESI-ILSI; D Barber Hernandez reports grants and personal fees from ALK, personal fees from Aimmune, outside the submitted work; O Palomares has received fees for giving scientific lectures from: Allergic Therapeutics, Amgen, AstraZenenca, Inmunotek S.L, Novartis and Stallergenes. Oscar Palomares has participated in advisory boards from Novartis and Sanofi Genzyme. O Palomares received research grants from Inmunotek S.L. under public collaborative projects from Spanish Ministry (MINECO)/CDTI: IPT-2012-0639-090000, IDI-20110410 and IDI-20141131 and from MINECO, SAF2014-52706-R. D. Ryan reports personal fees from Stallergenes, Thermo Fisher, MEDA outside of the submitted work. L Klimek reports grants and personal fees from ALK-Abelló, personal fees from MEDA, Sweden, grants and personal fees from Novartis, Switzerland, grants and personal fees from Allergopharma, Germany, grants and personal fees from Bionorica, Germany, personal fees from Boehringer Ingelheim, Germany, grants and personal fees from GSK, Great Britain, grants and personal fees from Lofarma, Italy, grants from Biomay, Austria, grants from HAL, Netherlands, grants from LETI, Spain, grants from Roxall, Germany, grants from Bencard, Great Britain, outside the submitted work; E Angier reports being Secretary of Primary Care Interest Group EAACI. ALK conference SOSA meeting 2015. Previous paid advisory board one each for MEDA 2012, Stallergenes, 2012, Schering Plough 2009 and one paid lecture by MEDA; T Casale reports grants and speaker fees from Stallergenes, outside the submitted work; M Fernandez-Rivas reports grants from European Union, grants from Instituto de Salud Carlos III, Ministerio de Ciencia, España, grants from Ministerio de Economia, España, personal fees from DBV, personal fees from Aimmune, Reacta Biotech, Schreiber foods, personal fees from ALK Abello, Merck, GSK, Allergy Therapeutics, non-financial support from EAACI, personal fees and non-financial support from Fundación SEAIC, other from Hospital Clínico San Carlos, and Universidad Complutense de Madrid. España , outside the submitted work; In addition, Dr. Fernandez Rivas has a patent PT0042/2013 issued; S Halken reports personal fees from ALK-Abelló, personal fees from Different companies e.g. MEDA, Stallergenes, Allergopharma and ALK-Abelló, from null, outside the submitted work; G Sturm reports grants from ALK Abello, personal fees from Novartis, personal fees from Bencard, personal fees from Stallergens, outside the submitted work; M Jutel reports personal fees from Allergopharma, Anergis, Stallergenes, ALK, LETI outside the submitted work. S Lau reports project related payment to Charité Medical University by Symbiopharm and Allergopharma. Honorarium by Symbiopharm and DBV. Drug Monitoring Committee Merck. E-M Varga reports lecture fees from ALK-Abello, Stallergenes; The other authors have no conflict of interest to disclose.




