AllergoOncology: Opposite outcomes of immune tolerance in allergy and cancer
Abstract
While desired for the cure of allergy, regulatory immune cell subsets and nonclassical Th2-biased inflammatory mediators in the tumour microenvironment can contribute to immune suppression and escape of tumours from immunological detection and clearance. A key aim in the cancer field is therefore to design interventions that can break immunological tolerance and halt cancer progression, whereas on the contrary allergen immunotherapy exactly aims to induce tolerance. In this position paper, we review insights on immune tolerance derived from allergy and from cancer inflammation, focusing on what is known about the roles of key immune cells and mediators. We propose that research in the field of AllergoOncology that aims to delineate these immunological mechanisms with juxtaposed clinical consequences in allergy and cancer may point to novel avenues for therapeutic interventions that stand to benefit both disciplines.
Abstract

Abbreviations
-
- AhR
-
- arylhydrocarbon receptor
-
- AIT
-
- allergen immunotherapy
-
- APRIL
-
- a proliferation-inducing ligand
-
- B-ALL
-
- B-cell acute lymphoblastic leukaemia
-
- Bregs
-
- B regulatory cells
-
- CCL1
-
- CC-chemokine ligand 1
-
- cHL
-
- classical Hodgkin's lymphoma
-
- COPD
-
- chronic obstructive pulmonary disease
-
- CTLA-4
-
- cytotoxic T-lymphocyte-associated protein 4
-
- CTL
-
- cytotoxic T cell
-
- DCs
-
- dendritic cells
-
- EGFR
-
- epidermal growth factor receptor
-
- FcγR
-
- Fc gamma receptor
-
- FLC
-
- free light chains
-
- GM-CSF
-
- granulocyte-macrophage-colony-stimulating factor
-
- i.v.
-
- intravenous
-
- IFNγ
-
- Interferon gamma
-
- Ig
-
- immunoglobulin
-
- ILCs
-
- innate lymphoid cells
-
- IL
-
- interleukin
-
- LCN2
-
- lipocalin-2 (human)
-
- MCP-1
-
- macrophage chemoattractant protein 1
-
- MDSC
-
- myeloid-derived suppressor cells
-
- MHC
-
- major histocompatibility complex
-
- MMP9
-
- matrix-metallopeptidase 9
-
- NGF
-
- nerve growth factor
-
- NSCLC
-
- non-small-cell lung cancer
-
- PAR
-
- protease-activated receptor
-
- PBMC
-
- peripheral blood mononuclear cells
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L
-
- programmed cell death ligand
-
- RCC
-
- renal cell carcinoma
-
- RORγt
-
- RAR-related orphan receptor gamma t
-
- ROS
-
- reactive oxygen species
-
- SCF
-
- stem cell factor
-
- SIT
-
- subcutaneous AIT
-
- TGFβ
-
- transforming growth factor-beta
-
- Th
-
- T helper
-
- TiBCs
-
- tumour-infiltrating B cells
-
- TLSs
-
- tertiary lymphoid structures
-
- TNFα
-
- tumour necrosis factor-alpha
-
- Tregs
-
- T regulatory cells
-
- TSLP
-
- thymic stromal lymphopoietin
-
- VEGF
-
- vascular endothelial growth factor
1 INTRODUCTION: INSIGHTS INTO IMMUNE TOLERANCE IN ALLERGY AND CANCER
The immune system strives to strike a fine balance to maintain homoeostasis. Therefore, after elimination of pathogens an immune regulation phase follows, facilitated by checkpoint molecules, CD4+ CD25+ FoxP3+ natural regulatory T cells (Tregs),1 regulatory subsets of B cells (Bregs) and dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs) and M2 macrophages, secreting immunomodulatory mediators such as IL-10 and transforming growth factor-β (TGFβ).2
In allergy, the immune system overreacts to innocuous allergens, mounting Th2 responses and B-cell class switching to IgE driven by cytokines such as IL-4 and IL-13. Allergic responses are further amplified by a failure to adequately launch immunomodulatory mechanisms. Allergen immunotherapy (AIT) may re-establish tolerance involving Tregs, IL-10 and TGFβ, and class switching to anti-inflammatory IgG4 and IgA.3
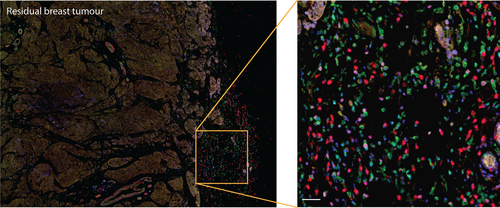
While in incipient cancer innate and adaptive immune responses may clear some tumour cells,4 cancer-initiating cells (CiC) soon instruct infiltrating immune effector cells to adapt regulatory phenotypes.5 Such active immune suppression functions are accompanied by tumour-supported accumulation of Tregs, B cells, DCs and M2 macrophages in its microenvironment, which may contribute immunoregulatory mediators such as IL-10 or VEGF, and B cells may express alternative Th2-biased IgG4 and IgA. Tregs have a metabolic advantage via FoxP3 expression in low-glucose, lactate-rich tumour environments.6 Cancer cells themselves are a prominent source of IL-10 providing “metastatic competence”.7 Hence, while immune infiltrates are abundant in many solid tumours (Figure 1), the tumour microenvironment may be instrumental in maintaining suppression and preventing immune clearance. Checkpoint inhibitor antibodies in clinical oncology act by directly targeting T-cell tolerance mechanisms with the aim of overcoming tumour-associated T-cell suppression. We here extract common features in the functions of immune modulation in allergy and cancer within the scope of AllergoOncology.
2 THERAPEUTIC INTERVENTIONS TO CONTROL IMMUNE TOLERANCE: TRANSLATIONAL INSIGHTS IN ALLERGY AND CANCER
2.1 Tolerance induction with allergen immunotherapy
As part of tolerance induction, AIT has been correlated with enhanced regulatory T- and B-cell responses,3, 8 and regulatory DCs,9 all potential sources of the immunomodulatory cytokine IL-10. A classical hallmark of AIT is the induction of allergen-specific IgG1 and anti-inflammatory IgG4. Allergen-specific IgGs may (i) act as blocking antibodies, trapping allergens before they can cross-link allergen-specific IgE; (ii) interact with inhibitory IgG receptor FcγRIIb and downregulate IgE-mediated signalling10; or (iii) promote differentiation of tolerogenic M2b macrophages.11 While investigation into immune tolerance in allergy provided some insights, it is unclear which of these mechanisms may drive tolerance or which specific biomarkers could be used to predict clinical response to AIT.12
2.2 The opposite side of the coin: clues into breaking tolerance in cancer
Tumour cells and tumour-infiltrating immune cells can help to shape a strong tolerogenic microenvironment that promotes tumour growth and allows metastatic spread. The question of how to break tumour tolerance has recently become more pertinent, catalysed by the clinical efficacy of checkpoint inhibitors in some tumour types, including melanoma.
Two checkpoint molecules, the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and the programmed cell death protein 1 (PD-1), play key roles in restricting T-cell activation and inducing immunological tolerance that contribute to immunosuppressive environments in cancer by multiple mechanisms. They restrict recruitment, activation and proliferation of cytotoxic CD8+ T cells,13 and their effects are amplified by tumour cells expressing CTLA-414 or PD-1 ligand (PD-1L).15 These findings opened new avenues for intervention by so-called checkpoint inhibitors.
The anti-CTLA-4 monoclonal ipilimumab was the first clinically applied antibody that conferred tangible improvements in the treatment of metastatic melanoma,16 with long-term survivors after 3 and 5 years.17 The anti-PD-1 inhibitor antibodies, nivolumab and pembrolizumab, also approved for the treatment of malignant melanoma, also demonstrated significant progression-free and overall survival benefits for patients with advanced melanoma. Toxic side-effects of checkpoint inhibitors in human patients include severe autoimmunity,18 underlining the dangers when interfering in immune regulation. Clinical trials in other malignant diseases are performed with anti-PD-1 antibodies nivolumab and pembrolizumab based on better efficacy and less toxicities compared with ipilimumab. Nivolumab is approved for metastatic non-small-cell lung cancer (NSCLC), renal cell carcinoma (RCC) and classical Hodgkin lymphoma (cHL)19; pembrolizumab for NSCLC, head and neck squamous cell carcinoma (HNSCC) and classical Hodgkin lymphoma (cHL).20 Furthermore, anti-PD-L1 (PD-ligand1) antibody avelumab is applied for the treatment of NSCLC,21 and others such as atezolizumab and durvalumab22 are under clinical investigation.
OncoImmunology (alias ImmunoOncology) explores host immune responses to tumours for uncovering insights that can lead to novel treatments.23 Similarly, the study of Th2-type immune responses in cancer in the emerging field of AllergoOncology24-26 may also uncover new approaches for future treatment interventions.
3 CELLULAR PLAYERS IN IMMUNE TOLERANCE IN ALLERGY AND CANCER (SEE OVERVIEW Table 1)
| Section | Cell type | Proposed roles in allergy | Proposed functions in cancer |
|---|---|---|---|
| 3.1 | Dendritic cells | Support conversion of T cells into Tregs | Depending on differentiation; Support conversion of T cells into immunosuppressive Tregs, promoting cancer progression |
| 3.2 | Macrophages |
M2a macrophages support allergic diseases M2b have immunoregulatory functions |
Depending on subtype; M1 macrophages support survival Low M1/M2 ratios associated with poor survival M2b associated with tolerogenic tumour microenvironment |
| 3.3 | Tregs |
Source of IL-10, supported by IL-10 Foster IgG4 production and suppress allergies |
Accumulate in cancer tissue Correlate with disease progression |
| 3.4 | Bregs |
Source and recipient of IL-10 Origin of IgG4 in allergen immunotherapy |
Source and recipient of IL-10 origin of IgG4 in cancer tissues, where they correlate with disease severity and poorer outcomes |
| 3.5 | Innate lymphoid cells (ILCs) | Source of Th2 cytokines and thus involved in initiation of allergy | Depending on subtype; ILC2 may in a tumour provide an immunosuppressive environment, like ILC3s which support IL-10 secretion |
| 3.6 | Mast cells | Major effector cells in allergy and source of IL-4 |
Controversial; Can engender pro- or antitumoral functions, depending on microlocalization and type of tumour A source of TNFα; Can express PD-L1 or PD-L2 and inhibit effector T cells. |
| 3.7 | Eosinophils | Involved in chronic allergic and atopic conditions and associated with curative processes | Controversial; Attracted by CCL1 in tumour; Can be pro- (Hodgkin′s lymphoma) or antitumorigenic (in solid tumours); May sense tumour cells in innate manner and kill in concert with Igs; May induce Tregs |
| 3.8 | Epithelial cells | Contribute to site-specific tolerance induction, mainly via DC interactions; Epithelial barrier disruption stimulates allergen entry and epithelial activation; Source of TSLP and IL-33, supporting Th2 responses | Source of immunosuppressive microvesicles, with a potential role in cancer promotion |
3.1 Dendritic cells
DCs are professional antigen-presenting cells with the unique ability to induce differentiation of naïve T cells into tumour antigen–specific or allergen-specific T helper cells or CTLs. Maturation, upregulation of costimulatory molecules (CD40, CD80, CD86) and secretion of pro-inflammatory cytokines (IL-1β, IL-12, IL-6, TNFα) are required for successful proliferative T-cell responses following DC-mediated antigen presentation.
However, DCs also exhibit regulatory functions. IL-10-treated DCs derived from atopic donors suppress the allergen-driven Th2-specific response or convert T cells into Tregs.27 In particular in the tumour microenvironment, myeloid DCs are converted into tolerogenic phenotypes promoting Tregs rather than T-effector cells as an escape mechanism from immune clearance,28 supported by TGFβ and IL-10.29 Tolerogenic DCs show low expression of costimulatory molecules, secrete low levels of IL-12 and produce IL-10. Therefore, while useful tolerance in allergen immunotherapy, antigen presentation by tolerizing DCs is harmful in cancer as it can prevent antitumour T-cell responses.
The immunosuppressive features of DCs may be overwritten by inflammatory signals. IgE-mediated antigen sampling is a particularly potent mechanism to activate primary T-cell responses,30 not inducing IL-10, and dampening LPS-induced IL-12 production31; irrespectively, it activates CTLs via cross-presentation and improves antitumour responses.30 Therefore, the current literature implies that IgE-mediated antigen presentation supports DC-based immunity rather than leading to DC-mediated tolerance.
3.2 Macrophages
The different functional phenotypes of macrophages depend on signals from the surrounding environment. A general classification mirroring the Th1/Th2 paradigm of classically pro-inflammatory (M1) and alternatively anti-inflammatory (M2) macrophages is broadly accepted.
Pro-inflammatory M1 cells are activated by mainly IFNγ, LPS and other TLR ligands. M1 were observed in exacerbation of lung injury and airway remodelling in allergic asthma via nitric oxide production.32 The presence of M1 macrophages in the tumour microenvironment has been associated with extended survival of cancer patients.
M2 macrophages comprise different activation phenotypes: M2a, M2b and M2c. M2a are triggered by IL-4 and IL-13 and positively correlate with the severity of airway inflammation in allergic asthma.33 In cancer, a low M1/M2a ratio was associated with poor prognosis in a variety of murine and human malignancies.
M2b and M2c are involved in immune regulation, tissue remodelling, angiogenesis and tumour progression. M2b are induced by IgG immunoglobulin complexes and LPS and are reported in the context of allergy as well as cancer.34 The inhibitory IgG receptor FcγRIIb (CD32) is a critical player for signal transduction and upregulation of IL-10 and CCL1 expression, while reducing IL-12. CCL1 secretion is critical to maintain the M2b phenotype in mice and humans.35 M2c are induced by glucocorticoids, TGFβ and IL-10, and support induction of Tregs,36 which in tumours correlates with disease progression and poor prognosis.
Immune complexes with an antitumour IgE antibody or cross-linking of surface-bound IgE can polarize monocytes and macrophages to upregulate CD80 and the pro-inflammatory mediator TNFα. TNFα can then stimulate production of the macrophage chemoattractant protein 1 (MCP-1) by both monocytes and tumour cells, and trigger recruitment of macrophages into tumour lesions and restriction of tumour growth.37 This macrophage TNFα/MCP-1 signalling has been reported in IgE-mediated clearance of parasites by macrophages and brings an additional dimension to the functions of IgE antibodies.38
Based on these multifaceted phenotypes of macrophages, blocking M1 pro-inflammatory or M2 anti-inflammatory macrophages may lead to opposite effects in cancer and allergy. Targeting macrophage functions with tumour antigen-specific IgE antibodies or vaccines that trigger IgE responses against cancer would require careful examination.
3.3 Tregs
The main mechanisms underpinning Treg immune effects include production of inhibitory cytokines (IL-10, TGFβ and IL-35), effector cell cytolysis (via secretion of granzymes A and B), direct targeting of DCs via the inhibitory PD-1 and CTLA-4 cell surface checkpoint molecules and metabolic disruption of effector cells (CD25, cAMP, adenosine, CD39 and CD73).39 FoxP3+-induced Tregs (iTregs) are mandatory, while thymic-derived natural (FoxP3+) nTregs are dispensable for oral tolerance. In addition, iTreg cells rather than nTreg cells are involved in the control of mucosal Th2 responses.40
In contrast in cancer patients, Tregs contribute to an immunosuppressive tumour microenvironment. Accumulation of Tregs in tumours can occur through their recruitment via chemokines (eg, CCL-17, CCL-22, CCL-5, CCL-28), while their expansion in situ or conversion of conventional T cells can be promoted by high local levels of cytokines, such as TGFβ.41 Specifically, the smoke compound acrolein promotes regulatory cells via the arylhydrocarbon receptor (AhR), thereby increasing malignant growth, while decreasing the risk for allergic sensitization.42 High numbers of infiltrating Tregs are associated with poor prognosis in many cancers, including ovarian, pancreatic, lung cancer, glioblastoma or melanoma.41 Therefore, cancer immunotherapy targeting Tregs while breaking tumour tolerance can also break tolerance to self,43 highlighting the fine balance between antitumour immune activation and the risk of autoimmunity.
3.4 B cells and Bregs
B cells play key roles in inflammation, antigen presentation and antibody production in the context of allergy. Their contributions in cancer are less well delineated.
Tumour-infiltrating B cells (TiBCs) have been detected in human solid malignancies, including melanoma,44, 45 breast, ovarian, cervical, colorectal and non-small-cell lung cancer. As in allergy,46 TiBCs show evidence of antigen-driven expansion and somatic hypermutation, implying active in situ antibody affinity maturation in the cancer tissue.45 The presence of TiBCs, and B cells in tertiary lymphoid structures (TLSs), is associated with improved prognosis in different cancer types. Increased survival has been observed when CD8+ cells are also detected in the same tumours, suggesting synergies between T and B cells and induction of adaptive immune response. TiBCs may mediate immune responses against tumours by several mechanisms: (i) TiBC-derived antibody activities, (ii) direct cytotoxicity by B-cell-secreted mediators, (iii) immunomodulation of other TILs and promotion of TLSs, or antigen presentation.47
B regulatory cells (Bregs) can mediate allergen tolerance by IL-10-dependent and IL-10-independent mechanisms.48 In cancer, Bregs may function in a similar manner to promote immune tolerance or potentiate Treg responses, leading to tumour progression.47, 49, 50 The latter is consistent with TiBCs found in close proximity to FoxP3+ T cells in melanoma lesions and in other tumour types.44 Specific compartments and actions of B cells may thus be targeted to improve treatment of allergic or malignant diseases.
3.5 Innate lymphoid cells (ILCs)
Innate lymphoid cells (ILCs) broadly mirror helper T-cell subsets, but they do not express specific antigen receptors. Based on their lineage-specific transcription factor and cytokine production, they are classified in 3 groups.51 ILC1s phenotypically like Th1, respond to IL-12, IL-15 and IL-18, and are defined by the production of IFNγ and expression of transcription factor T-bet. NK cells expressing eomesodermin and producing cytotoxic granzymes and perforin also belong to that group. ILC2s, which resemble Th2 cells, respond to epithelium-derived cytokines, such as IL-33, IL-25, TSLP, eicosanoids and IL-1β. The cells are defined by production of type 2 cytokines IL-4, IL-5, IL-9 and IL-13 and by the expression of the transcription factor GATA-3.
ILC2s are involved in the initiation of innate allergic inflammation and in its enhancement by interacting with other immune cells. They are stimulated by epithelial cells (through IL-33, IL-25, TSLP) or by proximal mast cells (via IgE-mediated eicosanoid release) that induce type 2 cytokine production from human ILC2s.51 On the other hand, ILC2s are negatively regulated by IL-33-activated mast cells that suppress them via Treg cell expansion or by KLRG1 (produced by ILC2 after stimulation with IL-33 or TSLP)/E-cadherin (expressed by keratinocytes) axes.
In cancer, IL-33 secreted by macrophages stimulates ILC2s and, in turn, the secretion of IL-13 and IL-5, which have pro-tumoral effects. ILC2s can also establish an immunosuppressive tumour microenvironment by amphiregulin secretion.52
ILC3s resemble Th17 and Th22 cells. They respond to IL-1β and IL-23 and are defined by the production of IL-17A and IL-22 and by the expression of RORγt.53 Furthermore, cells of the ILC3 subtype secrete IL-22 upon IL-23 stimulation by macrophages and have tumorigenic effects. On the other hand, ILC3s could induce tolerance by increasing IL-10 and retinoic acid secretion by DCs upon stimulation by microbiota and macrophages,54 or by enabling T-cell tolerance through the expression of MHC Class II in the absence of costimulatory molecules.55 Thus, among the ILC type, especially the ILC3s could favour tumour growth and tolerance.
3.6 Mast cells
Mast cells are major players in allergy, but also accumulate in the stromal and intratumoral tissues of a diverse array of cancers. Mast cells are chemoattracted by different factors such as stem cell factor (SCF), vascular endothelial growth factor (VEGF), chemokines, prostaglandins, leukotrienes, histamine and osteopontin56 in the tumour microenvironment. The controversial role of mast cells in tumorigenesis and tumour progression could possibly depend on their micro-localization and the type of tumour. Their contribution to human tumour growth has mostly been assessed by relating the number of mast cells in cancer tissue to the stage and/or prognosis of the disease, while generally no markers for functional activity were taken into account.56, 57 The net outcome of mast cell accumulation for the tumour cells may be determined by the condition of the tumour micromilieu shaping local pH and oxygen tension.56, 58, 59 Upon activation, mast cells can release a great number of prestored and newly synthesized mast cell mediators, which can stimulate tumour growth (see below).
Furthermore, mast cells express cell surface molecules, which upon contact with target cells inhibit cell activation or may polarize mast cells towards tolerance induction. Expression of PD-L1 or PD-L2 on mast cells may contribute to inhibition of T-cell activation in the tumour microenvironment.60 Furthermore, co-culture of human mast cells with immature DCs stimulates their expression of PD-1 and programs DCs towards tolerogenicity supporting induction of regulatory T cells.61 The impact of the mast cells checkpoint inhibitors for tumour development requires further research.
3.7 Eosinophils
Eosinophils were found mainly antitumorigenic in solid tumours such as bladder, gastrointestinal, melanoma, prostate, with only some studies showing a detrimental effect in favour of tumour growth such as in the case of Hodgkin lymphoma.62 Mechanistically, CCL1 produced by tumour cells can attract eosinophils (via CCR3) that may interfere with tumour growth.63 Tumour cells can stimulate eosinophils to produce IL-18, which in turn facilitates eosinophil-cancer cell interactions leading to tumour cell death.64 Production and secretion of ROS and cytotoxic mediators, typical eosinophil mediators, were found antitumorigenic in many cancer studies.65 Nevertheless, the growth factor APRIL could support growth of malignant plasma cells in bone marrow in a multiple myeloma mouse model.66 More recent studies place eosinophils as first line “sensors” for the general antitumour immune response, showing for example, that intratumoral eosinophils are crucial for the recruitment of antitumour CTLs in melanoma.67 On the other hand, lung eosinophils were shown to induce Treg infiltration and consequently support lung metastasis.68 Lastly, eosinophils are involved in curative processes induced by checkpoint inhibitor immunotherapies (eg, ipilimumab or pembrolizumab69).
Hence, numerous findings suggest that eosinophils can be an immunotherapeutic effector cell either being activated by immunotherapies such as checkpoint inhibitors, or granulocyte-macrophage-colony-stimulating factor (GM-CSF)-based vaccines,70 or perhaps by adoptive transfer of these cells in an appropriate setting.71
3.8 Epithelial cells
The essential contribution of the epithelial barrier to a balanced immune response is well established. For oral antigens, the site of antigen uptake and its particulate nature is decisive for the subsequent immune response.72 Antigen passage through mucus-producing goblet cells has been specifically associated with tolerance induction.73 In allergy as well as cancer, the cell surface receptor repertoire of epithelial cells includes the high-74 and the low-affinity IgE Fc receptors facilitating antigen passage and directing antigen presentation.75 The epithelium is additionally an important cytokine source, profoundly modulating the immune response (Table S1). In the intestinal tract, extracellular vesicles physiologically produced from intestinal epithelial cells contribute to innate immunosuppression,76 which may contribute to oral tolerance—or cancer progression.
4 MOLECULES IN IMMUNE TOLERANCE: FROM ALLERGY TO CANCER (SEE OVERVIEW IN Table 2)
| Section | Molecule | Function in allergy | In cancer |
|---|---|---|---|
| 4.1 | IgG4 antibodies | Induced in allergen immunotherapy; Function as blocking antibodies and via FcγRIIb |
Overexpressed in some malignancies Derived from intratumoral Bregs Correlate with progression |
| 4.2 | IgE and IgG repertoires | IgE: the major effector antibody in immediate-type allergies, via high-affinity binding to FcεRI; Responsible for antigen cross-presentation to T cells; IgE clones have increased persistence, more likely related to other switched isotypes, larger clonal families | IgE and IgG: monoclonality in myelomas; Small subclones re-emerged at relapse alongside a dominant clone in B-cell leukaemia |
| 4.3 | Free light chains (FLC) |
Increased polyclonal FLC levels in allergies Activate mast cells |
Increased FCL: biomarker for poor prognosis in basal-like breast cancer |
| 4.4 | Regulatory cytokines and chemokines | IL-10 and TGF-β have pivotal role in tolerance establishment to allergens; CCL1:CCR8 axis plays a master role in immune regulation | IL-10 and TGF-b derived from immune and cancer cells shape the immunosuppressive environment in cancer and correlate with disease progression; Controversial: the role of the CCL5:CCR5 axis; CCL3, -4, -5 derived from intratumoral myeloid-derived suppressor cells (MDSCs) can recruit Tregs |
| 4.5 | Mast cell mediators and receptors |
Secreted mediators and cytokines that support allergy and acute inflammation Interact with IgE and other isotypes |
Controversial; Can stimulate tumour growth (eg, histamine, NGF, IL-8), neovascularization (eg, VEGF, heparin, TGFβ), and suppress effector T cells, enhance Tregs via amphiregulin; On the contrary, can inhibit tumour growth, cause apoptosis (eg, via TNFα, IFNγ, PAR-1/2), and attract leucocytes (eg, IL-8, TNFα), inhibit metastasis via chondroitin sulphate |
| 4.6 | Lipocalins (LCN) | Sequester iron; LCN and iron levels decreased in allergy; Some allergens belong to the lipocalin family themselves | LCN and iron levels are upregulated in cancer; form complexes with matrix-metallopeptidase 9 |
4.1 IgG4 antibodies
IgG4 is the least abundant subclass of IgG in normal human serum, but it may reach elevated levels following chronic exposure to antigen, or during inflammation. IgG4 has unique structural characteristics that are responsible for its inability to fix complement, low affinity for activating FcγRs, and relatively high affinity for the inhibitory Fc receptor FcγRIIb.26 IgG4 has a unique ability to undergo Fab-arm exchange, resulting in the formation of bispecific antibodies which are functionally monovalent, and are therefore not able to cross-link antigens to form large immune complexes.44 For these reasons, IgG4 are widely considered to be anti-inflammatory antibodies, playing a positive and protective role in allergy and helminth infections. On the other hand, in IgG4-related diseases, such as sclerosing pancreatitis and autoimmune pancreatitis (AIP), pathogenic roles for IgG4 cannot be fully precluded yet. In contrast, the anti-inflammatory properties of IgG4 may have a negative impact in the context of cancer. As tumours are characterized by chronic inflammation with prolonged exposure to tumour-associated antigens, elevated IgG4 has been detected in extrahepatic cholangiocarcinoma, pancreatic cancer, melanoma45 and glioblastoma.77 Indeed, these tumour types are characterized by Th2-biased expression of mediators known to trigger B cells to produce IgG4. Tumour-specific IgG4 may act as a blocking antibody competing with inflammatory antibodies such as IgE or IgG1 for binding to antigen, or in form of immune complexes engage the inhibitory receptor FcγRIIb and thus dampen the inflammation. IgG4 antibodies in cancer may thus have a darker side, as compared to their association with tolerance induction to allergens.
4.2 IgE and IgG repertoires in allergy and cancer
New-generation sequencing for immunoglobulin repertoire analysis78 was applied in subcutaneous AIT79 and longitudinal studies of cancer progression.80 Also the IgG repertoire of healthy individuals contained antibodies to tumour-associated carbohydrate antigens, with a possible function immunosurveillance.81
A study of IgE sequences in the blood and nasal biopsies of eight allergic rhinitis patients undergoing SIT79 compared the repertoires at baseline and after 2 months and 1 year of SIT with the library of paired allergen-specific IgE heavy- and light-chain variable region fragment (scFv) sequences selected at baseline by phage display. The allergen-specific IgE clones showed increased persistence, higher likelihood of clones related to other switched isotypes, and larger clonal families. Related IgG clones may represent the putative competitors for allergen responsible for the acquired allergen tolerance.82, 83
Studies of the immunoglobulin repertoire in cancer are rare. The study of a multiple myeloma patient not only confirmed the monoclonality of the myeloma cells, previously observed in conventional low-throughput sequencing, but also demonstrated certain pitfalls in the massively parallel sequencing technology.80 Another study of 15 B-cell acute lymphoblastic leukaemia (B-ALL) patients tracked the clonal diversification of the malignant cells and found that large numbers of small subclones, present at diagnosis, re-emerged at relapse alongside a dominant clone.84 The technology detected minimal residual disease with unprecedented sensitivity and framed early cyto-reduction, such as surgery and radiation to reduce the tumour bulk, as an important determinant of long-term survival.
Immunoglobulin repertoire analysis by new-generation sequencing can in future provide a rich source of information relating to immuno-biology and immuno-therapeutics in allergy as well as cancer.
4.3 Free light chains (FLC)
Free light chains (FLC) are produced and secreted in excess to immunoglobulin heavy chains during synthesis of tetrameric immunoglobulins by plasma cells and can be detected in all body fluids. Significant increases in systemic and local polyclonal FLC levels have been measured in many chronic inflammatory diseases, including allergic diseases (such as asthma, rhinitis), chronic obstructive pulmonary disease (COPD), multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, diabetes and cancer.57, 85 FLC can induce inflammatory responses by mediating antigen-specific mast cell activation86 and also reduce neutrophil apoptosis and stimulate their release of pro-tumorigenic IL-8. The expression of FLC was found to be a biomarker for poor prognosis in basal-like breast cancer.87 In a preclinical melanoma model, it was further demonstrated that FLC-induced mast cell activation-stimulated tumorigenesis.87 As FLC were found in the stromal tissues of a great number of human tumours, one could speculate that overall FLC may contribute to cancer growth and progression.
4.4 Regulatory cytokines and chemokines
Without doubt among cytokines, IL-10 and TGFβ are pivotal in the establishment of immune tolerance, and their production by many different cell types is desirable in allergy3 but unwanted in cancer, as discussed above. For instance, CCL1 is a chemokine expressed by monocytes and especially by the tolerogenic M2b macrophage subpopulation, after engagement of FcyRIIb and LPS or by IL-1β stimulation.35, 88 CCL1 may be potentiated by the expression of its cognate receptor CCR8 on CCR8+ FoxP3+ Treg cells, and by the CCL1:CCR8 axis in immune regulation.89
Growing interest has recently emerged on the role of the CCL5:CCR5 axis (and other CCL5 receptors, CCR1 and CCR3; and CCR5 ligands, CCL3 and CCL4) in cancer progression, but also in the possibility to exploit this chemokine-receptor interaction to combat cancer. Depending on the tumour environment, CCL5 has been implicated in antitumour adjuvanticity but also in carcinogenesis. Intratumoral myeloid-derived suppressor cells (MDSCs) can mediate recruitment of Tregs through the secretion of CCL3, CCL4 and CCL5 acting on the CCR5.90 Specifically tailored interventions on CCR5 expressed by tumour cells or by tumour stromal cells resulted in antitumour effects.91, 92 Interestingly, CCL5 and CCR5 are involved in immune tolerance and in the recruitment and activation of MDSCs.93, 94 This CCL5:CCR5/MDSC axis may provide yet another important link between allergy and cancer. An example may be the association of chronic food allergy and the development of colorectal cancer via mast cell-mediated recruitment of MDSCs.95 Therefore, while the acute allergic reactions per se via the cytokine and chemokine systems should stimulate the immune system and elevate tumour surveillance, any prolonged chronic inflammation supports tumour development.
4.5 Mast cell mediators and receptors
As discussed above, despite their pro-inflammatory functions, mast cells can have a prominent role in the induction of immune tolerance. Among the prestored and newly synthesized factors, some stimulate tumour growth (eg, histamine, nerve growth factor [NGF], IL-8), neovascularization (eg, VEGF, heparin, TGFβ), and can restrict T-cell responses.96 On the other hand, mast cells can also secrete mediators which are detrimental to the tumour by inducing growth inhibition, cellular disruption and apoptosis (eg, TNFα, IFNγ, activation of PAR-1/2), and by attracting inflammatory leucocytes (eg, IL-8, TNFα), and inhibiting metastasis (eg, chondroitin sulphate). Of note, the mast cell-derived enzymes chymase and tryptase can be both beneficial and detrimental.
Histamine can modulate different immune cells via the histamine 2 (H2) receptor. This could have juxtaposed functions, such as enhanced mobilization of DCs, reduction in cytolytic activity of NK cells and modification of MDSCs.97 Interestingly, ranitidine, an H2 receptor antagonist, was inhibited lung metastasis and breast cancer development in mouse models.98 Mast cells can also release immunosuppressive IL-10 and TGFβ,97, 99 tryptophan hydroxylase-1 (thp-1) and amphiregulin.100, 101 thp-1 deficiency in mast cells results in slower tumour growth kinetics.100 The exact mechanism by which thp-1 regulates immune responses is still unclear. Amphiregulin stimulates the epidermal growth factor receptor (EGFR), enhances Treg function and contributes to the local immunosuppression in the tumour micromilieu.101 All these suggest an important role of mast cell mediators in immunoregulation.
4.6 Lipocalins
Many innate defence proteins are differentially regulated in allergy and cancer, for instance lipocalin-2 (LCN2; NGAL). LCN2 is highly expressed in the liver, bone marrow, spleen, salivary gland, the colon, the lung and the kidney. LCN2 is upregulated in various cancer types and has been proposed as a cancer biomarker.102-104 LCN2 binds iron through iron-chelating compounds.105 As such, it is involved in numerous processes including innate immunity, apoptosis and renal development. It can also form complexes with the matrix-metallopeptidase 9 (MMP9, a 92-kDa gelatinase), itself being a poor prognostic factor in several cancer types.106 Increased iron raises the cancer risk and as a participant in iron homoeostasis also LCN2 is upregulated in various cancers.107 Contrastingly, decreased LCN2 and serum iron are associated with allergy.108 Some exogenous antigens have a striking structural resemblance with human LCN2,109, 110 suggesting potential interference with the iron loading of endogenous LCN2 and affecting its immunomodulatory potency. Taken together, LCN2 could assume divergent immune roles in allergy and cancer.
5 ANIMAL MODELS AND IMMUNE TOLERANCE IN ALLERGY AND CANCER
The induction of allergy, often followed by re-establishment of tolerance, are important goals in allergic animal models, mostly mice.111, 112 A range of humanized mouse models (immunocompromised mice engrafted with nonmalignant human peripheral blood lymphocytes or mononuclear cells or hematopoietic stem cells) are used for studies on allergies.113 Comparisons among allergy mouse models are difficult as the responses vary widely.114 Moreover, components of the human allergic response (mast cells, allergen-specific IgE and eosinophils) are unnecessary for allergy induction in certain mouse models, especially allergic asthma models. Brown Norway rats are helpful as they develop food allergy upon high-frequency intragastric dosing of allergen without adjuvant.114 However, large amounts of antigens are needed.111, 114 Dogs spontaneously develop allergies, but drawbacks are the high cost, limited number of strains, and greater interanimal variation compared to rodents.111 Increasingly, allergic canine patients are introduced in comparative allergy studies.112 Swine share dog's advantages and disadvantages plus the limitation of long dosing times required for sensitization.111
In cancer animal models, tolerance to implanted or artificially expressed malignant cells is needed from the onset of the experiment, followed by either tumoricidal approaches or efforts to break tumour tolerance such as by immunotherapeutic approaches. Immunocompromised mice bearing human xenograft tumours have been extensively used to study anticancer drugs,115 but lack an adaptive immune response. This can be overcome using immunocompetent mice or rats bearing syngeneic tumours, which respond to a broad variety of immuno-stimulatory approaches such as antibodies (including IgE), antibody fusion proteins and cancer vaccines.115-117 However, differences to the rodent immune system limit the translation of these models to humans.115 Transgenic mice originally developed to study allergic responses and therapeutics, for instance those expressing specific IgE Fc receptors, may be used in the cancer setting. Alternatively, humanized mice with human immune cells have been employed.115, 118 These mouse models can be challenged with human tumours to evaluate immuno-stimulatory anticancer agents.118 Hurdles of humanized mice are the suboptimal engraftment of nonmalignant cells and their inadequate maturation in cases where stem cells are engrafted; however, humanized mice are promising models for both studies on allergies and malignancies whose improvement continues.118 Dogs are an up-and-coming comparative model in AllergoOncology due to their high incidence of spontaneous malignancies in an intact immune system setting, and because they can sometimes develop allergies119; however, there are limited canine-specific/cross-reactive reagents and epitopes characterized for immunotherapy use.120 Cynomolgus monkeys are often used to evaluate toxicity of anticancer agents due to their phylogenetic proximity to humans. Differences in the immune system compared to humans can still be problematic, especially when examining checkpoint inhibitors and other immuno-stimulators,121 and this model is expensive and not practical to address antitumour activity.
6 CONCLUSION
Insights from research in allergen immunotherapy and cancer immunology strongly suggest that the same key immune cells are involved in immune tolerance induction in allergy and also in cancer (Table 1; Figure 2). DCs, as well as other cells including mast cells, may not only play pivotal roles in CTLA-4 and PD-1 mediated Treg activation in allergy, but also can help shape a strong immunosuppressive microenvironment with detrimental effects in cancer. Notably, tumour antigen–specific IgE could break DC-mediated tolerance and also repolarize macrophages to a phenotype that can promote antitumour responses. Particularly, among innate cells, M2b and ILC3 subtypes are expressed in cancer and correlate with disease progression and poor prognosis.
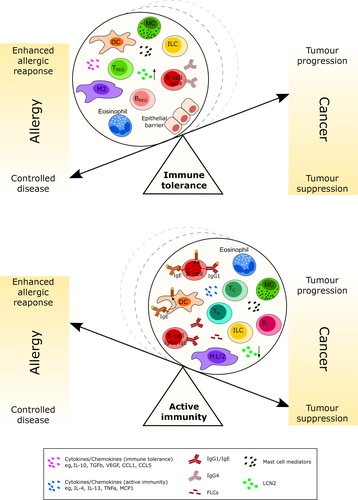
Tregs and Bregs, possibly via IL-10 and other molecules (Table 2), can also contribute to an immunosuppressive tumour microenvironment and can be promoted by environmental triggers through AhR. Mast cells and eosinophils express cell surface molecules, which upon contact with target cells, can polarize these cells towards tolerogenic phenotypes. Mast cells simultaneously stimulate tumour growth via factors such as histamine or NGF, and manipulate T-cell responses in favour of immunosuppression. IL-10 and TGFβ, CCL1 and CCL5 and their axes to CCR8 and CCR5 could support tolerance induction. The contrasting immune effects of single molecules are illustrated by the increased LCN2 levels in cancer, while decreased in allergy.
In contrast to their beneficial effects in AIT, the IgG4 subclass and also FLCs may have poignant significance in cancer. In contrast to allergy, little is known about the specificity of immunoglobulins in cancer, but repertoire analysis by new-generation sequencing provides a future source of information. For all AllergoOncology approaches, including those interrogating immune tolerance, emerging allergy and oncology know-how must be combined to design optimal in vivo models.
Collectively, there are many parallels to be drawn between tolerance mechanisms at play in allergy and cancer, albeit with contrasting implications on disease progress and patient outcomes. The pursuit of further insights into these parallel mechanisms with juxtaposed clinical consequences has the potential to lead to novel therapeutic interventions to benefit both disciplines.
ACKNOWLEDGMENTS
The AllergoOncology Task Force was financed by the European Academy for Allergy and Clinical Immunology (EAACI). The authors would like to thank EAACI for their financial support in the development of this Task Force report. The authors acknowledge support by the Austrian Science Fund FWF grants CCHD W1205-B09, F4606-B28, P23398-B11 (EJJ); FWF grant KLI284 (EU); Israel Cancer Association #20161131 and Israel Science Foundation #472/15 (FLS). The authors acknowledge support by Cancer Research UK (C30122/A11527; C30122/A15774) (SNK, HJB, DHJ); The Academy of Medical Sciences (SNK, DHJ); CRUK/EPSRC/MRC/NIHR KCL/UCL Comprehensive Cancer Imaging Centre (C1519/A10331) (SNK, DHJ); the Medical Research Council (MR/L023091/1) (SNK); Breast Cancer Now (147) (SNK, SI); CRUK/NIHR in England/DoH for Scotland, Wales and Northern Ireland Experimental Cancer Medicine Centre (C10355/A15587) (SNK); NIH/NCI grants R01CA181115 (MLP and TRD); and R21CA193953 (MLP and TRD). The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) based at Guy's and St Thomas’ NHS Foundation Trust and King's College London (IS-BRC-1215-20006) (SNK, SC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Erika Jensen-Jarolim and Sophia N. Karagiannis wrote the Abstract, part 1 (Introduction) and part 6 (Conclusion), designed Tables 1 and 2 and contributed to specific parts below, coordinated all texts, edited and compiled them into the final article. Part 2 (Therapeutic interventions) was written by Erika Jensen-Jarolim and Josef Singer; Part 3 (Cellular players) was authored by the following contributors: Dendritic cells, Edda Fiebiger and Rodolfo Bianchini; Macrophages, Rodolfo Bianchini and Giulia Pellizzari; Tregs, Liam O'Mahony, Jozef Janda and Franziska Roth-Walter; B cells and Bregs, Sophia N. Karagiannis and Heather Bax; Innate Lymphoid Cells (ILCs), Rodolfo Bianchini and David Dombrowicz; Mast cells, Frank Redegeld and Francesca Levi-Schaffer; Eosinophils, Francesca Levi-Schaffer and David Dombrowicz; Epithelial Eva Untersmayr and Jozef Janda; Part 4 (Molecules) was authored by the following contributors: IgG4 antibodies, Sophia N. Karagiannis, Silvia Crescioli, Debra H Josephs; IgE and IgG repertoire, Hannah Gould; Free light chains, Frank Redegeld; Cytokines/Chemokines, Frank Redegeld, Luca Vangelista, Rodolfo Bianchini; Mast cell mediators, Frank Redegeld; Lipocalins, Franziska Roth-Walter; Part 5 (Animal models), Tracy R. Daniel-Wells, Manuel L. Penichet. Figures were prepared by Sophia N. Karagiannis, Heather J Bax, Silvia Crescioli, Sheeba Irshad, Debra H Josephs and Giulia Pellizzari. The final manuscript has been approved by all authors.




