The potential of anti-infectives and immunomodulators as therapies for asthma and asthma exacerbations
Abstract
Asthma is responsible for approximately 25,000 deaths annually in Europe despite available medicines that maintain asthma control and reduce asthma exacerbations. Better treatments are urgently needed for the control of chronic asthma and reduction in asthma exacerbations, the major cause of asthma mortality. Much research spanning >20 years shows a strong association between microorganisms including pathogens in asthma onset, severity and exacerbation, yet with the exception of antibiotics, few treatments are available that specifically target the offending pathogens. Recent insights into the microbiome suggest that modulating commensal organisms within the gut or lung may also be a possible way to treat/prevent asthma. The European Academy of Allergy & Clinical Immunology Task Force on Anti-infectives in Asthma was initiated to investigate the potential of anti-infectives and immunomodulators in asthma. This review provides a concise summary of the current literature and aimed to identify and address key questions that concern the use of anti-infectives and both microbe- and host-based immunomodulators and their feasibility for use in asthma.
Abstract

Abbreviations
-
- ABPA
-
- allergic bronchopulmonary aspergillosis
-
- ACQ
-
- Asthma Control Questionnaire
-
- AE
-
- Asthma exacerbation
-
- ARI
-
- acute respiratory illnesses
-
- BL
-
- bacterial lysates
-
- DPT
-
- diphtheria, pertussis, tetanus
-
- EAACI
-
- European Academy of Allergy & Clinical Immunology
-
- FEV1
-
- forced expiratory volume
-
- GCs
-
- glucocorticoids
-
- IFNs
-
- interferons
-
- IPD
-
- invasive pneumococcal disease
-
- LAIV
-
- live attenuated influenza vaccine
-
- LRTI
-
- lower respiratory tract infection
-
- MMR
-
- measles, mumps and rubella
-
- ORMDL3
-
- orosomucoid 1-like 3
-
- pDCs
-
- plasmacytoid dendritic cells
-
- PPSV-23
-
- pneumococcal polysaccharide 23-valent vaccine
-
- RSV
-
- respiratory syncytial virus
-
- RV
-
- rhinovirus
-
- SAFS
-
- severe asthma with fungal sensitization
-
- siRNA
-
- small interfering RNA
-
- TIV
-
- trivalent split-virus influenza vaccination
-
- TLR
-
- toll-like receptor
-
- URTIs
-
- upper respiratory tract infections
1 INTRODUCTION
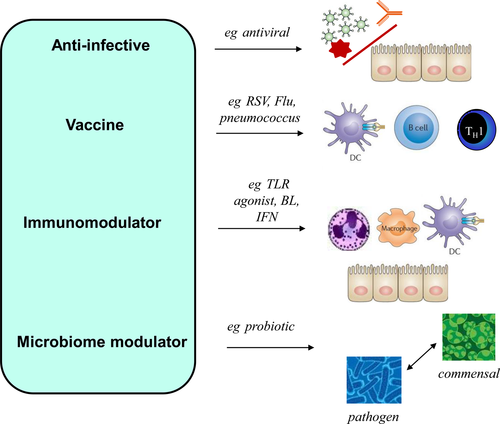
Research in asthma over the last 20 years or more repeatedly shows an overwhelming link between the actions of microorganisms and asthma.1, 2 From acting as direct pathogens, to educating the immune system and preventing opportunistic pathogen colonization through occupying specific niches, it is now well accepted that viruses, bacteria and fungi are positively associated with asthma onset,3, 4 asthma severity,5, 6 asthma exacerbation (AE)7, 8 and asthma management9 and even secondary prevention strategies of asthma (summarized in Table 1). Despite these important associations, the use of anti-infectives (antibiotics, antivirals, antifungals, vaccines) that specifically target known pathogens, or drugs that are based on or exploit microbe-host receptor interactions (toll-like receptor agonists, bacterial lysates) or are immunomodulators (vitamin D), and/or may work in part by altering our associated microbiology (probiotics) are, with the exception of severe asthma, seldom considered in asthma treatment, prevention and guidelines. These treatment options are summarized in Figure 1.
| Pathogens | Pathogen type | Notes |
|---|---|---|
| RSV | Virus | Paediatric RSV infection is associated with asthma onset, severe bronchiolitis, wheeze and AE |
| RVs | Virus | Associated with asthma onset, trigger of AE, also associated with bronchiolitis, wheeze |
| Human metapneumoviruses | Virus | Associated with childhood wheeze and AE |
| Influenza viruses A & B | Virus | Associated with adult AE and fatal asthma |
| Parainfluenza 1, 2 & 3 | Virus | Associated with AE |
| Streptococcus pneumoniae | Bacteria | Associated with wheeze in children increased carriage in asthma by 16S rRNA abundance |
| Haemophilus influenzae | Bacteria | Wheeze in children, increased carriage in asthma observed by 16S rRNA abundance |
| Morrexella catarrhalis | Bacteria | Associated with wheeze in children |
| Chlamydophila pneumoniae | Atypical bacteria | Associated with AE and wheeze in children |
| Mycoplasma pneumoniae | Atypical bacteria | Associated with AE and wheeze in children |
| Aspergillus fumigatus | Fungus | Important causative agent of allergic bronchopulmonary aspergillosis (ABPA) and severe asthma with fungal sensitization (SAFS) |
| Moulds | Fungi | Can produce aerosolized allergen common in asthma epidemics associated with thunder storms, involved in severe asthma with fungal sensitization (SAFS) |
The European Academy of Allergy & Clinical Immunology (EAACI) Task Force on Anti-infectives in Asthma was initiated in 2014 to ask open questions about the potential use of anti-infectives and immunomodulators as treatments for asthma and AE. We thus provide a thorough review of the field (Table 2 for search methods and terms), and specifically, have considered several important points in relation to the use and implementation of anti-infectives in asthma, thus identifying important challenges for the field. In our investigation, we chose to include any such studies of treatment that is based on microorganisms or host molecules, or exploits microbe-host interactions and thus alters the host response or microbiome.
| Section | Search terms & methods |
|---|---|
| Time Frame: The years between 1990 and present were rigorously searched for all sections. Where appropriate, additional older papers were also included. | |
| Antibiotics | PUBMED was searched for combinations of appropriate terms antibiotics, asthma, macrolide, reverse causality, microbiome |
| Antivirals | PUBMED was searched for combinations of appropriate terms: antiviral, pleconaril, soluble ICAM-1, tremacamra, amantadine, rimantadine, monoclonal antibody, palivizumab, motavizumab, gammaglobulin, interferon, RNA polymerase inhibitor, vapendavir, favipiravir, inhibitor of viral protein synthesis, rupintrivir ribavirin, small interfering RNA, siRNA, ALN-RSV01, neuraminidase inhibitor, zanamivir, oseltamivir, laninamivir, peramivir and respiratory, Gilead and Alios. |
| Vaccines | PUBMED was searched for combinations of appropriate terms: vaccines, influenza pneumococcus, rhinovirus, RSV, LAIV, TIV, IPD, PPSV-23 |
| Immunomodulators | PubMed and Academic Search Complete, CENTRAL, Health Source: Nursing/Academic Edition, MEDLINE and Cochrane databases were searched for combination of appropriate terms: probiotics, prebiotics, bacterial lysates, vitamin D, asthma, toll-like receptor, TLR3, TLR7/8, TLR9 |
| Antifungals | PUBMED was searched for combinations of appropriate terms: fungal antibiotics, fungicide, fungal infection, asthma, SAFS, ABPA, fungus, Aspergillus fumigatus, amphotericin B, azole |
In this review, we offer our findings on the above points and consider the role of anti-infectives, immunomodulators and alteration of host microbiology in both asthma development and AE. We include findings from recent clinical trials and discuss the relative merits of these approaches in the light of the many challenges facing asthma research and the state of the art of the field. This review thus provides important insights aimed at young researchers and clinicians and also experienced researchers in the field to inform and stimulate scientific discussion of this important topic.
2 ANTIBIOTICS
2.1 Antibiotic use in pregnancy, early life and childhood
Internationally, current guidelines do not support the use of antibiotics for asthma, yet despite this recommendation, antibiotics are prescribed too often as a general treatment for lower respiratory tract infections (LRTIs), general chest infections and even asthma. Overall, antibiotic use is associated with asthma risk rather than protection at most stages of human development, including pregnancy,10, 11 early life12 and childhood,13 although why this is so is a subject widely debated.
In Denmark, antibiotic use in pregnancy use was shown to increase risk of AE in five-year-old children by twofold if used in the third trimester.10 Antibiotic exposure in foetal life was associated with an increased risk of asthma in cohort analyses, and this association more than tripled if antibiotics were used to treat respiratory tract infections rather than antibiotics used for either urinary tract or skin infections. These associations decreased, however, when sibling analysis was included (when nonaffected siblings are used as controls).11 Early antibiotic use is also believed to increase asthma risk by two- to threefold in seven- to eight-year-olds.13
The potential negative impact of antibiotics was explored in a birth-cohort study at age 11 from Manchester, United Kingdom.12 There was a significantly higher risk of physician-confirmed wheezing after antibiotic prescription and a twofold increase in severe wheeze or AE after antibiotic prescription. In children who wheezed, the risk of AE and admissions to hospital were also significantly increased in the 2 years after the first antibiotic prescription. Children who received antibiotics in infancy had significantly lower induction of cytokines from PBMCs (taken at age 11) stimulated ex vivo with viruses, but surprisingly, not bacteria. The authors concluded that an increased susceptibility to viral infections is associated with both early-life antibiotic prescription and asthma risk, although the authors could not exclude reverse causation, in that antibiotic use or asthma influences PBMC responses to viruses later in life. There was also a significant association with antibiotic prescription in the first year of life in that children carrying a G allele in ZPBP2 (SNP rs11557467) and those carrying a C allele in the gene encoding orosomucoid 1-like 3 (ORMDL3) (SNP rs4795405) were at higher risk of being prescribed antibiotics. Although its function is not completely clear, ORMDL3 is expressed on the endoplasmic reticulum in bronchial epithelial cells and is thought to activate the unfolded protein response pathway. The association of antibiotic use with ORMDL3 is of particular excitement, as this gene has recently been highlighted in Genome-Wide Association Studies in asthma14 and in the risk of virus-induced wheeze.15
2.2 Antibiotics and modulation of the microbiome
The microbiome describes the bacteria, fungi and other microorganisms that are present in the environment and co-exist within our bodies. The respiratory microbiome can now be studied by 16S rRNA sequencing, and certain microbial phyla or genera are thought to be harmful or protective.5, 16 Antibiotics may negatively affect bacterial ecology in early life and this in turn affects asthma development.10 In retrospective studies, the association between antibiotic use and increased risk of asthma or wheezing in children is further confused due to the potential of reverse causation.12, 17 In experimental animals, the negative impact of antibiotics has already been shown, and long-term oral antibiotic treatment affects the gut microbiome, which in turn affects lung immunity to influenza virus.18 Indeed, how diet and the gut microbiome affect mucosal immunity including respiratory immunology is now a subject of wide interest;19, 20 hence antibiotics through regulating the gut microbiota thus directly affect the development of the immune system. The potential of antibiotic-induced changes in the developing microbiome to be directly responsible for asthma or AE risk is yet to be formally proven, however.
2.3 Macrolide antibiotics
Macrolide antibiotics are a class of antibiotic commonly prescribed for respiratory tract infections or inflammatory respiratory disorders. Macrolides may have additional properties to their bacteriostatic function, such as anti-inflammatory21 and even antiviral activity.22, 23 Despite their ability to inhibit both bacteria and virus infections, only a few studies have tested macrolides as therapies for asthma or AE. Guidelines state very little on macrolide use in asthma, and studies have shown positive effects on severe and neutrophilic asthma, yet the evidence supporting this is conflicting. These studies in both stable asthma and AE have been recently reviewed,24 and the data suggest macrolide-responsive subgroups (eg neutrophilic asthma) may exist.25, 26 Additionally, recent studies also report a reduction in severe LRTI rates27 and asthma-like symptoms28 for children with azithromycin. For such studies where macrolides exhibited positive effects, it is difficult to ascertain a responsible mechanism. It is possible that a combination of antibacterial, antiviral and anti-inflammatory properties may all contribute. Further carefully designed studies capable of appropriate sampling to obtain mechanistic insights are needed before this is clarified. The use of macrolides could further be tested in human challenge studies rather than those relying on natural infection. Macrolide use in asthma also suffers from the potential of encouraging antibiotic-resistant bacteria, and microbiologists have also questioned how these drugs may disturb the microbiome.29, 30
2.4 Summary and outlook
Antibiotics are associated with asthma risk and their use should be discouraged for asthma or wheeze-like illnesses. A possible explanation for this association is that antibiotics affect the microbiome in a negative way and thus increase susceptibility to disease. With the realization that the microbiome is key in controlling host immunity early in life, and the design of supportive animal studies that have modelled this association and identified protective genera,31 this idea has merit but the overall hypothesis remains to be thoroughly tested in human clinical models. Antibiotics may also be associated with asthma via other, as yet to be identified mechanisms, and likely involve reverse causation. The narrower antibacterial spectrum of some macrolide antibiotics, combined with their other advantageous properties, suggests that these may have use in AE but their use remains controversial.
3 ANTIVIRALS
3.1 Specific antivirals targeting viral attachment or entry
The mechanisms of action of antivirals, which have shown clinical efficacy in respiratory tract infection, are summarized in Figure 2. The prophylaxis with intramuscular/intravenous antibody palivizumab has been shown to effectively decrease respiratory syncytial virus (RSV)-induced bronchiolitis-related hospitalization, need for mechanical ventilation and recurrent wheezing.32 Interestingly, it has also decreased recurrent wheezing induced by other viruses postbronchiolitis. The second-generation monoclonal antibody motavizumab has also markedly reduced hospitalization with RSV.33 GS-5806 inhibits RSV entry by blocking viral envelope fusion with the host cell membrane. Recently, it was shown to impressively decrease the viral load and the severity of upper respiratory tract illness in a challenge study.34 ALX-0171 is a promising trivalent nanobody, which is designed to target the RSV-F protein for delivery via inhalation.35
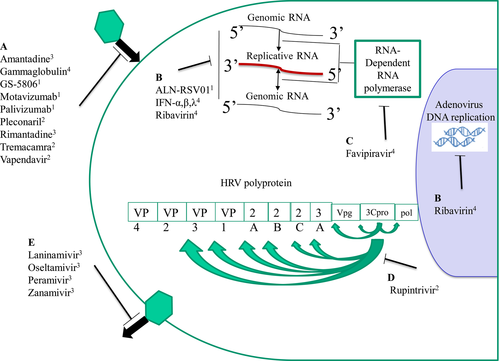
For picornaviruses, pleconaril works by inhibiting attachment of the virus to the cell and/or uncoating viral RNA. Oral pleconaril has reduced the duration and severity of colds due to picornavirus infections and has good tolerability36; however, it has not been approved for clinical use due to adverse events. Vapendavir, a novel oral capsid inhibitor, has antiviral activity against many enteroviruses and may have additional activity against rhinoviruses (RVs). It has shown good safety and tolerability profiles37 and is currently a large phase IIB trial. An intranasal recombinant soluble ICAM-1, tremacamra, has effectively decreased clinical symptoms in experimental RV infection, but the development programme has been discontinued.38 Only a few patients benefit from the M2 ion channel inhibitors, amantadine and rimantadine, drug resistance is high, and they have significant adverse events.39 Neutralizing antibodies against RSV, coronaviruses and influenza viruses are being developed.
3.2 Intracellular inhibitors of virus replication
Inhibiting virus replication through interfering with viral enzymes active within cells poses additional problems in drug discovery; however, several useful inhibitors for respiratory tract viruses have found their way into phase I/II clinical trials. Few, however, have been specifically tested in asthma. Favipiravir is a novel antiviral compound that selectively and potently inhibits the RNA-dependent RNA polymerase of many RNA viruses including influenza virus, enteroviruses and paramyxoviruses.40 A phase III clinical trial of favipiravir for influenza therapy has been just completed in Japan and the United States. Rupintrivir is a potent, irreversible inhibitor of RV 3C protease. It has a broad antipicornaviral spectrum, but the development programme has been discontinued.41 The nucleoside inhibitor ribavirin is a synthetic purine nucleoside analogue exhibiting antiviral activity against a broad range of both DNA and RNA viruses in vitro.42 It is the only antiviral agent currently available against RSV infection, but its use has many concerns. ALS-008176 is an oral RSV replication inhibitor (a cytidine nucleoside analogue). In randomized, double-blind, clinical trial in healthy adults inoculated with RSV, more rapid RSV clearance and a greater reduction in viral load were observed in the groups of patients treated with ALS-008176 than in the placebo group.43 Small interfering RNA (siRNA)44, 45 is a novel technology for the development of antiviral agents. An aerosolized therapeutic siRNA, ALN-RSV01, was recently shown to downregulate replication of human RSV infection in adults experimentally infected with wild-type RSV.46 It was well tolerated, effective as well as decreased the incidence and progression of bronchiolitis obliterans syndrome in lung transplant recipients with naturally occurring RSV infection.47
The development of cidofovir and its derivative, brincidofovir, broad-spectrum antivirals active against five families of dsDNA viruses (including adenoviruses), are currently in phase III clinical trials.48
3.3 Inhibitors of viral release
The neuraminidase inhibitors prevent influenza virus release from infected cells and infection of adjacent cells. Oral oseltamivir and inhaled zanamivir are recommended for the treatment and chemoprophylaxis of influenza in children and adults.49 Inhaled laninamivir has shown good safety and efficacy profiles in the treatment for influenza in patients with chronic respiratory tract diseases and has been approved in Japan.50 Single dose of intravenous peramivir has been shown to be noninferior to oseltamivir and to have good safety profile also in patients with chronic respiratory tract diseases.51
3.4 Interferons and other nonspecific antivirals
Biologically, interferons (IFNs) are induced within hours of infection52 and consequently induce the expression of hundreds of antiviral effecter molecules blocking virus replication.53 IFN therapy is an important consideration in asthma as several studies show that asthmatics lack adequate type I (IFN-α/IFN-β) and type III (IFN-λ) expression (reviewed in Ref. 2). Case reports have suggested IFN-α to be clinically effective in the treatment of difficult-to-control asthma, severe adult respiratory syndrome virus infection and persistent RV infections in hypogammaglobulinaemia patients. Analysis of a subgroup of those with more difficult-to-control asthma in a clinical trial has suggested that inhaled IFN-β may be a potential treatment for virus-induced asthma exacerbation.54 IFN-λ has similar antiviral properties in vitro as the type I IFNs.55 IFN-λs (IL-28A, IL-28B and IL-29) may also have promise as an antiviral or antiasthma drug.56 IFN-γ is considered predominantly as immunomodulatory agent and has additional properties to being antiviral.
3.5 Summary and outlook
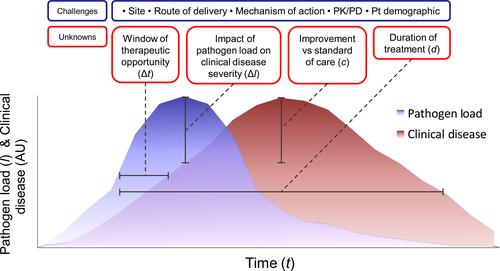
Influenza virus, RSV and RV infections make up the predominant clinical burden of respiratory viral illnesses. For influenza virus infection, approved treatments exist, but for RSV infection, there is only prophylaxis available for high-risk infants. These approaches are yet to be tested specifically in asthma. Intensive research is needed to develop clinically feasible antivirals against RV, as it is the main trigger of asthma. Another important point is suitable models to test new antivirals, and how these are implemented in antiviral testing and development. Recruiting and testing large patient numbers of asthmatics with a specific endophenotype in clinical trials of natural infections is logistically difficult. Virus challenge models that are available for RV57, 58 and RSV34 may avoid some of these constraints, as lower numbers of patients are employed, but they are yet to be used specifically to test antivirals in asthma. This is an important discussion point, and the challenges and unknowns associated with these infection models are presented in Figure 3. These general points can be equally applied to other microorganisms.
4 ANTIFUNGALS
4.1 Severe asthma with fungal sensitization (SAFS) & Allergic bronchopulmonary aspergillosis (ABPA)
Up to 50% of adult asthmatics who remain inadequately controlled despite maximal doses of inhaled corticosteroids are sensitized to one or more filamentous fungi such as Aspergillus fumigatus. This understanding has led to the term “severe asthma with fungal sensitization” (SAFS), which describes a grouping of asthmatics with severe disease but lacking some of the serological and radiological features of the related and more aggressive allergic bronchopulmonary aspergillosis (ABPA) (eg IgE <1000 IU/mL, absence of bronchiectasis). Globally, almost 5 million asthmatics have evidence of ABPA, whilst many more have SAFS.59 Corticosteroids remain the backbone of treatment in both SAFS and ABPA; however, steroid side-effects including osteoporosis and diabetes have driven the search for alternative therapeutic options. Of these, the azole antifungal agents have been most widely investigated following the assumption that the allergic airways inflammation is primarily driven by the presence of airway fungal infection.
4.2 Azoles and other antifungals
The effectiveness of itraconazole in the treatment for ABPA has been confirmed in two randomized, placebo-controlled trials60, 61 leading to the recommendation for azole use in asthma-ABPA by the Cochrane collaboration.62 Pooled data from these studies suggest itraconazole is effective in around 60% of asthma-ABPA patients.63 Newer triazoles including voriconazole and posaconazole have also been studied in ABPA with promising results. However, the greater incidence of drug toxicity with voriconazole, and substantial financial costs of both voriconazole and posaconazole limit their current widespread use.
More recently, the use of azoles has also been studied in SAFS. In the first such study by Denning et al,64 58 patients with SAFS were randomized to treatment with itraconazole or placebo. A significant improvement was seen in the primary end-point (quality of life score). However, whilst 60% of SAFS patients were identified as responders to itraconazole, five of 29 treated patients were discontinued due to side-effects and half the patients had evidence of cortisol suppression as a result of drug-drug interactions. In addition, a similar trial of voriconazole in a group of SAFS patients failed to show a significant clinical benefit, leaving the future of azole treatment in SAFS uncertain.65
A possible alternative to azole therapy is the antifungal amphotericin B, which was first used in 1959 for a case of chronic pulmonary aspergillosis. Whilst published data remain largely limited to case reports, in a recent study of patients with SAFS or ABPA, nebulized amphotericin B failed to produce a clinical benefit in 18 of 21 subjects with over half having to discontinue treatment due to bronchospasm.66 Consequently, the prospect of amphotericin B use in asthma remains unlikely.
The majority of reports to date have investigated the use of antifungal agents in adult asthmatics; however, the potential for their use in children has also been highlighted in a recent paediatric study describing sensitization to fungal allergens in 59% of severe asthmatic children.67 Unfortunately, reports of azole use in asthmatic children are limited to a few subjects only leading to the current joint ERS/ATS guideline recommending the consideration of treatment only after detailed evaluation in a specialist severe asthma centre.68
4.3 Summary and outlook
In summary, to date, there have only been a handful of placebo-controlled trials of azoles in asthma and all have suffered from being relatively small in size and with varying treatment durations. It remains unknown whether any potential benefit relates to their antifungal activity or to alterations in GC metabolism; in addition, the optimal duration of therapy provides a further unknown. Data on the alternative antifungal agent inhaled amphotericin B are even more limited. In all cases, safety concerns require antifungal agents to be used with caution in asthma in a specialist centre where close monitoring is readily achievable. As our understanding of the heterogeneity of asthma continues to develop, it is possible that subgroups within ABPA/SAFS will emerge in whom treatment with antifungal therapy will be predictably beneficial. However, we still remain some distance away from this goal at present.
5 VACCINES
5.1 Influenza vaccines
Influenza infections can precipitate acute AEs and may be more severe among asthma patients69 for whom annual vaccination against seasonal influenza is therefore recommended. There is, however, uncertainty about the degree of protection that either inactivated or live attenuated influenza vaccination (LAIV) afford against influenza-associated AE.70 There are only limited studies of influenza-related AE following trivalent split-virus influenza vaccination (TIV). These studies did not find significant effects of TIV on the number, severity or duration of AEs during influenza infection, but vaccinated children displayed lower symptom scores during influenza-positive weeks. More studies are needed to validate these findings. A comparison of LAIV with TIV also failed to show significant differences in AE numbers in adults or children (>6 years).70 Thus, the potential role of influenza vaccines in AE prevention requires further in-depth study, in virologically confirmed influenza and with improved definition and characterization of asthmatic subgroups.
Conversely, influenza vaccination itself has been alleged to cause AEs. Three large cross-over trials show no evidence of increases in AEs in the two weeks following TIV in adults or children.71 Concerns that LAIV could increase AEs, wheezing episodes and hospitalizations in children70 were allayed the absence of an association of LAIV with AE in high-risk children.72 The SNIFFLE trials, combined, assessed LAIV in egg-allergic children, including 634 children with asthma/recurrent wheeze (79.7% on daily preventer treatment).73 Immediate systemic reactions did not occur, but for 6.6% of children with asthma/recurrent wheeze diagnosis, parents reported wheeze within 72 hours of LAIV. However, most wheeze episodes only required routine treatment, there was no hospitalization, and no increased respiratory tract symptoms in the 4 weeks after LAIV. Based on these findings that suggest that LAIV is well tolerated in children with well-controlled asthma/recurrent wheeze, immunization advice for the United Kingdom now recommends LAIV for 2- to 18-year-old children, unless they have severe or acutely exacerbated asthma.
Novel experimental strategies to develop a universal influenza vaccine aim to stimulate immune responses to the conserved stalk of influenza haemagglutinin,74 in theory allowing for cross-immunization against several influenza virus subtypes.
5.2 Pneumococcal vaccines
The presence of Streptococcus pneumoniae (pneumococcus) has been linked to AE, and in children, early-life pneumococcal colonization is associated with an increased risk of wheezing and asthma later in childhood.4 Furthermore, asthma is a risk factor for invasive pneumococcal disease (IPD),75 warranting pneumococcal vaccination, which is recommended for all people with asthma over 6 years of age if they did not receive routine childhood pneumococcal immunization.76 The pneumococcal polysaccharide 23-valent vaccine (PPSV-23) offers protection against the most common invasive serotypes from 6 years of age, whilst pneumococcal conjugated vaccines are effective against early childhood IPD, antibiotic-resistant strains and prevent nasopharyngeal carriage. Studies in asthmatic children and adolescents addressing pneumococcal colonization, IPD burden, immune responses to pneumococcal vaccination and its potential benefit against AE are currently lacking.77 In a 2002 Cochrane review,78 only one study involving PPSV-23 vaccination of asthmatic patients was eligible for inclusion and reported fewer AEs following vaccination. Another study did not observe a difference in the risk of hospitalization due to pneumococcal pneumonia following PPSV-23 vaccination comparing asthma patients to control subjects.79
5.3 Experimental vaccines for RSV and RV
Vaccines for RV and RSV that are associated with AEs and asthma inception are lacking. RSV vaccine research has been hampered by formalin-inactivated RSV immunization trials in infants and children in 1966, which resulted in increased disease severity upon first natural RSV infection, with hospitalization of 80% of vaccinees and 2 deaths.80 Candidate vaccines against RSV, currently in clinical trials, include live attenuated viruses, gene-based vectors, RSV-F-nanoparticles and RSV-F subunit vaccines. Preclinical studies have identified a highly conserved RV capsid protein that induces cross-reactive cellular and humoral immune responses to heterologous RV strains,81 and may allow the development of a broadly cross-reactive subunit RV vaccine.
5.4 Other routine childhood vaccines
There has been concern over potential pro-allergic and pro-asthmatic effects of childhood immunizations, in particular regarding measles, mumps and rubella vaccine (MMR). However, the suspected association between MMR and asthma (since been discredited) was based on a small study comparing anthroposophic to nonanthroposophic children and was not confirmed in a subsequent multinational study in these populations.82 A wealth of studies has also failed to show evidence of pro-asthmatic effects of vaccination against poliovirus, pertussis, tetanus, hepatitis B or Haemophilus influenzae. In contrast, significant evidence suggests antiasthmatic effects of childhood vaccinations. Inverse associations between childhood asthma prevalence and high cumulative vaccine doses,83 pertussis vaccination84 and MMR85 have been found and reduced asthma hospitalization rates and medication use in MMR-vaccinated children.86
The immunogenic potential of different vaccine formulations in asthma has been questioned, but normal antibody responses to pertussis, varicella, hepatitis B, measles and rubella vaccination have been reported. Mumps antibody titres after MMR were lower and measles antibodies waned more quickly from 9 years of age in asthmatics. Treatment with inhaled glucocorticoids (GCs) did not affect the antibody response to hepatitis B or varicella immunization, but these were impaired by oral GC therapy.
5.5 Summary & Outlook
The vaccinations discussed are safe and effective in asthma, may help prevent asthma development, and pneumococcal and annual influenza vaccination in particular should be offered to asthmatics. Future vaccines against RV and RSV, which are the main triggers for AEs and have been linked to asthma inception, should help reduce asthma morbidity and mortality.
6 IMMUNOMODULATORS THAT ALTER HOST RESPONSES
6.1 Bacterial lysates
Bacterial lysates (BL) have been extensively used in Europe to effectively reduce the number of seasonal acute respiratory illnesses (ARI). BL are microbial products that when given orally, may exhibit certain immunostimulatory and immunomodulatory effects.87 Their effectiveness was confirmed in numerous interventional trials (reviewed in ref. 88). BL have been successfully tested in preventing wheezing attacks provoked by ARIs in preschool children demonstrating a 38% reduction in symptomatic wheezing and a decrease in the number of URTI.89
There is only one interventional clinical study to date, demonstrating a promising antiallergic potential of an orally applied BL.90 Therefore, one may speculate that bacteria-derived preparations may become an interesting class of immune modulators for the future.
6.2 Toll-like receptor (TLR) agonists
Recognition of invading microbes is controlled by a range of innate pattern recognition receptors including TLRs which are directed at highly conserved molecular motifs expressed on the invading pathogen. This ancient surveillance network provides a form of first line of defence in the airway.91 Signalling through TLRs produces a broad range of pro-inflammatory cytokines, chemokines and importantly, antimicrobial proteins. Therefore, precise targeting of individual TLR pathways could provide a mechanism of promoting specific immunity, allowing for tailored immunomodulation, an approach that is highly attractive in chronic diseases such as asthma where numerous immunological disparities are evident.
A number of approaches targeting TLRs have shown efficacy in suppression of airways inflammation, granulocytic influx and airways hyper-responsiveness in various animal models. These have proved a platform for progression into clinical studies. Therapeutic and prophylactic administrations of TLR agonists for TLR4,92 TLR7,93, 94 TLR895 and TLR996 have been employed broadly in the atopic inflammatory disease setting, particularly in rhinitis as allergen immunotherapeutic agents due to the immunomodulatory potential of signalling via these pathways. The capacity for TLR agonists to elicit robust immunological responses has led to their exploitation as adjuvants for novel vaccine approaches with proven efficacy and longevity.97, 98 To date, a small but growing number of studies have been conducted applying TLR signalling approaches in the context of asthma and AE.
The TLR9 agonist CYT003A (formerly QbG10), a bacteriophage capsid filled with A-type CpG, has shown significant clinical benefit when used alone or in combination with specific allergen extracts for the treatment of rhinitis99 and rhinoconjunctivitis.100 Moving forward to a phase 2a proof of concept double-blind, placebo-controlled trial in 63 mild-to-moderate atopic asthmatics on steroids, incorporating a controlled GC withdraw period, repeated subcutaneous of the TLR9 agonist allowed for considerable or complete withdrawal of inhaled GCs whilst asthma conditions remain stable or even improved.101 The proposed mechanism is the restoration of the Th1/Th2 balance. However, supporting evidence is yet to be provided. A follow-up, double-blind, placebo-controlled study carried out in poorly controlled, moderate-to-severe asthmatics where CYT003A was administered as an add-on to current GCs and β2-agonist therapy showed no significant advantage over placebo in relation to the primary outcome of change from baseline in Asthma Control Questionnaire (ACQ) or in secondary outcomes of change from baseline in prebronchodilator forced expiratory volume (FEV1).102 These data suggest that whilst CYT003A may have a use in initial control of disease in GC-sensitive patients, it has no efficacy as an add-on therapy for more severe patients. In addition, more prolonged administration of the TLR9 agonist, assessing its ability to readdress the supposed Th2 skewing, was not conducted. Additional concerns stem from recent reports of potential impairment of TLR9 function in PBMCs in severe asthmatic patients.103
6.3 Vitamin D
Evidence is accumulating on the potential of vitamin D in immunoregulation, particularly in lymphocyte function and cytokine production, suggesting its potential in modifying asthma incidence and severity.104 The association between low levels of vitamin D and asthma has been supported by many observational and epidemiologic studies.105 A recent meta-analysis of epidemiological studies demonstrated a positive association of vitamin D deficiency and asthma (RR 1.68 95% CI 1.3-2.2).106 Parallel-conducted, prospective, observational studies on vitamin D supplementation in infancy, however, showed rather conflicting data and do not support these implications.107 Other studies in pregnant woman, including two large clinical trials, did not find protective effects on offspring wheeze.108, 109 Interpretations of existing evidence argue an advantage of vitamin D supplementation.107 Though, another current systematic review with meta-analysis in paediatric asthmatics noted a major reduction in AE with vitamin D supplementation (RR 0.41, 95% CI 0.27-0.63),110 an effect that is attributed to both an anti-infective and immunostimulatory activity of vitamin D; however, these effects are challenged in the view of the most recent reports.111
Vitamin D exhibits immunostimulating effects upon both innate and adaptive immunity and demonstrates antiviral effects against a vast variety of viruses in vitro. Therefore, attempts were made to determine whether vitamin D supplementation prevents acute respiratory tract infections in healthy and asthmatic children. According to a systematic review,112 children previously diagnosed with asthma may experience a 74% reduction in the risk of AE (RR 0.26, 95% CI 0.11-0.59).112 More studies are needed to provide a larger and more powerful meta-analysis before the benefits of vitamin D can be stated with confidence.
6.4 Summary and outlook
Whilst the efficacy of disease-modifying specific TLR stimulation and use of BL in stable asthma continues to be explored, there is a clear agenda for assessment of these agents in prevention of AE. Benefits for the use of TLR agonists include the potential ability to reshape long-standing immune responses/polarity; good PK/PD profiles; good stability and ease of delivery; and reduced risk of adverse effects. Several controlled clinical trials have been undertaken to explore the effect of vitamin D supplementation on asthma prevention and control, as well as respiratory tract infections. In view of the recent randomized controlled trials, vitamin D cannot be recommended as adjunctive therapy for asthma or asthma prevention. Nevertheless, its preventive activity, particularly in AEs, seems promising.
7 MICROBIOME MODULATORS-PROBIOTICS
7.1 Prevention of infection
Probiotics are preparations of live microorganisms that may colonize the intestinal tract and/or compete with pathogens, and may also stimulate the immune system. An interesting aspect of the immunostimulatory effects of probiotics is their ability to lower the number of upper respiratory tract infections (URTIs) that may lead to AE. In this respect, probiotics were superior to placebo in diminishing the incidence of acute URTIs in the general population.113, 114 Despite several methodological issues, this concept definitely warrants further investigations in asthma.
7.2 Probiotics may modulate asthma and/or allergy
Animal studies have shown that modification of the microbiota modulates the systemic immune responses of the host, resulting in lower sensitization rates and reducing allergic inflammation.115 However, interventional trials in infants receiving both pre- and probiotics in allergy and/or asthma prevention provided equivocal results.116 Whilst moderate positive effects in infant eczema were found, a lack of evidence that probiotics prevent any other allergy including asthma in three other similar meta-analyses remains a matter of concern.117-119 In view of two well-conducted meta-analyses, an opportunity of asthma/wheezing prevention with probiotics seemed to not be plausible.118, 119 One may speculate, however, that the studies to date may not have used the right probiotic, the right dose, the right timing or duration and/or population. Therefore, more studies are still needed.
7.3 Summary and outlook
Evidence to date suggested that modulation of the gut microbiome may represent an interesting therapeutic or preventative opportunity for the prevention of allergic asthma and AE, whilst clinical trials do not confirm initial enthusiastic expectations. In view of the recent systematic reviews, probiotics cannot be recommended as adjunctive therapy for asthma or asthma prevention. Though, recent technological developments that permit identification of the most promising microbial strains and their products that may exhibit more profound positive effects will keep this area active and interesting to follow.
8 CONCLUSIONS
With the exception of antibiotics and antifungals, current guidelines do not take into account the potential of specific or broader-spectrum anti-infectives or immunomodulatory agents in asthma or AE. Despite wide interest and active research in this area, the basis for this is likely due to a lack of clinical studies that allow robust or clear conclusions to be made regarding efficacy. For the clinical studies that have been performed with available anti-infectives or immunomodulatory agents, conclusions are often contradictory or show a lack of robust evidence supporting an effect. Reasons for these outcomes include a small number of studies, differences in study design making direct comparisons difficult or studies that are underpowered for primary or secondary endpoints, or subgroup analysis. As shown in Table 3, for asthma there is a lack of studies in infancy and only occasional studies focusing in the elderly or taking into account older people. There is a clear and explicit need for not only more, large clinical studies investigating these agents in asthma, but thoughtfully designed studies with the ability to address definitive clinical efficacy and understand detailed mechanisms of action within the context of this complex heterogeneous disease. Only then, when appropriate supportive clinical studies are performed, can guidelines responsibly include educated approaches for the use of anti-infectives and immunomodulatory-based treatments in asthma control and management. With the increase in new human challenge models for respiratory tract viruses, coupled with the arsenal of small-molecule anti-infectives and biologics available, for antivirals, the future heralds a promising outlook.
| Age group | Treatment option | Supporting evidence |
|---|---|---|
| Pregnancy | ||
| Infancy/Preschool Age | Antivirals | 32, 33 |
| Macrolides | 27, 28 | |
| Immunomodulators | 86, 89 | |
| Vaccines | 84 | |
| School Age | Antivirals | 49 |
| Antifungals | 49 | |
| Immunomodulators | 110, 112 | |
| Vaccines | 76, 78 | |
| Probiotics | 113 | |
| Adulthood | Antivirals | 36, 46, 54 |
| Macrolides | 25, 26 | |
| Antifungals | 60, 61 | |
| Immunomodulators | 99-101, 110 | |
| Probiotics | 113 | |
| Elderly | Macrolides | 121 |
| Vaccines | 122 | |
| Immunomodulators | 123 | |
| Probiotics | 113 |
There is also a substantial body of literature investigating preclinical development of anti-infectives and immunomodulatory drugs and preparations in animal models, with many studies endeavouring to better understand the scientific principles behind their mechanism(s) of action. This is an absolute necessity in the anti-infective drug discovery pipeline; however, there is some confusion regarding how best to progress these anti-infectives in these studies in clinical trials, or alternatively, how interpretations of preclinical work can best inform on future clinical trials is a subject of wide debate. Much consideration still needs to be given to how future drug targets related to anti-infectives are progressed, and how drug discovery programmes are best implemented to exploit these.
ACKNOWLEDGMENTS
This manuscript is the result of a Task Force by the Interest Group on Infections and Allergy (IG Infections and Allergy) of the European Academy of Allergy and Clinical Immunology (EAACI) and received funding from the EAACI and was also supported by RSF Grant N° 14-15-00894.
CONFLICT OF INTEREST
All authors are members of EAACI and declare no other competing interests in respect to this publication.
AUTHOR CONTRIBUTIONS
Musa Khaitov (Task Force chair) and Michael Edwards (Task Force secretary) initiated this TF in 2014 to assess the potential use of anti-infectives as treatments for asthma and asthma exacerbations. M. Edwards, R. Walton, D. Jackson, W. Feleszko, C. Skevaki, T. Jartti, H. Makrinoti, A. Nikonova, I. Shilovskiy, J. Schwarze, S. L. Johnston and M. Khaitov contributed to this manuscript with their expertise. All authors participated in the discussion and approved the final version of this position paper.




