Novel approaches and perspectives in allergen immunotherapy
Abstract
In this review, we report on relevant current topics in allergen immunotherapy (AIT) which were broadly discussed during the first Aarhus Immunotherapy Symposium (Aarhus, Denmark) in December 2015 by leading clinicians, scientists and industry representatives in the field. The aim of this symposium was to highlight AIT-related aspects of public health, clinical efficacy evaluation, mechanisms, development of new biomarkers and an overview of novel therapeutic approaches. Allergy is a public health issue of high socioeconomic relevance, and development of evidence-based action plans to address allergy as a public health issue ought to be on national and regional agendas. The underlying mechanisms are in the focus of current research that lays the ground for innovative therapies. Standardization and harmonization of clinical endpoints in AIT trials as well as current knowledge about potential biomarkers have substantiated proof of effectiveness of this disease-modifying therapeutic option. Novel treatments such as peptide immunotherapy, intralymphatic immunotherapy and use of recombinant allergens herald a new age in which AIT may address treatment of allergy as a public health issue by reaching a large fraction of patients.
Abstract

Abbreviations
-
- AEC
-
- allergen exposure chamber
-
- AIT
-
- allergen immunotherapy
-
- AR
-
- allergic rhinitis
-
- CSMS
-
- combined symptom and medication score
-
- DC
-
- dendritic cell
-
- EAACI
-
- European Academy of Allergy and Clinical Immunology
-
- EMA
-
- European Medicines Agency
-
- Fet A
-
- fetuin A
-
- GAP
-
- Grazax Asthma Prevention
-
- HRQL
-
- Health-Related Quality of Life
-
- IL
-
- interleukin
-
- ILIT
-
- intralymphatic immunotherapy
-
- IT
-
- immunotherapy
-
- NCT
-
- nasal challenge test
-
- OIT
-
- oral immunotherapy
-
- SCIT
-
- subcutaneous immunotherapy
-
- SLIT
-
- sublingual immunotherapy
-
- SPIRE
-
- synthetic peptide immunoregulatory epitopes
-
- SPT
-
- skin prick test
-
- VAS
-
- visual analog scale
-
- WHO
-
- World Health Organization
Allergies have become a public health concern of pandemic proportions that affect >150 million Europeans. More alarming, their prevalence and impact are on the rise. It has been predicted that within the next few decades, up to half of the European population may at some point in their lives experience some type of allergy 1. Allergen immunotherapy (AIT) is the only currently available medical intervention that can limit the natural course of the disease 2. Years of preclinical research, clinical trials, systematic reviews and meta-analyses have convincingly shown that AIT can achieve significant reduction of symptoms of patients, improving the allergic individuals’ quality of life, changing the course of the disease and reducing the long-term costs and burden of allergies 2-5. These effects of AIT are of utmost importance from the public health point of view.
Nevertheless, despite these advances during the past century (Fig. 1) AIT usage varies across the globe with market penetration rates (the fraction of possible sales achieved) from <1% in emerging markets in Asia to 20% in the United States 6-8, so there is clearly room for improved allergy management and new innovation in the field to unleash this potential. Within this context, leading researchers from academia and industry discussed the latest advances in the field at the first Aarhus Immunotherapy Symposium on 2 December 2015. This review summarizes the main findings from the symposium on the current understanding of allergic disease mechanism, new emerging technologies, development of diagnostic and therapy monitoring technologies and disease management programmes including prevention.
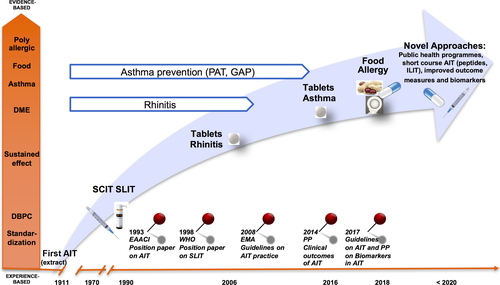
Tolerance induction and prevention in allergy – a public health issue
The prevalence of allergic diseases has increased in many industrialized and urbanized countries during the last 50 years. Although the origin of allergy remains unresolved, increasing evidence indicates that modern living in an urban environment is deprived of environmental protective factors that are fundamental for normal tolerance induction 9. The concept of induction of immune tolerance has become a prime target for prevention and treatment strategies for many chronic inflammatory diseases such as allergy, asthma and autoimmunity in which dysregulation of the immune system plays an essential role.
There are few nationwide, comprehensive public health programmes on allergic disorders with defined goals and systematic follow-up 10. One practical example is the Finnish Allergy Programme 2008–2018. It is based on the idea that the allergy epidemic in modern societies is caused by inadequately developed or broken tolerance 11. Whilst allergies have traditionally been associated with industrial countries, they are also endemic in the developing world. Recent reports indicate that the prevalence of allergic disease in the Asia–Pacific region has reached the highest levels in 50 years 12, a trend also observed in tropical South-East Asia 13, 14. Although a similar set of allergens to those eliciting responses from populations in western regions has been proposed, the course, specificity and complexity of the allergic response in tropical countries may differ substantially from that in temperate zones. In a recent study of two independent cohorts of ethnic Chinese living in Singapore, the allergic response in a tropical urban environment was dominated by house dust mites 15. This monospecific IgE sensitization translates into increased prevalence of allergic airway diseases, which now impact a large proportion of the population in Singapore. It presents a unique opportunity to treat and manage these patients with AIT.
As the first allergic sensitization is a strong predictor for ensuing sensitizations, it should be treated with (i) AIT in addition to (ii) individual guided self-management, (iii) symptomatic treatment and possibly with (iv) avoidance of allergens that clearly worsen the symptoms. The WHO AIT guidelines from 1998 state that AIT acts globally on IgE-mediated inflammation in various organs 2. Allergen immunotherapy could be administered in primary health care setting with enough know-how according to the national AIT guidelines based on international guidelines, as well as student and occupational health care where feasible 16. Subcutaneous immunotherapy (SCIT) has proven value but requires specialist supervision due to associated risks of severe allergic reactions. The sublingual route is an effective and safer alternative suitable for daily self-administration.
From the public health point of view, an important fact is that allergic rhinitis is a risk factor for asthma. Allergen immunotherapy is considered to prevent asthma in patients treated with AIT for allergic rhinitis in clinical trials 17 as well as in real-world settings 18. Confounding by indication cannot be excluded but would lead to an underestimation of the true preventive effects of AIT.
The Grazax Asthma Prevention (GAP) trial, investigating the preventive effect on asthma development of grass AIT tablet in children aged from 5 to 12 years with grass pollen-induced allergic rhinitis (AR), is the first double-blind, placebo-controlled randomized trial to assess the preventive effects of AIT 19. Although the trial did not achieve its primary endpoint of preventing asthma, the asthma diagnosis criteria used in the time to onset analysis were rather rigid and dependent on objective tests of reversibility. Even so children treated with the grass tablet AIT had a significantly reduced risk of experiencing asthma symptoms and a reduced use of asthma medication not only during the 3 years of AIT but also for 2 years after discontinuation. They also had a beneficial effect on their allergic nasal and eye symptoms throughout the 5 year study period 20. Thus, the results of the GAP trial confirmed the disease-modifying effect of grass tablet AIT on grass pollen-induced asthma and rhinoconjunctivitis in children.
Early introduction of allergenic food has been shown to be good primary prevention of peanut allergy 21, which may face difficulties when introduced in primary care 22. Despite the lack of effect in the intention-to-treat analysis and reluctance to introduce solids as early as at 3 months of age, the reduction in prevalence by 2/3 in the per-protocol analysis when ingesting 2 g of peanut or egg protein is encouraging. Oral immunotherapy (OIT) of IgE-mediated food allergy has been shown to desensitize individuals at risk of or having experienced severe allergic reactions against peanut 23, egg 24, 25 and cow's milk 26. However, these oral desensitization protocols were experimental, had low success rates, carried significant risks of inducing anaphylaxis and, in contrast to immunotherapy for inhalant allergens, have not been shown to induce long-term tolerance after discontinuation. Although the data are encouraging, current guidelines confine OIT for food to research protocols in the hands of trained allergy specialists and are not recommended for routine clinical practice.
Allergen immunotherapy has not yet received adequate attention from European institutions, and thus far, too many allergic patients in the general population remain unaware of the benefits of AIT 3. It is time to re-evaluate the allergy paradigm and implement new kinds of actions as allergic individuals are becoming a significant minority of Western populations, and their number is increasing worldwide. National and regional action plans, such as the Finnish Allergy Programme 2008–2018 11, are needed to meet this challenge.
Evaluation of efficacy in AIT trials
To advocate that AIT resolves the unmet need of allergy in public health, good, standardized assessment of clinical efficacy is mandatory. Throughout the last decade, increasing emphasis has been put on clear guidance in standardization of clinical trials in the field of AIT 27, 28. Besides these academic positions, the European Medicines Agency (EMA) 29 and the Center for Drug Evaluation and Research of the US Food and Drug Administration 30 have published regulatory guidelines which outline standards for the clinical development and documentation of new products in clinical trials. The relevant EMA guideline 29 requires that the primary endpoint in AIT trials has to ‘reflect both, symptom severity as well as the intake of rescue medication’ and, moreover, specifies several secondary outcome parameters. However, it also highlights that at present ‘no validated symptom score exists’ and ‘different approaches to combine symptom score and intake of rescue medication are possible’. Recently, a Task Force initiative from the European Academy of Allergy and Clinical Immunology (EAACI) recommended a standard for the primary endpoint for future randomized controlled trials in AIT for allergic rhinoconjunctivitis, the ‘combined symptom and medication score’ (CSMS; Table 1 31). Besides these parameters, several secondary endpoints such as Health-Related Quality of Life Questionnaires, visual analog scales or ‘Global Assessments’ are also used in AIT trials 32, 33. Among these secondary endpoints, allergen provocation tests (conjunctival, bronchial or nasal provocations) can be performed that directly measure the allergen sensitivity (and possible changes throughout the course of AIT) in the allergic target organ 29. Therefore, the EMA also recommends provocation tests to be used as primary endpoints in dose-finding trials of AIT 29 (EMA register of clinical trials at www.clinicaltrialsregister.eu). For pivotal phase III trials, these tests ‘can give additional information but are no surrogate markers and cannot replace the measurement of clinical symptoms’ 29. Exposure in an allergen exposure chamber (AEC), through standardized protocols under controlled environmental conditions (temperature, humidity), is an attractive alternative. Their technical validation underlines their principal advantage compared to other challenge methods 34. These models have been used to demonstrate the proof of concept, onset of action and magnitude of clinical effects 35-37. A clear unmet need in the future is a thorough technical standardization and (clinical) validation within and between different AEC models 31, 34.
| (A) Symptom score | ||
| Nasal symptoms | (Score 0–3) | 0 = no symptoms |
| 1 = mild symptoms (sign/symptom clearly present, but minimal awareness; easily tolerated) | ||
| 2 = moderate symptoms (definite awareness of sign/symptom that is bothersome but tolerable) | ||
| 3 = severe symptoms (sign/symptom that is hard to tolerate; causes interference with activities of daily living and/or sleeping) | ||
| Itchy nose | 0–3 | |
| Sneezing | 0–3 | |
| Runny nose | 0–3 | |
| Blocked nose | 0–3 | |
| Conjunctival symptoms | Itchy/red eyes | 0–3 |
| Watery eyes | 0–3 | |
| (Total) daily symptom score (dSS)a | 0–3 (max score is 3, i.e. 18 points/divided by six symptoms) | |
| (B) Medication score | ||
| Oral and/or topical (eyes or nose) nonsedative H1 antihistamines (H1A) | 1 | |
| Intranasal corticosteroids (INS) with/without H1A | 2 | |
| Oral corticosteroids with/without INS, with/without H1A | 3 | |
| (Total) daily medication score (dMS) | 0–3 (max score is 3) | |
| (C) Combined symptom and medication score | ||
| CSMS | dSS (0–3) + dMS (0–3) | 0–6 |
- a Max score 18/6 (i.e. four nasal symptoms, max score 12; and two conjunctival symptoms, max score 6) is optimal for studies of seasonal pollinosis. This could possibly be modified for studies of perennial allergies (e.g. in mite-allergic patients), for example max score 12/4 (i.e. four nasal symptoms with omission of eye symptoms). By assigning 0–3 for all individual symptoms and dividing by total number of symptoms, the symptom range 0–3 and maximum symptom score 3 would remain the same.
Mechanisms of AIT
Long-term clinical improvement associates with humoral and cellular modifications to the allergen-specific immune response 38. Allergen immunotherapy may reduce the risk of progression from allergic rhinitis to asthma 17. There are some data to suggest that AIT may reduce the risk of developing new sensitizations to allergens, although further studies are needed to clarify this 39, 40. A greater understanding of the underlying mechanisms (Fig. 2) following the allergen-specific interventions of SCIT and sublingual immunotherapy (SLIT) is important for better understanding of the disease, for the development of predictive biomarkers and to assist the rational design and testing of novel more effective and safer immunotherapy strategies.
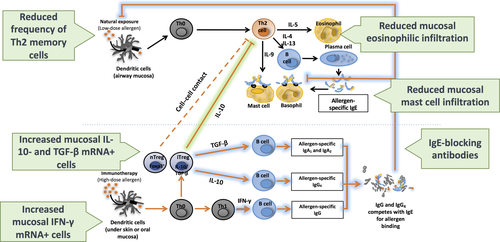
Allergic rhinitis is characterized by IgE synthesis, mast cell activation and tissue eosinophilia, events under the regulation of Th2 cytokines that are produced preferentially by CD4+ helper Th2 cells but also by mast cells, basophils and innate lymphoid cells (ILC2s). Subcutaneous immunotherapy is associated with a decrease in effector cells in target organs, transient increases in allergen-specific IgE followed by blunting of seasonal increases in IgE and a marked increase in IgG, particularly IgG4 41 (Fig. 2). For example, grass pollen SCIT resulted in a decrease in numbers of mast cells 42 and eosinophils 43 in the skin at sites of suppressed allergen-induced late cutaneous responses. Subcutaneous immunotherapy suppressed late nasal responses that paralleled decreases in local c-kit+ mast cells 44, eosinophils 45 and basophils 46 in the nasal mucosa. Recent studies have shown suppression of basophil activation 47, 48 and a decrease in circulating ILC2s 49.
Akdis et al. 50 first introduced the concept of early involvement of regulatory T (T reg) cells. Bee venom SCIT resulted in increased IL-10 production from both blood T and B cells. T reg involvement was confirmed by flow cytometry following grass and house dust mite SCIT 51, 52. Local induction of T regs following grass SCIT was shown by increases in IL-10+ 53 and TGF-beta+ T cells 54 in the nasal mucosa that accompanied increases in serum IgG4 and IgA (in keeping with their known properties in promoting preferential switching of B cells in favour of IgG4 and IgA) and an increase in local nasal FOXP3+CD25+, IL-10-producing T cells 55 by immunofluorescence histology.
The mechanism of SLIT has been shown to be broadly similar to SCIT; in particular, SLIT also induces IL-10 production by T cells 56. Nasal and conjunctival eosinophils and adhesion molecules decrease following mite SLIT 57. Serum allergen-IgG increased after birch SLIT. Higher local numbers of CD4+FOXP3+ and CD25+FOXP3+ T cells were found in the sublingual mucosa and increases in peripheral allergen-specific IgG (IgG1 and IgG4) and IgA2 following grass SLIT 58, 59. Blood Th1 cells increased and blood Th2 cells decreased following birch SLIT 60. Peripheral CD25+FOXP3+, presumed T reg cells, increased after SLIT 61, findings confirmed in relation to grass SLIT 62. Two recent studies showed that birch 63 and mite 64 SLIT resulted in early increases in T regs at 4–6 weeks and delayed increases in allergen-specific Th1 cells at 12 months. The mechanism of SCIT and SLIT may differ in terms of kinetics, quality and quantity of circulating antibody. Transient early increases in specific IgE are greater after SLIT compared to SCIT, whereas both inhibit seasonal increases in IgE to the same degree. Conversely, immunoreactive IgG levels are approximately 10-fold less for SLIT compared to SCIT 59, 65, whereas both are accompanied by equivalent increases in serum IgG-associated IgE-blocking activity. This disparity suggests that IgG antibodies produced by SLIT may be more functionally active, with greater avidity or affinity for allergen. Although speculative, this could possibly occur as a consequence of more efficient antigen processing and/or T–B cell cooperation in the local environment of the sublingual mucosa and draining cervical lymph glands 66.
The LEAP and LEAP ON studies have brought major advance to our understanding of primary prevention of food allergy, as the number of high risk individuals regularly exposed to allergen acquired fivefold less food allergy than control individuals who avoided allergen, as suggested by international guidelines 21.
The demands to OIT are high as consumption of allergen may result in severe symptoms 67. Adverse side-effects during conventional OIT can be severe, and only half of the enrolled patients reach maintenance dose 23, 68. Half again could be maintained on maintenance dose for planned duration of treatment, and between a quarter and half of these patients had sustained unresponsiveness or developed persistent tolerance 24, 67. To improve this performance, treatment is attempted with hypoallergic allergens 25 or under a blanket of anti-IgE treatment to limit side-effects 26. Persistent biomarker changes associated with successful primary prevention and OIT are a reduction of skin prick test (SPT) response and improvement in basophil reactivity and sensitivity 67, 69.
Biomarkers for diagnosis and monitoring in AIT trials
Patients with an indication for AIT respond with individual side-effect profiles, degree of symptom relief and persistence of treatment effect (Table 2) 70-74. This may be due to the diversity of their pattern of IgE sensitization (i.e. involvement of seasonal vs perennial allergens, mono- vs polysensitization), varying genetic susceptibility and comorbidities. Identification of biomarkers that predict benefit of AIT for the individual is therefore of high priority. According to the EMA guideline, biomarkers can be analysed in dose-finding studies but need to be validated 29.
| Categories of biomarkers | Applications | Candidate biomarkers |
|---|---|---|
| Biomarkers for diagnosis | Stratify patients to predict disease severity and/or progression |
Patterns of IgE sensitization 119 Ex vivo basophil responsiveness to the therapeutic allergen 73 |
| Biomarkers predictive of AIT safety |
Reduce risk and/or severity of side-effects Improve patient compliance |
Ex vivo basophil responsiveness to the therapeutic allergen 73 |
| Biomarkers of AIT efficacy (predictive or follow-up) |
Improve efficacy by selecting patients more likely to benefit from AIT Improve patient compliance Reduce cohort size needed for clinical development Confirm or not treatment efficacy after few weeks (early onset of efficacy) Document whether immune protection has been reached (support decision to pursue or stop AIT) Confirm lasting protection after stopping AIT (support decision to resume or not AIT) Adjust treatment modalities (e.g. dosing, immunization scheme) |
Fetuin A isoforms 74 Reduction of ex vivo basophil sensitivity to the therapeutic allergen 48, 73 Changes in T-cell or dendritic cell polarization reflecting a reorientation of Th2 responses towards regulatory/suppressive responses 78, 79 Induction of allergen-specific IgG4s and blocking antibodies 76 |
Knowledge of mechanisms underlying AIT has been translated into developing biomarkers beyond specific IgE to predict efficacy and side-effects, and to monitor clinical response to and persistence of immunotherapy. Basophil sensitivity 48 and IgG-associated serum inhibitory activity for IgE-facilitated binding of allergen–IgE complexes to B cells (IgE-FAB) correlated closely with clinical response to immunotherapy 75. The latter assay has been standardized 76 and translated into a cell-free solid phase ELISA-based assay 77. Allergen-stimulated basophil activation has been validated 73.
Unbiased identification of new biomarkers of AIT efficacy
Unbiased approaches have been used to find biomarkers for change in dendritic cells (DCs) during AIT. A 4-month SLIT course in grass pollen-allergic patients induced regulatory dendritic cells (DC regs) expressing high levels of C1Q and stabilin 78, paralleled by a decrease in the blood of pro-allergic DC2 markers such as CD141 and OX40L, which support differentiation of Th2 cells 79. This switch of the DC reg/DC2 balance was detected in peripheral blood by quantitative PCR only in patients exhibiting clinical responses.
A sialylated variant of fetuin A (Fet A), which is expressed at high levels in pretreatment sera from grass pollen-allergic patients who benefited from SLIT, but not in samples from nonresponders, was also identified by an unbiased approach 74. Fet A appears to modulate inflammation due to its capacity to interact with a broad variety of ligands including calcium, TGF-β, the insulin receptor, fatty acids and TLR4 and is involved in multiple inflammatory conditions and cancer 80. The novel link between Fet A and allergy was confirmed in preclinical models where post-translational modifications of Fet A could modulate allergic inflammation 74.
Basophil activation testing as a new biomarker in diagnosis for and monitoring of AIT
Basophil sensitivity and reactivity to allergen in a basophil activation test can measure effect of AIT in response to allergen exposure at the effector cell level in an accessible ex vivo assay 73.
In a study in ultrarush venom immunotherapy, AIT induced desensitization of basophils in response to both the treatment allergen and anti-IgE stimulation after 5 days of updosing 81. This unspecific desensitization was reproduced in vitro 82. After 1 year of venom AIT, basophil response to submaximal doses of allergen decreased fourfold in adults 83 and children 84. In subjects suffering from seasonal pollen-allergic rhinitis, SCIT induces a strong decrease in basophil sensitivity during the updosing phase 85. These early changes in basophil sensitivity were shown to predict clinical outcome in the following pollen season 48 and are maintained throughout maintenance therapy and even after treatment cessation 86. A protocol of preseasonal injections also reduced the basophil response 87.
One study comparing effects of SCIT and SLIT in grass pollen-allergic patients found a slower and smaller, yet still significant decrease in basophil sensitivity in the SLIT-treated group 88, and another using diamine oxidase as a read-out for basophil degranulation found comparable changes in both SCIT- and SLIT-treated patients 47; other studies did not find a clear change in the basophil allergen response 89. Oral immunotherapy induced a reduction in the basophil reactivity to allergen in children treated with peanut 90 and egg 24.
In SCIT, the decrease in basophil sensitivity seems to reflect the induction of allergen tolerance and may be useful to predict clinical outcome. In SLIT, intralymphatic immunotherapy (ILIT) and OIT, the role of basophil response to allergen is less clear. Further larger studies are needed to standardize basophil testing, to confirm the predictive value of basophil sensitivity during AIT and to establish basophil reactivity or sensitivity as a biomarker.
Novel approaches for AIT
A better understanding of mechanisms has been translated into novel immunotherapy approaches. Targeting IgE by anti-IgE antibody in combination with AIT reduced the incidence of side-effects during immunotherapy and resulted in prolonged suppression of IgE-FAB for longer than the half-life of anti-IgE, suggesting this strategy may have a durable effect 91. The combination of anti-IL-4 with AIT was effective in suppressing circulating Th2 cells and allergen-induced late responses although it had no obvious advantages over allergen extract alone 92. Targeting immune deviation using the TLR4 agonist monophosphoryl lipid A in combination with AIT was effective with four preseasonal injections without an increase in side-effects 93. The use of Bacterial DNA oligonucleotides rich in CpG sequences, covalently linked to the major ragweed allergen Amb a 1, was effective, possibly by inducing T regs and/or immune deviation although this has not been pursued 94. Further strategies such as targeting epithelial cytokines thymic stromal lymphopoietin and IL-33 in combination with AIT or possibly the combined use of probiotics with AIT are yet to be tested. Strategies to reduce allergic adverse events in AIT include the use of engineered recombinant hypoallergenic molecules, allergen multimers and allergen fragments including peptides. These approaches rely on alterations to the structure of the allergen protein reducing the ability to cross-link IgE whilst maintaining the ability to target allergen-specific T cells and induce immune modulation. The following emerging approaches aim at optimizing delivery and immunization schemes towards shorter course therapy to improve compliance by applying targeted delivery to immune compartments or peptide-based technologies.
Peptide immunotherapy
One such approach is the use of short soluble synthetic peptides containing the immunodominant T-cell epitopes of major allergen proteins. Peptides are selected so as to lack the length and three-dimensional structure to cross-link IgE molecules on the surface of mast cells and basophils, whilst retaining the ability to be recognized by and modulate allergen-specific T cells. The mechanisms of action of peptide immunotherapy may involve the induction of antigen-specific hyporesponsiveness (anergy), deviation of the T helper cytokine production profile from Th2 to Th1, clonal deletion (e.g. through exhaustion) and the induction T cells with regulatory function, perhaps mediated by IL-10 and TGF-β 38.
Clinical efficacy of Fel d 1 synthetic peptides (Cat-SPIRE) was evaluated in a phase 2b clinical trial in an AEC 95. Treatment was safe, well tolerated and reduced symptoms of rhinoconjunctivitis. Administration of eight intradermal injections of 3 nmol synthetic Fel d 1 peptides with 2 week intervals resulted in a greater improvement in symptoms scores than placebo 95.
A further phase 2b trial evaluated efficacy 18–22 and 50–54 weeks after treatment with Fel d 1 synthetic peptides. Reduced symptom scores were observed at both time points with the 50- to 54-week follow-up achieving statistical significance, despite the lack of treatment intervention for the preceding 9 months 36. After approximately 2 years, the changes in TRSS remained at the same level as earlier, providing evidence of enduring efficacy 96. Most recently, the results of a phase 3 trial (EudraCT Number: 2012-001733-13) involving over 1400 subjects showed that treatment with peptides was safe and well tolerated. However, the trial failed to demonstrate clinical efficacy in the field. Although active treatment resulted in an approximately 60% reduction in mean symptom and medication scores, a similar effect was observed in the placebo-treated group.
Safety and efficacy of grass pollen synthetic peptides were evaluated in an AEC. Subjects were exposed to grass pollen for 3 h per day on four consecutive days. Subjects treated with eight administrations of 6 nmol grass-peptide immunotherapy (dosed at 2-weekly intervals) reported a mean 42% change in TRSS compared with the placebo group. Forty-four per cent of subjects in this active treatment group considered themselves ‘very much better’ after treatment, compared with 22% of subjects in the placebo group (P < 0.01) 97.
Contiguous overlapping peptides
AllerT contains three overlapping 49–71 amino acid peptides with no ability to form the original Bet v 1 three-dimensional structure causing birch pollen-induced rhinitis/rhinoconjunctivitis 98. This removes the need of going through the slow progressive dose-escalation process required with conventional AIT and allows the administration of doses up to 10-fold more allergen equivalent than with conventional AIT. These long peptides have markedly reduced IgE binding and do not induce anaphylaxis in sensitized mice 99. A phase I study demonstrated increases in IL-10, IL-5 and IL-13 within weeks followed by 40-fold increases in IgG4 that remained elevated for more than 3 years 98.
In a phase II b study, treatment with long peptides resulted in significant reductions in the combined rhinoconjunctivitis symptom and medication score compared to placebo, as well as significant reductions in secondary endpoints 100 that persisted in the subgroup that agreed to a second follow-up year 101. Based on data from 1569 injections in 335 participants, long contiguous overlapping peptides seem to be well tolerated. Most reported adverse events were mild to moderate with no anaphylactic reactions and no immediate systemic reactions within 30 min of injections. Most participants completed the course of five injections. Four subjects experienced WAO grade 3 systemic reactions after 30 min (equivalent to 2.5/1000 injections) and 5% of subjects a >30% drop in FEV1. These reactions occured between 4 and 6 h after injection and were most pronounced early in the desensitization process. They were likely to be T cell-mediated rather than IgE-dependent anaphylaxis. A new larger European field-based clinical trial is to be implemented during 2016 (EudraCT 2016-000076-23).
Intralymphatic immunotherapy
In the first open randomized study, ILIT was compared with SCIT in 165 patients with grass allergy 102. There was a quick onset of increased tolerance to skin prick tests and nasal provocations. There was a significant reduction in rhinitis symptoms that was long-lasting and comparable to the observed clinical effect of SCIT. Another randomized double-blind trial compared ILIT to placebo in 15 participants grass and birch allergy 103. A significant and clinically relevant reduction in self-reported symptoms was seen in the ILIT group. There was reduction in allergen-specific IgE and a decreased inflammatory response in the nose during provocation tests.
Recombinant cat dander allergen (MAT-Fel d 1) has been investigated with ILIT in a randomized double-blind placebo-controlled study with 20 participants. Primary outcome was response to titrated nasal provocation test. The study demonstrated a 74-fold increase in nasal tolerance and also an increase in specific IgG4 104.
Conflicting results were published in 2013 in a randomized double-blind placebo-controlled study in 45 patients with grass allergy 105. There was no difference reduction in the combined symptom medication score (SMS), respiratory quality of life questionaire (RQLQ) or skin prick test results, whereas an increase in allergen-specific IgG4 was observed after ILIT. One possible explanation for the lack of clinical efficacy may have been that the dosing interval was 2 weeks, whereas in all other studies the interval was 4 weeks.
In a pilot study with seven patients, the frequency of allergen-specific non-IgE+ plasmablasts increased significantly 1 week after allergen injection, and tolerance to nasal provocation as well as titrated skin prick test response increased after the pollen season 106. In a follow-up randomized controlled trial, 36 participants with grass pollen-induced rhinitis were randomized 2 : 1 in favour of the active treatment (three injections of 1000 SQU Alutard). Patients on active treatment had significantly fewer symptoms in the first season after treatment 107. In a more recent double-blind placebo-controlled study with 36 patients, ILIT against birch and grass allergy significantly improved self-reported treatment outcomes 108, whereas there were no differences between the ILIT and placebo group in response to nasal allergen provocation and no differences in specific IgE or IgG4. A subgroup of treated patients with good clinical response had increased the affinity of IgG4 for grass allergen to offer better protection. In the first double-blind placebo-controlled trial of adolescents and the first on the American continent, treatment was safe but did not achieve statistical significance 109. The reason may be low compliance when reporting total symptom score; only 73% of participants entered data on ≥50% of days in the peak pollen season. This underscores the need for a pervasive monitoring tool for AIT.
Given the limited number of studies and conflicting results, there is a need for adequately powered trials of ILIT and studies with prolonged follow-up to assess potential for long-term tolerance.
Recombinant allergens
Molecular allergy has characterized most major allergen components of common inhalants with important implications for diagnosis and therapy 110. For example, Phleum p 1 (Phl p 1) and Phl p 5 are the dominant major grass pollen allergens and detection of IgE to Phl p 1 and Phl p 5 confirms clinically relevant sensitivity, whereas specific IgE to Phl p 12 is likely due to cross-reactivity with the birch-derived profilin Bet v 2. Such a patient may exhibit an irrelevant, false-positive skin test to birch pollen extract, due to the presence of IgE to the irrelevant cross-reacting profilin Phl p 12. Such considerations are helpful in selecting suitable patients and the relevant allergen for AIT 111. Recombinant allergens either singly as in the case of Bet v 1, the major birch pollen allergen 112, or as the relevant recombinant grass allergen mixture (Phl p 1, Phl p 2, Phl p 5a, Phl p 5b, Phl p 6) 113 have been shown to be highly effective in randomized controlled trials. Recombinant allergens may also be genetically modified to reduce IgE binding and allergenicity 114 with great potential for safer immunotherapy. The recombinant vaccine protein BM32, encompassing non-IgE binding domains from grass allergens Phl p 1, 2, 5 and 6 fused with a hepatitis B viral surface protein, is currently in phase 2 studies 115, 116. Patients treated with this vaccine raised IgG antibody to allergens and hepatitis B viral sequences encoded by the recombinant proteins. The immune response to both allergen 115 and virus 116 was protective in vitro, and patients had reduced allergy symptoms in an AEC challenge 115. Recombinant approaches may ultimately allow tailor-made immunotherapy for individuals 117, which will not induce novel sensitizations to irrelevant allergens found in extracts.
Conclusion
Allergic diseases are underdiagnosed and undertreated. Allergen immunotherapy has not yet received adequate attention from European (public) institutions, and many allergic patients remain unaware of the potential benefit of AIT. Areas for improvement and innovation are found through a better understanding of the mode of action and dose effects, which will lead to improved delivery and immunization schedules. More effective preparations with faster onset and reduced doses are likely to improve compliance. A previous trial of AIT for asthma prevention had methodological limitations, so the recent results from the randomized, double-blind placebo-controlled GAP trial are encouraging. With improvement in grading of evidence, standardizing clinical outcomes, we can expect better studies in general in this area. Identification of biomarkers for improved patient selection and monitoring will increase the value and usefulness of AIT.
In summary, it is time to re-evaluate the allergy paradigm and implement new treatment approaches with a special focus on AIT as allergic individuals are becoming a significant minority of Western and global populations. Innovation at all levels and national and regional action plans, such as the Finnish Allergy Programme, are needed to meet the challenge. This first interdisciplinary Aarhus Immunotherapy Symposium brought together specialists from different disciplines to document exciting developments in the field of AIT and provided hope for improvement of this therapeutic option for allergic patients in the future.
Author contributions
EV, OP, PM, JMS, SHS, LOC, KS, ML and SRD contributed specific sections of the manuscript. PS and HJH wrote introduction and conclusion. All authors reviewed the final document.
Conflicts of interest
Drs. Hoffmann, Schmid and Cardell have nothing to disclose. Dr. Valovirta reports grants and personal fees from ALK-Abello, personal fees from TEVA and Stallergenes-Greer and personal fees and other from MEDA, outside the submitted work. Dr. Pfaar reports grants and personal fees from ALK Abelló, Allergopharma, Stallergenes-Greer, Allergy Therapeutics/Bencard Allergie gmbH, Anergis S.A., Lofarma, Biotech Tools S.A., Laboratorios LETI/LETI Pharma, Novartis Pharma; HAL Allergy Holding B.V./HAL-Allergie gmbH, grants from Biomay, Nuvo and Circassia; and personal fees from MEDA Pharma, Sanofi Aventis, Mobile Chamber Experts (a GA2LEN Partner) and Pohl-Boskamp, outside the submitted work. Dr Durham has received research funding from ALK Denmark, Merck USA and Regeneron USA and consulting fees from Merck, Stallergenes, Allergy Therapeutics, Circassia, Anergis, Biomay and Boehringer Ingelheim. Dr. Skaarup reports fees from UsABCD for lectures on clinical ultrasound. Dr Simonsen is an employee of Anergis SA. Dr. Larché reports grants and personal fees from Adiga Life Sciences and grants, personal fees and other from Circassia Ltd/Circassia Pharmaceuticals plc, during the conduct of the study; personal fees from UCB, Sanofi, Aravax Pty, Brandon Capital Ventures and Air Canada, outside the submitted work; in addition, Dr. Larché is an inventor on 17 patent families (pending or issued) held by Circassia Ltd. Dr. Moingeon and Dr. Sørensen are employees of Stallergenes-Greer.




