Pediatric solid organ transplant recipients demonstrate robust cell-mediated and humoral responses to three doses of mRNA SARS-CoV-2 vaccine
Abstract
Pediatric solid organ transplant recipients (pSOTR) often demonstrate suboptimal vaccine responses and are not included in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine efficacy trials. This population has shown variable humoral immunity following SARS-CoV-2 vaccination, and no studies have assessed cell-mediated responses after SARS-CoV-2 vaccination in pSOTR. SARS-CoV-2-specific interferon-gamma release assay (IGRA), immunoglobulin G (IgG), and receptor-binding domain (RBD)-angiotensin-converting enzyme 2 (ACE2) blocking antibody (Ab) were measured in pSOTR aged 5–17 years after 2–3 doses of SARS-CoV-2 mRNA vaccine. In all, 33 subjects were included, with 25 tested after the second dose of mRNA vaccine (V2) and 21 tested after the third dose of mRNA vaccine (V3). Of the 19 subjects who had IgG testing after V3, 100.0% (19/19) had a positive IgG response. Of the 17 subjects who had IGRA testing after V3, 94.1% (16/17) had a positive IGRA response. RBD-ACE2 blocking antibody increased significantly from V2 to V3 (p = .007). Subjects <1 year from transplant demonstrated a significantly larger increase in RBD-ACE2 blocking Ab from V2 to V3 than did those >1 year from transplant (p = .05). SARS-CoV-2 vaccination induces humoral and cell-mediated responses in the majority of pSOTR, with improved quantitative humoral response after three doses.
Abbreviations
-
- Ab
-
- antibody
-
- ACE2
-
- angiotensin-converting enzyme 2
-
- COVID-19
-
- coronavirus disease 2019
-
- IFN
-
- interferon
-
- IgG
-
- immunoglobulin G
-
- IGRA
-
- interferon-gamma release assay
-
- MMF
-
- mycophenolate mofetil
-
- PCR
-
- polymerase chain reaction
-
- pSOTR
-
- pediatric solid organ transplant recipients
-
- RBD
-
- receptor-binding domain
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
-
- SOTR
-
- solid organ transplant recipients
-
- V2
-
- 2nd dose of mRNA vaccine
-
- V3
-
- 3rd dose of mRNA vaccine
1 INTRODUCTION
Solid organ transplant recipients (SOTR) demonstrate decreased immune responses to vaccination, and pediatric SOTR (pSOTR) have not been enrolled in any phase of clinical trials for novel vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Current recommendations for immunocompromised children <18 years of age include administration of three primary doses and one booster dose of messenger RNA (mRNA) vaccine.2 However, most data informing vaccination schedules in immunocompromised children are extrapolated from data in immunocompromised adults, most of which are humoral immunity data that do not account for cell-mediated immune responses.
In adult SOTR, SARS-CoV-2 vaccine immunogenicity studies have demonstrated variable humoral responses, with seropositivity up to 54% after the second dose of mRNA vaccine (V2) and a significant increase in seroconversion rates in SOTR after the third dose (V3).3-8 Early immunogenicity data in pSOTR suggest that humoral responses to SARS-CoV-2 vaccine are more robust than those in adult SOTR, with seroconversion rates as high as 73% after two doses of mRNA vaccine.9 Seroresponse rates vary between recipients of different organs; pediatric heart transplant recipients demonstrate 70% seroresponse,10 while adolescent kidney transplant recipients demonstrated only 52%.11
These humoral immunity data provide some support for existing vaccine recommendations in pSOTR, but correlates of protection against SARS-CoV-2 are still not fully understood. Receptor-binding domain (RBD)-angiotensin-converting enzyme 2 (ACE2) blocking antibody (Ab) may be an important marker of neutralizing activity12 and is a potential predictor of disease severity.13 Cell-mediated responses also play a vital role in vaccine-induced immunity against viral pathogens, but are consistently low or absent (30–65%) in adult SOTR.3, 4, 14-16 To date, SARS-CoV-2-specific cell-mediated immune responses have not been studied in vaccinated pSOTR.
In this study, we assessed SARS-CoV-2-specific humoral responses, RBD-ACE2 blocking Ab activity, and, for the first time, SARS-CoV-2-specific cell-mediated responses in pSOTR after V2 and V3. We found that overall responses were higher than those reported in adult SOTR. We also found that RBD-ACE2 blocking Ab responses increased significantly from the second to third dose of vaccine, which may support current recommendations for a third dose in this population.
2 MATERIALS AND METHODS
2.1 Study design
A convenience sample of solid organ (liver, heart, kidney, lung, or liver-intestine) transplant recipients aged 5–17yo was recruited via clinic communications during May 2020–April 2022 after vaccination with ≥2 doses of mRNA vaccine. Recipients were excluded after receiving any SARS-CoV-2-specific convalescent plasma or monoclonal antibody. Clinical data, including age, sex, date of transplantation, dates of vaccine doses, and maintenance immunosuppressant medications, were collected. History of COVID-19 was established based on chart review and SARS-CoV-2 polymerase chain reaction (PCR) results available through Stanford's electronic medical records (EMR) system or through Care Everywhere, a regional shared EMR network. National Institutes of Health criteria were used to determine severity of disease.17 In subjects transplanted within the 12 months prior to vaccination, induction immunosuppressive agents were recorded. When available, immunosuppressant levels drawn within 2 weeks of vaccine-response testing were collected.
SARS-CoV-2-specific immunoglobulin G (IgG), RBD-ACE2 blocking Ab, and SARS-CoV-2-specific interferon (IFN)-gamma(𝛾) release assay (IGRA) were performed on subjects' serum samples ≥2 weeks after V2 and/or V3. For individual subjects with quantitative data after both V2 and V3, change in RBD ACE-2 blocking Ab and change in IFN-γ response were defined as the subject's absolute change in quantitative response between V2 and V3.
2.2 Laboratory testing
Anti-SARS-CoV-2 Spike S1 domain IgG ELISA was performed on lithium heparin plasma using the EUROIMMUN instrument and reagents (Lübeck, Germany) per the manufacturer's instructions. The anti-SARS-CoV-2 IgG antibody level was reported as the ratio of optical density (OD) of the sample over the OD of the calibrator. The ratio was defined as follows: <0.8 negative; ≥0.8 to <1.1 borderline; ≥1.1 positive. RBD-ACE2 blocking Ab was measured in subjects with positive IgG via an RBD-ACE2 competition ELISA assay.13 Plasma samples were incubated in wells coated with SARS-CoV-2 spike RBD protein prior to the addition of ACE2-mFc that binds to any open RBD sites. Horseradish peroxidase-conjugated goat anti-mouse IgG (Invitrogen) was then added with subsequent OD measurement (Molecular Devices), with higher measurements indicating more open RBD sites and reduced anti-RBD antibodies in the plasma. OD values were then converted to a range of percentages to reflect blocking activity.
Stanford's laboratory-developed IGRA18 was performed as follows: freshly collected blood (>3 ml) in a lithium heparin tube was (1) left unstimulated as negative control, stimulated with (2) a single SARS-CoV-2 peptide pool (Miltenyi Biotec, Bergisch Gladbach, Germany) for CD4+ T cells and (3) two SARS-CoV-2 peptide pools for CD8+ T cells, and (4) stimulated with mitogen as positive control. A single CD4+ T cell megapool (CD4+ pool) consisted of 221 predicted HLA class II CD4+ T-cell epitope peptides covering the entire viral proteome except for the spike protein which was covered with 253 15-mer peptides overlapping by 10 residues. Two CD8+ T cell megapools (CD8+ pools A and B) together consisted of 628 predicted HLA class I CD8+ T-cell epitopes from the entire SARS-CoV-2 proteome. After incubation for 24 hours, IFN-γ concentration in the plasma fraction was measured with an automated ELISA instrument in international units (IU) per ml. Positive IFN-γ response was defined as: Nil ≤8.0 and SARS-COV-2 Antigen minus Nil >0.35. Negative IFN- γ response was defined as: Nil≤8.0 and SARS-COV-2 Antigen minus Nil<0.35 and Mitogen-Nil ≥ 0.5.
2.3 Statistical analysis
Fisher's exact test was performed on categorical variables. Welch's T-test and the Mann–Whitney U-test were performed for comparison of normally distributed continuous variables and non-normally distributed continuous variables, respectively. Kruskal–Wallis one-way analysis of variance was used to compare IFN-γ response and RBD-ACE2 blocking Ab levels between groups or between time points. Paired testing was used for subgroup analyses.
The study was approved by Stanford University's institutional review board (IRB #59811). Informed consent was obtained in person, verbally, or via a RedCap electronic consent form; the Stanford IRB reviewed and approved the verbal consent process and electronic consent form.
3 RESULTS
3.1 Baseline characteristics
A total of 33 subjects were enrolled, including 17 liver, 7 kidney, 7 heart, 1 lung, and 1 liver/intestine transplant recipients. Median age at first dose was 15 years (IQR 12–16 years), and 4 subjects were under 12 years old. In all, 19 (57.6%) were males. All subjects received only BNT162b2 vaccine. Median time from transplantation to vaccination was 7.0 years (IQR 1.5–13.5 years), with 6 subjects (18.2%) vaccinated within the first year after transplantation. In all, 31 (93.9%) subjects were on tacrolimus, and 13 (39.4%) were also on mycophenolate. All six subjects vaccinated within the first year after transplantation received anti-thymocyte globulin as induction immunosuppression, and two of the six (33%) also received methylprednisolone. Two subjects were diagnosed with SARS-CoV-2 infection via nasopharyngeal PCR prior to vaccination.
3.2 Humoral and cell-mediated responses after vaccination
Of the 33 enrolled subjects, 25 underwent laboratory testing after V2, 21 underwent testing after V3, and 13 underwent testing after both V2 and V3 (Table 1). After V2, all subjects had IgG testing, and after V3, 19 subjects had IgG testing and 2 had IGRA testing only. Laboratory testing was performed an average of 9.24 weeks (SD 4.76) after V2 and 9.29 weeks (SD 6.12) after V3.
| Demographics | Post V2 n/total tested (%) | Post V3 n/total tested (%) | |||
|---|---|---|---|---|---|
| Positive IgG | Positive IGRA | Positive IgG | Positive IGRA | Overall n (% of total) | |
| Age at vaccination | |||||
| 5–11 | 1/1 (100.0) | 1/1 (100.0) | 3/3 (100.0) | 2/3 (66.7) | 4 (12.1) |
| 12–17 | 20/24 (83.3) | 16/22 (72.7) | 16/16 (100.0) | 14/14 (100.0) | 29 (87.9) |
| Sex | |||||
| Male | 12/14 (85.7) | 11/13 (84.6) | 12/12 (100.0) | 10/11 (90.9) | 19 (57.6) |
| Female | 9/11 (81.8) | 6/10 (60.0) | 7/7 (100.0) | 6/6 (100.0) | 14 (42.4) |
| Organ transplanted | |||||
| Liver | 12/12 (100.0) | 10/12 (83.3) | 9/9 (100.0) | 9/9 (100.0) | 17 (51.5) |
| Kidney | 6/7 (85.7) | 5/6 (83.3) | 6/6 (100.0) | 3/3 (100.0) | 7 (21.2) |
| Heart | 2/4 (50.0) | 1/3 (33.3) | 4/4 (100.0) | 4/5 (80.0) | 7 (21.2) |
| Lung | 0/1 (0.0) | 0/1 (0.0) | — | — | 1 (3.0) |
| Liver/intestine | 1/1 (100.0) | 1/1 (100.0) | — | — | 1 (3.0) |
| Time since transplant | |||||
| <1 year | 3/5 (60.0) | 3/5 (60.0) | 4/4 (100.0) | 3/3 (100.0) | 6 (18.2) |
| ≥1 year | 18/20 (90.0) | 14/18 (77.8) | 15/15 (100.0) | 13/14 (92.9) | 27 (81.8) |
| Immunosuppressant regimen | |||||
| Tacrolimus only | 10/10 (100.0) | 8/10 (80.0) | 9/9 (100.0) | 10/10 (100.0) | 15 (45.5) |
| Tacrolimus + MMF | 7/10 (70.0) | 6/9 (66.7) | 7/7 (100.0) | 5/6 (83.3) | 11 (33.3) |
| Tacrolimus + MMF + prednisone | — | — | 2/2 (100.0) | — | 2 (6.1) |
| Everolimus only | 1/1 (100.0) | 1/1 (100.0) | 1/1 (100.0) | 1/1 (100.0) | 1 (3.0) |
| Tacrolimus + prednisone | 0/1 (0.0) | 0/1 (0.0) | — | — | 1 (3.0) |
| Tacrolimus + sirolimus | 2/2 (100.0) | 1/1 (100.0) | — | — | 2 (6.1) |
| Sirolimus only | 1/1 (100.0) | 1/1 (100.0) | — | — | 1 (3.0) |
| Total | 21/25 (84.0) | 17/23 (73.9) | 19/19 (100.0) | 16/17 (94.1) | 33 |
Overall, a majority of subjects had positive IgG and IGRA responses after V2 and V3, with significant increases in quantitative responses from V2 to V3. Positive antibody responses improved from 21/25 (84.0%) after V2 to 19/19 (100.0%) after V3, and RBD-ACE2 blocking Ab demonstrated a significant increase from a mean blocking activity of 52.8% after V2 to 80.5% after V3 (p = .007, Figure 1). The proportion of positive IGRA responses increased from 17/23 (73.9%) after V2 to 16/17 (94.1%) after V3, although the increase in SARS-CoV-2-specific IFN-γ response values from a mean of 2.26 IU/ml after V2 to 4.10 IU/ml after V3 was not significant (p = .11, Figure 2). All subjects showed adequate response to the nonspecific mitogen control built into the IGRA, confirming good overall T-cell function.
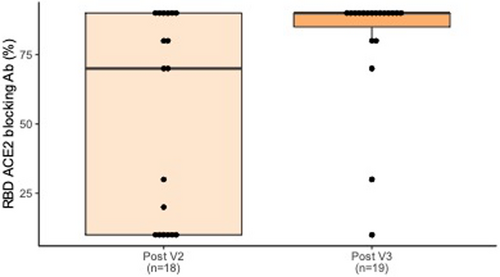
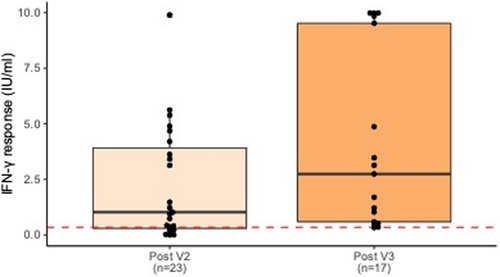
In the six subjects who were <1 year from transplant at time of first vaccine dose, RBD-ACE2 blocking Ab increased from a mean of 2.0% to 85.0% from V2 to V3 (p < .001). Similarly, IFN-γ response increased from a mean of 0.80 IU/ml to 7.81 IU/ml from V2 to V3 (p < .01). In this subgroup, the average time between V2 and laboratory testing was 7.83 weeks (SD 3.44), and the average time between V3 and testing was 11.71 weeks (SD 8.38).
3.3 Immune response by modifying factors
Positive SARS-CoV-2 IgG and IGRA after V2 were not associated with antimetabolite use, number of immunosuppressants, type of organ, or age at vaccination. Because all subjects had a positive SARS-CoV-2 IgG after V3, Fischer's exact test could not be performed to detect differences between groups.
Clinical factors associated with the degree of quantitative response after V2 or V3 were also assessed. Following V2, liver transplant (vs. kidney transplant), antimetabolite use, and being more than 1 year from transplant (vs. <1 year from transplant) were associated with higher quantitative RBD-ACE2 blocking Ab (p < .01, p < .05, p < .05, respectively). There were no significant differences in RBD-ACE2 blocking Ab by age at vaccination or serum tacrolimus level. There were also no significant differences in the quantitative IFN-γ response values by these modifying factors after V2. Following V3, there were no significant differences in the quantitative RBD-ACE2 blocking Ab or IFN-γ response values by organ type, immunosuppressant regimen, age at vaccination, time since transplant, or serum tacrolimus level.
3.4 Change in response by modifying factors
Of the 13 subjects who had testing after both V2 and V3, nine subjects had quantitative response measurements at both timepoints. In subgroup analyses for these nine subjects, the average change in RBD-ACE2 blocking Ab was greater in kidney transplant recipients (62.0%) than in liver transplant recipients (17.5%), though this benefit did not reach significance (p = .06). Neither immunosuppressant regimen nor tacrolimus level was associated with change in RBD-ACE2 blocking Ab or change in IFN-γ response. Following V3, subjects <1 year from transplant saw a greater change in RBD-ACE2 blocking Ab response (73.3%), compared to subjects ≥1 year from transplant (26.7%) (p = .05).
3.5 Clinical outcomes
Seven subjects developed SARS-CoV-2 infection after vaccination (five after V3 and two after V2). Of these breakthrough cases, two were asymptomatic, three had mild illness, one had severe illness, and one did not have enough clinical information available via EMR to determine disease severity. No subjects required hospitalization. All but one breakthrough case occurred in December 2021 or later, and median time after SOT was 6 years (IQR 1–14 years). One study subject died during the study period, though not due to SARS-CoV-2 infection; this subject had received two doses of vaccine.
4 DISCUSSION
To our knowledge, this is the first study reporting cell-mediated responses and quantitative RBD-ACE2 blocking Ab after SARS-CoV-2 vaccination in pSOTR. We found that all SOTR 5–17 years of age developed an antibody response after V3, and all but one developed a SARS-CoV-2-specific cell-mediated response.
Quantitative humoral responses increased significantly between V2 and V3. Despite a near-doubling in quantitative cell-mediated responses between V2 and V3, this increase did not reach significance; the study may not have been powered to detect differences in SARS-CoV-2-specific IFN-γ response values. Compared to those ≥1 year from transplant, those who were vaccinated in the first year after transplant saw a significantly greater improvement in quantitative responses between V2 and V3. There were no significant differences in quantitative responses after V3 in our subgroup analyses, despite some differences between groups after V2, which suggests that V3 may catch up certain subgroups, such as kidney transplant recipients or those on antimetabolites. Taken together, our qualitative and quantitative vaccine response data provide support for the recommendation for three primary doses in pSOTR, particularly for those who are at risk for diminished immune response, such as those recently transplanted or on antimetabolite therapy. Our findings also underscore the importance of quantitative immune response measurements, particularly in this vulnerable population, to develop a more nuanced understanding of the magnitude of response. Specific quantitative measurements such as those utilized in this study may aid in determining specific markers of immunity via longitudinal analyses.
Despite the presence of adequate immune responses, some subjects did develop COVID-19 after vaccination, nearly all of which were during the Omicron variant surge. Importantly, the majority had asymptomatic or mild disease, and the subject with severe illness was more than 5 months post V3 at the time of SARS-CoV-2 diagnosis. Waning vaccine effectiveness after V3 has been demonstrated in adults during the recent Delta and Omicron variant surges, with only 50% vaccine effectiveness at 5 months or more post V3.19
Limitations of this study include its small study size, which may not have been sufficiently powered to detect differences between some subgroups. We were not able to perform baseline serologic testing prior to vaccination. Some subjects with positive SARS-CoV-2 IgG were missing RBD-ACE2 blocking Ab data points due to a brief lapse in the availability of this assay. Due to subject and laboratory test scheduling, only a subset of our subjects was tested at both timepoints.
It remains unclear which of the parameters tested in this study represents a reliable correlate of immunity; identification of such a marker requires pairing of serial measurements of humoral and cell-mediated responses with longer-term clinical outcomes data. Until such an immune correlate is identified, pSOTR should continue risk mitigation measures such as masking and social distancing.
We believe this study contributes to the limited existing data regarding immune responses to SARS-CoV-2 vaccines in pSOTR, by providing a more complete examination of immune response in this population, and by generating pSOTR-specific data supporting the benefit of a three-dose primary series of mRNA vaccines. Future directions include determining the durability of immune response in these subjects, further characterizing responses of pSOTR between 6mo and 11 years via larger study samples, and assessing the frequency of breakthrough infection and risk factors for breakthrough disease. Additional measurements of phenotypic and functional lymphocyte markers, coupled with clinical outcomes data, could also aid in identifying correlates of immunity. Larger prospective cohorts of pSOTR are required to assess the immunogenicity of SARS-CoV-2 vaccine in the first year post-SOT, as well as the immunogenicity of booster doses.
ACKNOWLEDGMENT
The authors wish to acknowledge the solid-organ transplant teams at Lucile Packard Children's Hospital Stanford for their support in study recruitment.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




