Donor-derived acute myeloid leukemia in solid organ transplantation
Luigi Marchionni and Francisco Pereira Lobo contributed equally to this article.
Abstract
We report the transmission of acute myeloid leukemia (AML) undetected at donation from a deceased organ donor to two kidneys and one liver recipients. We reviewed the medical records, and performed molecular analyses and whole exome sequencing (WES) to ascertain AML donor origin and its molecular evolution. The liver recipient was diagnosed 11 months after transplantation and died from complications 2 months later. The two kidney recipients (R1 and R2) were diagnosed 19 and 20 months after transplantation and both received treatment for leukemia. R1 died of complications 11 months after diagnosis, while R2 went into complete remission for 44 months, before relapsing. R2 died 10 months later of complications from allogenic bone marrow transplantation. Microsatellite analysis demonstrated donor chimerism in circulating cells from both kidney recipients. Targeted molecular analyses and medical records revealed NPM1 mutation present in the donor and recipients, while FLT3 was mutated only in R1. These findings were confirmed by WES, which revealed additional founder and clonal mutations, and HLA genomic loss in R2. In conclusion, we report the first in-depth genomic analysis of AML transmission following solid organ transplantation, revealing distinct clonal evolution, and providing a potential molecular explanation for tumor escape.
Abbreviations
-
- AML
-
- acute myeloid leukemia
-
- ARCH
-
- age-related clonal hematopoiesis
-
- BM
-
- bone marrow
-
- CHIP
-
- clonal hematopoiesis of indeterminate potential
-
- CNA
-
- copy number alterations
-
- CNV
-
- copy number variation
-
- HLA
-
- human leukocyte antigen
-
- RDW
-
- red blood cell distribution width
-
- RTC
-
- regional transplantation center
-
- Tx
-
- organ transplantation
-
- VEP
-
- VARIANT EFFECT PREDICTOR
-
- WES
-
- whole-exome sequencing
1 INTRODUCTION
Transplantation (Tx) in patients with organ failure is a lifesaving procedure that carries intrinsic medical risks. Besides rejection, and development of opportunistic infections and cancers due to immuno-suppression, there is also the possibility of donor-transmitted malignancies. Such incidence ranges between 0.01 and 0.06%— melanoma, colon, and lung cancer being the most common transmitted tumors.1-3 Donor-derived cancers can either develop early—that is, during the first few weeks after transplantation—or at a later time. In the first instance, tumors are transplanted together with the organ, engrafting notwithstanding HLA mismatch, possibly due to recipient's immunosuppression. Late-onset donor-derived cancers are less well understood and more difficult to recognize and treat.
In July 2013, nephrologists at an Italian hospital reported to the Regional Transplantation Center (RTC) the possible transmission of donor-related Acute Myeloid Leukemia (AML) in the kidney recipients from a common donor. Upon transplantation history evaluation, it was discovered that the liver recipient had already died from AML in November 2012. Hence, we undertook a complete review of donor's and recipients' clinical history, and performed comprehensive molecular analyses to ascertain the AML origin, confirm its transmission from the donor, and tease apart the distinct evolution paths in the recipients. We report here our findings.
2 CASE REPORTS
2.1 Donor
In October 2011, a 79-year-old female was diagnosed with an intracerebral hematoma with intraventricular hemorrhage. Upon neurologic symptom progression, monitoring for death certification by neurological criteria was initiated. All required donor-eligibility screenings were performed: no symptoms, clinical signs, imaging, or laboratory records suggested any condition precluding organ donation. Briefly, the medical history reported treatment with nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and low molecular weight heparins for an arm fracture, and a recent weight loss was attributed to a family bereavement. Under existing guidelines, there was no clear indication for deeper hematologic evaluations, since the hematology analyzer did not report cell distribution abnormalities, beside mild leukocytosis with high neutrophil-lymphocyte ratio and an increase in monocytes with normal platelets count (Table 1), all common findings in heart beating donors. In conclusion, risk assessment according to existing guidelines,4 did not reveal any evidence in the medical history and laboratory workup that suggested the presence of hematological diseases, the donor was assessed at standard risk, and for such reasons, an AML diagnosis was missed. Due to the donor's age, only the two kidneys and the liver were recovered, while the other organs and tissues were unsuitable for transplantation.
| Cell type | Value |
|---|---|
| Red blood cells | 3.12 × 106/ml |
| Hemoglobin | 9.4 g/dl |
| Hematocrit | 28.2% |
| Platelets | 313 × 10 3/ml |
| White blood cells | 18.9 × 10 3/ml |
| Polymorphonuclear leukocytes (PMNs) | |
| Neutrophils | 74.3% |
| Lymphocytes | 4.1% |
| Monocytes | 20.4% |
| Eosinophils | 1.1% |
| Basophils | 0.1% |
| Molecular markers | Donor | R1 post-Tx | R2 post-Tx | R3 post-Tx |
|---|---|---|---|---|
| Sex | Female | Male | Female | Female |
| Donor chimerism in blood % (microsatellites) | NA | 83 | 29 | ND |
| Chromosome X centromeres by FISH | NA | Positive | ND | ND |
| NPM1 mutation | Positive | Positive | Positive | Positive |
| NPM1 mutation % | 17 | 49 | 24 | ND |
| FLT3 mutation | Absent | Positive | Absent | Absent |
| FLT3 mutation % | 0 | 44 | 0 | ND |
- Note: Samples for R1 and R2 post-transplant were collected at the time of the AML diagnosis, while the molecular information for R3 was retrieved from clinical records since no viable DNA was recovered after Tx.
2.2 Transplant recipients
All transplantations were successful, and no major post-surgical complications occurred. Two recipients (R1, a 57-year-old male, and R2, a 55-year-old female) received the kidneys, a third recipient (R3, a 62-year-old female) received the liver. From the histocompatibility standpoint, the two kidney transplantations were partial HLA-A, B, and DR matches, while the liver recipient was a full mismatch (Table S1). Immunosuppressive therapy for all recipients included—in accordance to best practices—induction with anti-CD25 antibodies before transplantation followed by tacrolimus, mycophenolate mofetil, and prednisone. After diagnosis of AML, immunosuppression was reduced to prednisone.
The two kidney recipients were followed by the same team, while the liver recipient was followed in a different hospital. R1 was diagnosed with AML in May 2013, received treatment, but died of complications in April 2014. R2 was diagnosed with myeloid sarcoma of the transplanted kidney—an extramedullary manifestation of NPM1-mutant AML,5 a diagnosis revised to AML upon bone marrow biopsy in June 2013. R2 was treated for leukemia, achieving complete remission until relapse in February 2017. In May 2017, R2 received an HLA-A, B, C, DR, and DQ identical, unrelated, allogeneic bone marrow transplant, but died of treatment-induced complications in December 2017. R3 was diagnosed with AML in September 2012 and died in November 2012 for complications during AML treatment. The clinical follow-up timeline is shown in Figure 1.
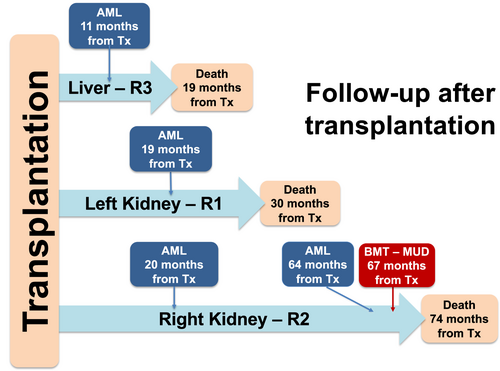
3 METHODS
3.1 Clinical data review
Donor's and recipients' transplantation and medical records were retrieved to collect all available clinical history and results from diagnostic evaluations. All available information was reviewed independently.
3.2 DNA purification
DNA from buffy-coats was available in the RTC archive for the donor and the three recipients before transplantation. For the kidney recipients, DNA was obtained from BM aspirates and buffy-coats at the time of AML diagnosis and during follow-up. For R3, despite an attempt to recover DNA from a formalin-fixed paraffin-embedded liver biopsy, no viable DNA was available after transplantation for further investigations.
3.3 Mutation analysis
We analyzed AML pathognomonic molecular alterations, including mutations in the Fms-like tyrosine kinase 3 (FLIT3) and nucleophosmin (NPM1) genes, using DNA from the donor and R1 and R2 samples obtained before and after kidney transplantation, and during follow-up for AML. NPM1 and FLT3 mutation status for R3 were obtained from clinical records.
3.4 Chimerism analysis
We performed microsatellites Short Tandem Repeat analysis to study the presence of distinct DNA genotypes after transplantation. We also performed Fluorescence In Situ Hybridization (FISH) analysis with probes specific to the X chromosome centromeres to detect the presence of female donor's sex chromosomes in the R1 male recipient.
3.5 Exome sequencing
Due to DNA quality, we were able to perform whole exome sequencing (WES) for the donor, all recipients before transplantation, and the kidney recipients after transplantation. All procedures were performed according to manufacturers' recommended protocols as described in the Supplementary Methods section.
3.6 Sequence pre-processing
Reads quality assessment was performed using the fastqc package. We used BWA to map reads to the hg38 reference genome. Dedupping, indel realignment, and base quality recalibration were performed using Picard and GATK best practices6, 7 (Supplementary Methods and Table S2 A–C).
3.7 Variant calling
We identified high-quality variants (minimum coverage of 10 reads, variant allele frequency ≥5%, supported by at least 2 reads, alternative allele frequency pre-transplantation <2%) with FreeBayes.8 The resulting variants were annotated to COSMIC (v85), dbSNP (v144), and for functional consequences using Variant Effect Predictor (VEP) Tool9 and SnpEff.10 We used Pindel11 to identify large deletions and insertions.
3.8 Copy number variation
We used CNVkit12 to perform segmentation of tumor/normal log2 ratio and detect copy number alterations (CNA) in the donor and R1 and R2 samples post-transplantation. This analysis was performed with and without correction for normal DNA contamination. We analyzed the loss of heterozygosity using custom scripts.
3.9 Computational analyses
All computational analyses downstream variant detection were performed using R/Bioconductor packages and ad-hoc scripts. A summary of the analytical pipelines is shown in Figure S1. For additional details about protocols and methods used see the Supplementary Material section.
3.10 Study approval
The study was conducted in accordance with the Institutional Ethics Committee guidelines and recommendations.
4 RESULTS
4.1 Targeted analyses
We performed targeted molecular analyses to identify AML-related somatic alterations before transplantation (donor, R1, R2, and R3), at diagnosis (R1 and R2), and during clinical follow-up for AML (R1 and R2). We identified heterozygous NPM1 mutations in the donor, and both R1 and R2 at diagnosis (17%, 49%, and 24%, respectively). The presence of NPM1 mutation in R3 was indirectly confirmed from medical records. We also identified a heterozygous FLT3 internal tandem repeat (ITD) mutation in R1 at diagnosis, but not in the donor or R2. Microsatellites analysis on post-transplantation samples identified donor DNA in BM aspirates of both R1 and R2 (83% and 29%, respectively), while FISH revealed a female karyotype in the male recipient after transplantation. Collectively, these findings document the presence of donor's leukemic DNA in the recipients after transplantation (Table 2 and Supplementary Material).
4.2 Somatic variants
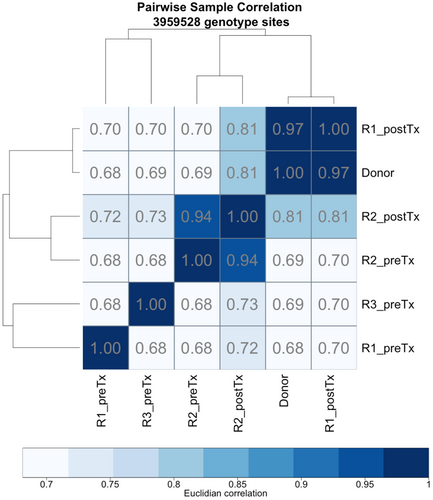
We performed WES to obtain a detailed characterization of the mutational landscape. Summaries for high-quality variants are reported in Table S3 A–D. Overall, pair-wise correlations based on these variants revealed that post-transplantation samples are highly similar to the donor (R ≅ 0.97 for R1 post-TX and R ≅ 0.81 for R2 post-TX, Figure 2). These findings agree with targeted analyses, confirming the donor origin of AML (Table 1).
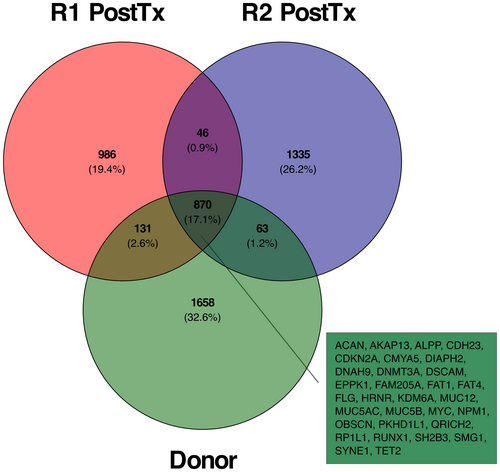
Through WES, we also identified the variants shared between the donor and the recipients, as well as those present in some of the samples. Among the 870 shared mutations, 485 were previously reported in COSMIC (Figure 3). This analysis confirmed the NPM1 frameshift mutation identified by targeted sequencing, and revealed additional somatic mutations in genes previously reported as AML drivers,13-16 including TET2, DNMT3A, RUNX1, CDKN2A, ASLX1, and BCOR among others. Finally, we were also able to confirm the FLT3 ITD mutation in R1 post-transplantation using Pindel11 (Table 1, Figure 4A, and Table S11).

4.3 Copy number analysis
The analysis of segmented coverage by cnvKit12 demonstrated low total CNA burden and the absence of focal gains or deletions, suggesting a cytogenetically normal AML17 in the donor and kidney recipients. We also performed loss of heterozygosity analysis using alternative allele frequencies for informative variants, which suggested a sub-clonal loss of chromosome 6 short arm (encompassing the HLA region) in R2 post-transplantation (Figure 4B and Supplementary Material).
5 DISCUSSION
We have documented for the first time AML transmission from a single donor to three distinct recipients through solid organ transplantation, and its independent clonal evolution, by multi-level evidence encompassing targeted molecular analyses and whole exome sequencing. Although we were unable to detect AML in the donor at donation, collectively, our findings support the notion of a direct engraftment of leukemic cells already present in the donor, rather than the evolution of pre-leukemic cells in the recipients.
The set of AML pathognomonic mutations shared among the donor and the recipients encompassed NPM1, RUNX1, DNMT3A, and TET2, in agreement with their “founding” nature,13-16 whereas the FLIT3 ITD mutation was only detected in one of the kidney recipients, in agreement with AML clonal selection in this subject. Additional evidence of distinct clonal evolution was provided by the identification of sub-clonal inactivating mutations in other AML drivers (like ASLX1 and BCOR), in important cancer genes (e.g., WT1, ATM, TWIST1, or NOTCH3), and in several genes for which a role in AML still needs to be defined.
Similarly, also copy number analysis results were consistent with an independent AML evolution in the two kidney recipients. While the total CNA burden was low—in agreement with the observed mutational spectrum and consistent with cytogenetically normal AML17—the assessment of informative allele frequencies revealed a sub-clonal loss of chromosome 6 short arm (encompassing the HLA loci) in one of the recipients. These findings might suggest an additional explanation—in addition to standard immunosuppressants—for tumor cells escaping allorecognition, consistent with findings of loss of the HLA incompatible haplotype in relapsing AML after haploidentical bone marrow transplantation.18
Our study supports the notion that AML can be transmitted also through solid organ transplantation, and not only through bone marrow and hematopoietic stem cells infusion. Donor-derived acute leukemia and myelodysplastic syndromes are well-documented complications in recipients of bone marrow and peripheral and umbilical cord blood, with an incidence ~0.5%.19 This complication results from the engraftment of premalignant clones, carrying mutated hematologic cancer genes (like TET2 and DNMT3A),20 that undergo proliferative and self-renewal stress during engraftment. This condition, known as clonal hematopoiesis of indeterminate potential (CHIP),21 is a strong risk factor for developing an hematologic cancer, and it is observed in 10% of individuals 65 years or older, while it is rarely seen in young people—hence it is also referred to as age-related clonal hematopoiesis (ARCH).22 Recent studies of CHIP/ARCH mutational landscape have revealed that progression to AML is associated with a higher number of mutations, the involvement of specific genes, and also higher mutation frequencies, suggesting a greater clonal expansion.14, 15
AML occurrence in recipients of solid organ transplantation has been already described. A case of acute promyelocytic leukemia was reported in 199923 in a 57-year-old woman 2 years after receiving the liver of a 16-year-old male who died of head injury. The authors demonstrated donor's origin of the AML, but in retrospective analyses found no evidence of leukemia in the donor, concluding that the disease resulted from donor cell transformation after transplantation. Furthermore, this report did not include any information about the recipients of the other organs (if any) and their outcomes. A second case was reported in 2013,24 when a 69-year-old woman died of AML complications 2 years after a double kidney transplantation. Since no signs of leukemia were found in the donor and the liver recipient did not develop AML, also in this case the authors concluded that the disease arose in the recipient. On the contrary, in the cases we have described, the leukemic clones were already present in the donor, as demonstrated by the presence of founder mutations and by their transmission to all three recipients.
Another interesting aspect to consider is the time lapsed before the AML manifested itself in the recipients. Given the extraordinariness of this event, with obvious limitations, we can only refer to what is known about the engraftment of human tumors into immunocompromised mice, in which a lag time of months is not at all infrequent (see Chen et al25). To this end, important aspects in determining engraftment time are the number of injected cells and their viability. We have no information about these factors, but we can assume that the absolute number of leukemic cells within the transplanted organs was low, with some variability—also in terms of viability—between the liver and kidneys, due to the intrinsic differences in volume and microenvironment between these organs. It is worth noting that the liver recipient died earlier than the kidney ones, who were, in turn, diagnosed close in time, suggesting that these latter patients might have received similar numbers of leukemic cells that engrafted in comparable times, notwithstanding the different hosts. Finally, the most relevant difference with xenograft models, is that the recipients were not immunodeficient and that they were only incompletely immunosuppressed. Hence, to fully explain why their immune system did not reject the tumor, we can postulate that additional mechanisms of immune evasion, impossible to ascertain by WES, were also at play (e.g., the expression of immune check-points molecules). Among these, it is interesting to note that tumor cells of one of the recipients lost at least one HLA haplotype.
The incidence of AML following solid organ transplantation is yet to be studied in detail. In a 2014 review,26 Rashidi and colleagues reported 51 AML cases following organ transplantation, of which four, all occurring after liver transplantation, were deemed to be donor-derived. For the additional cases more recently reported, donor origin was not investigated.27 Given the fact that hematopoietic stem cell can survive in transplanted organs,28 the increasing use of older donors, and the prevalence of ARCH later in life, it is not surprising to start observing donor-derived and, with this report, donor-transmitted leukemias also after organ transplantation. The true extent of this complication—ranging from 0.18% to 0.8%26—might be somewhat underestimated, since leukemias in the recipients can also result from immunosuppression, and usually only cases of multiple transmissions from single donors are fully investigated. Notably, this is also true for breast29 and other cancer types,2, 29, 30 suggesting the importance of continuous improvements to the reporting guidelines.
For AML and other leukemias, since ARCH/CHIP increases with age, one could speculate that older donors should be considered at high risk. While this is extremely important in bone marrow transplantation, there are no data suggesting that ARCH/CHIP clones will engraft in solid organ transplantation recipients. Hence, despite our findings, we believe that excluding donors based on age is not warranted, since this would result in an unacceptable reduction of the donor pool with a consequent reduction in transplantation survival benefits. Most importantly, under existing guidelines, cancer transmission risk is overestimated, resulting in the exclusion of high-risk donors that could be instead safely used.2, 30
A possible solution could be updating current guidelines to include hematologic tumor screenings for older donors, although systematically performing such testing would be burdensome, since currently—at least in the Italian experience—more than half of deceased donors are already over 65 years of age, suggesting the need for donor risk stratification strategies. Although blood smears do not always allow for cancer detection and bone marrow biopsies aren't usually feasible in emergency settings, our findings strongly suggest that for older potential donors with hematological abnormalities or alterations suggestive of cancer deeper evaluations by an hematologist would be warranted.
It would be different if rapid molecular tests—compatible with donation—were available to exclude cancerous cells presence. While genomic sequencing is feasible for living donors, in transplantation from deceased individuals this is not possible due to the required fast turn-around time. Nevertheless, with current technological advancements, is plausible that including such cancer detection tests in the donor workup is not far in the future. To this end, while such molecular information cannot yet inform donor risk assessment, it could be already useful for implementing enhanced follow-up protocols of the recipients receiving organs from older donors. Finally, it is worth mentioning that predictive cancer risk models based on health records and other clinical parameters—as shown for red blood cell distribution width in AML15—already exist, potentially enabling a better donor stratification into risk groups without any delay and at no additional cost.
In conclusion, this is the first report and in-depth genomic characterization of AML transmission following solid organ transplantation. We do not have a clear explanation for the time required for the AML to develop in the recipients, except that the number of AML cells transferred into recipient must have been quite small. Our results from whole exome sequencing highlight the potential molecular mechanisms explaining the evolution of the tumor and its escape from immune response in the recipients. Furthermore, all our molecular analyses indicate that leukemic cells were already present in the donor at the time of death, underscoring the need for continuous improvements of donor screening, risk stratification, and recipient follow-up protocols. Since the current donor pool is shifting toward an older age, these efforts are critical to continue minimizing the risk of transmission of cancer and other diseases through organ transplantation.
ACKNOWLEDGMENTS
We are indebted to Drs. L. Gondek, M. Loda, and D. Rigamonti for their invaluable help in discussing our analyses and findings during the study and the preparation of the manuscript. Our work was supported by a “Progetto Dipartimenti di Eccellenza 2018-2022” grant awarded by the Italy Ministry of Education to the Department of Medical Sciences of the University of Turin; by a grant from Regione Piemonte, Italy; and by the National Institute of Health (NIH-NCI) grant R01CA200859. This study was financed in part by the "Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior – Brasil (CAPES) – Finance Code 001.”
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Open Research
DATA AVAILABILITY STATEMENT
Data generated in this manuscript are available from the corresponding authors upon reasonable request in order to comply with the institutional ethics regulations and policies to protect patient privacy.




