CXCL10 and IL-6: Markers of two different forms of intra-amniotic inflammation in preterm labor
Abstract
Problem
To determine whether amniotic fluid (AF) CXCL10 concentration is associated with histologic chronic chorioamnionitis in patients with preterm labor (PTL) and preterm prelabor rupture of the membranes (PROM).
Method of Study
This study included 168 women who had an episode of PTL or preterm PROM. AF interleukin (IL)-6 and CXCL10 concentrations were determined by immunoassay.
Results
(i) Increased AF CXCL10 concentration was associated with chronic (OR: 4.8; 95% CI: 1.7-14), but not acute chorioamnionitis; (ii) increased AF IL-6 concentration was associated with acute (OR: 4.2; 95% CI: 1.3-13.7) but not chronic chorioamnionitis; and (iii) an increase in AF CXCL10 concentration was associated with placental lesions consistent with maternal anti-fetal rejection (OR: 3.7; 95% CI: 1.3-10.4). (iv) All patients with elevated AF CXCL10 and IL-6 delivered preterm.
Conclusion
Increased AF CXCL10 concentration is associated with chronic chorioamnionitis or maternal anti-fetal rejection, whereas increased AF IL-6 concentration is associated with acute histologic chorioamnionitis.
1 Introduction
Preterm labor is a syndrome characterized by the combination of increased uterine contractility, cervical remodeling (ie, ripening and dilatation), and decidual membrane activation, caused by multiple pathologic processes.1-12 One of the mechanisms of disease implicated in preterm parturition is a breakdown of immune tolerance, which may evolve into maternal anti-fetal rejection.13-30
The fetus and placenta express both maternal and paternal antigens; therefore, they are semiallografts.31-42 The placenta is considered to be the most successful transplant in nature, a biological adaptation accomplished by immune tolerance.43-48 Tolerance is a specific immunological term that refers to “the active state of antigen-specific non-responsiveness”49 leading to diminished reactivity to paternal antigens expressed by the placenta and/or fetus; it is considered key for successful pregnancy.31, 33, 39, 40, 50-53 The mechanisms responsible for tolerance in pregnancy include the following: (i) T-cell chemokine gene silencing in the decidual cells;54 (ii) a suppressive role of regulatory T cells;52, 55-64 (iii) expression of non-classical major histocompatibility complex molecules on trophoblast cells that do not elicit a maternal immune response;65-70 (iv) changes in tryptophan catabolisms;71-75 (v) T-cell apoptosis;76, 77 (vi) complement;78-90 and (vii) costimulatory molecules such as the programmed death ligand.91-93 Other mechanisms for tolerance are not currently understood. The interested reader is referred to recent contributions by Sing Sing Way's laboratory28, 39, 94-96 and Adrian Erlebacher.31, 42, 53
In transplantation medicine, failure of tolerance is responsible for graft rejection characterized by an infiltration of the recipient's CD8+ (cytotoxic) T cells into the graft and an overexpression of C-X-C motif chemokine 10 (CXCL10), a marker of allograft rejection.97-102 In obstetrics, rejection as a mechanism of disease has been largely overlooked. However, recent evidence suggests that maternal anti-fetal rejection is operative in a subset of patients with spontaneous preterm labor,15, 16, 20, 26, 103 preterm prelabor rupture of the membranes (PROM),20 fetal death,17, 25 recurrent abortion,19 and other obstetrical syndromes.14, 18, 21-24, 103 Maternal lymphocytes (akin to a transplant recipient) can infiltrate the chorioamniotic membranes (fetal tissue or semiallograft), lead to chronic chorioamnionitis,15, 26 and induce trophoblast apoptosis, which, when excessive, can result in graft failure (eg, membrane rupture or activation of the decidual membrane and the initiation of labor).15, 104 The chemotactic signal inducing the migration of maternal T lymphocytes into the chorioamniotic membranes appears to be present in the amniotic cavity. One such chemokine is CXCL10,15, 104, 105 and an increased concentration of this chemokine in the amniotic fluid has been characterized by our group to represent a distinct form of intra-amniotic inflammation, which is associated with chronic inflammatory lesions of the placenta and a novel form of fetal inflammatory response syndrome (FIRS) or FIRS type 2.21
This distinct form of intra-amniotic inflammation differs from the intra-amniotic inflammatory process observed in patients with preterm labor due to microbial invasion of the amniotic cavity (MIAC). Microorganisms and their products can induce a robust intra-amniotic inflammatory response characterized by an elevation in amniotic fluid interleukin (IL)-6 concentration,106-130 and neutrophil chemokines, such as IL-8,111-113, 131-140 as well as other inflammatory mediators capable of inducing the onset of labor.118, 135, 141-180 Recently, we provided an analysis of the protein inflammatory network for this condition.181 The histologic hallmark of MIAC is acute histologic chorioamnionitis, defined by the infiltration of maternal neutrophils into the chorioamniotic membranes.109, 182-196 Related lesions are chorionic vasculitis197 and the spectrum of lesions observed in cases of funisitis.155, 198-203, 196, 204 Therefore, at this time, at least two major types of intra-amniotic inflammation appear to occur in the context of spontaneous preterm labor—one associated with MIAC or induced by danger signals124, 205-209 and the other associated with chronic inflammatory lesions of the placenta (often attributed to maternal anti-fetal rejection).
The concentration of the T-cell chemokine CXCL10 (IP-10) is considered a marker for chronic inflammatory lesions associated with allograft rejection and chronic chorioamnionitis in the case of pregnancy. In contrast, IL-6, IL-8, IL-1, and TNF-α are examples of cytokines involved in acute inflammatory lesions of the placenta.109, 128, 130, 196, 210
The objective of this study was to determine the prevalence and clinical significance of an elevated CXCL10 concentration in the amniotic fluid of patients with a diagnosis of either preterm labor with intact membranes or preterm PROM and whether an elevation in CXCL10 concentration is associated with chronic chorioamnionitis, as an increased concentration of IL-6 and CXCL10 is frequently observed in patients with intra-amniotic infection and acute histologic chorioamnionitis.
2 Materials and methods
2.1 Study population
A nested retrospective cohort study was conducted by searching the clinical database and Bank of Biological Materials of Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (Detroit, MI) to identify patients with a diagnosis of spontaneous preterm labor with intact membranes or preterm PROM. Patients were included if they met the following criteria: (i) singleton gestation; (ii) episode of preterm labor and intact or ruptured membranes; and (iii) transabdominal amniocentesis performed between 20 and 35 weeks of gestation for microbiological studies. Patients were excluded if chromosomal or structural fetal anomalies or placenta previa was present. All patients provided written informed consent. The use of biological specimens and clinical data for research purposes was approved by the Institutional Review Boards of NICHD and Wayne State University.
2.2 Biological samples and analysis
Amniotic fluid was transported in a capped sterile syringe to the clinical laboratory where it was cultured for aerobic and anaerobic bacteria, including genital mycoplasmas. Evaluations of the white blood cell count, glucose concentration, and Gram stain of the amniotic fluid were performed shortly after collection. Amniotic fluid was centrifuged at 1300 g for 10 minutes at 4°C shortly after collection and stored at −70°C until analysis. Concentrations of IL-6 and CXCL10 in the amniotic fluid (ng/mL) were determined by the enzyme-linked immunosorbent assay test, using immunoassays obtained from R&D Systems (Minneapolis, MN, USA). The assay time, volume, and other characteristics for each method have been previously described.15, 123, 124, 169, 205-207
2.3 Clinical definitions
Gestational age was determined by the last menstrual period and confirmed by ultrasound examination, or by ultrasound examination alone if the sonographic determination of gestational age was not consistent with menstrual dating.211 Preterm labor was diagnosed by the presence of at least two regular uterine contractions every 10 minutes in association with cervical changes in patients with a gestational age between 20 and 36 6/7 weeks that led to preterm delivery (defined as birth prior to the 37th week of gestation). Preterm PROM was diagnosed by a sterile speculum examination with documentation of the pooling of amniotic fluid in the vagina in association with a positive nitrazine test and/or positive ferning test when necessary. Elevated amniotic fluid IL-6 concentration (≥2.6 ng/mL) was used to define intra-amniotic inflammation.176, 205-208, 212-215 MIAC was defined as a positive amniotic fluid culture. Intra-amniotic infection was defined as the combination of MIAC and intra-amniotic inflammation. An elevated amniotic fluid CXCL10 concentration as a marker of subclinical intra-amniotic inflammation was defined as ≥2.2 ng/mL, which is above the 95th percentile among patients with uncomplicated term deliveries.169
The diagnosis of acute histologic chorioamnionitis was based on the presence of acute inflammatory changes in the extraplacental chorioamniotic membrane roll and/or chorionic plate of the placenta, using the criteria previously described.188-190, 192, 196, 216, 217 The grading and staging of placental lesions consistent with amniotic fluid infection was defined according to the Amniotic Fluid Infection Nosology Committee of the Perinatal Section of the Society for Pediatric Pathology as reported by Redline et al.188 Acute funisitis was defined as the presence of neutrophils in the wall of the umbilical vessels and/or Wharton's jelly.188, 196, 197 Chronic placental inflammatory lesions included the following: (i) chronic chorioamnionitis; (ii) villitis of unknown etiology (VUE); and (iii) chronic deciduitis. Chronic chorioamnionitis was diagnosed when lymphocytic infiltration into the chorionic trophoblast layer or chorioamniotic connective tissue was observed.14, 15, 26, 218 VUE was defined as the presence of lymphohistiocytic infiltration, in varying proportion, of the placental villous tree.14, 219 Chronic deciduitis was diagnosed as the presence of lymphocytic infiltration into the decidua of the basal plate.220 Lesions consistent with maternal anti-fetal rejection proposed by our group included chronic chorioamnionitis, VUE, or chronic deciduitis with plasma cells.14, 16
2.4 Study groups
Participants were allocated into four study groups, according to whether they had an increase in amniotic fluid CXCL10 concentration and/or an increase in amniotic fluid IL-6 concentration: (i) normal amniotic fluid IL-6 and CXCL10 concentrations; (ii) an isolated increase in amniotic fluid IL-6 concentration; (iii) an isolated increase in amniotic fluid CXCL10 concentration; and (iv) an increase in both amniotic fluid IL-6 and CXCL10 concentrations. The cutoff has been derived from previous studies.109, 111, 169
2.5 Study outcomes
The primary outcome of this study was the presence or absence of acute or chronic chorioamnionitis, defined as (i) the absence of both acute and chronic chorioamnionitis; (ii) acute chorioamnionitis ≥stage 2 in the absence of chronic chorioamnionitis; (iii) chronic chorioamnionitis in the absence of acute histologic chorioamnionitis ≥stage 2; and (iv) the presence of both acute (≥stage 2) and chronic chorioamnionitis. The presence of placental lesions associated with maternal anti-fetal rejection was examined as a secondary outcome.217
2.6 Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normality of arithmetic data distributions. The Kruskal-Wallis test and the Mann-Whitney U test were used to make comparisons among and between groups for arithmetic variables. The chi-square test or Fisher's exact test was used for comparisons of proportions. Multinomial logistic regression models were fit to examine magnitudes of association with primary and secondary outcomes, adjusting for gestational age at amniocentesis. Statistical analysis was performed using SAS 9.4 (Cary, NC, USA). Confidence intervals (95% CI) that do not include the null hypothesis (ie, an odds ratio [OR] of “1.0”) are considered statistically significant.
3 Results
3.1 Clinical characteristics
One hundred and sixty-eight women with either preterm labor with intact membranes (72%) or preterm PROM (28%) were included in this study. Table 1 displays the clinical characteristics of the participants; 88% were African American, 34% were nulliparous, and 83% delivered preterm (<37 weeks of gestation). The median gestational age at amniocentesis was 30 weeks (interquartile range: 27-32 weeks), and amniotic fluid cultures were positive in 20% of the study participants. Placental lesions associated with acute and chronic histologic chorioamnionitis and maternal anti-fetal rejection were observed in 49% (82/168), 27% (45/168), and 41% (69/168) of the study population, respectively.
| Descriptive characteristics | Median (IQR) or % (n) |
|---|---|
| Maternal age (y) | 24.0 (20.3-30.0) |
| Body mass index (kg/m2) | 25.0 (21.0-30.9) |
| Nulliparity (%) | 33.9% (57/168) |
| Gestational age at amniocentesis (wk) | 30.2 (26.6-32.3) |
| African American ethnicity (%) | 88.1% (148/168) |
| Amniotic fluid WBC count (cells/mm3) | 3.5 (0-39.3) |
| Amniotic fluid glucose (mg/dL) | 23.0 (10.0-30.0) |
| Positive amniotic fluid culture (%) | 20.2% (34/168) |
| Amniocentesis-to-delivery interval (d) | 4 (1-26.8) |
| Preterm delivery (%) | 83.3% (140/168) |
| Gestational age at delivery (wk) | 32.1 (27.7-34.9) |
| Birthweight (g) | 1822.5 (1086.3-2472.5) |
| Acute histologic chorioamnionitis (%) | 48.8% (82/168) |
| Chronic chorioamnionitis (%) | 26.8% (45/168) |
| Placental lesions associated with maternal anti-fetal rejection (%)a | 41.1% (69/168) |
- Data presented as median (IQR) or % (n).
- IQR, interquartile range; WBC, white blood cell.
- a Placental lesions associated with maternal anti-fetal rejection: chronic chorioamnionitis, villitis of unknown etiology (VUE), and chronic deciduitis with plasma cells.
3.2 Amniotic fluid CXCL10 and IL-6 concentrations according to placental pathologic lesions and outcome of pregnancy
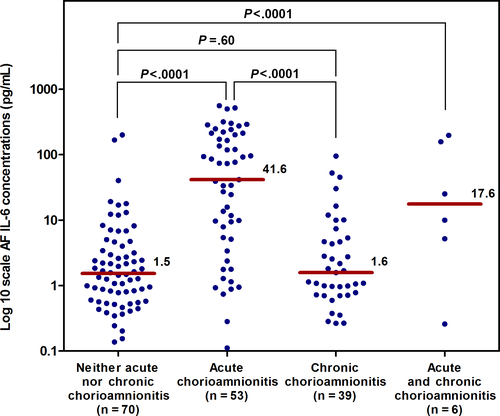
Amniotic fluid CXCL10 concentrations were highest in patients with chronic chorioamnionitis (Figure 1), whereas amniotic fluid IL-6 concentrations were highest in patients with acute chorioamnionitis ≥stage 2 (Figure 2). Clinical characteristics and the prevalence of acute and chronic inflammatory placental lesions for the four study groups, defined according to the amniotic fluid CXCL10 and amniotic fluid IL-6 concentrations, are shown in Table 2.

| Outcomes | Normal AF IL-6 and CXCL10 (n=53) | Isolated increase in AF IL-6 (n=26) | Isolated increase in AF CXCL10 (n=30) | Increase in both AF IL-6 and CXCL10 (n=59) |
|---|---|---|---|---|
| GA at amniocentesis (wk) | 31.3 (28.4-32.7) | 30.6 (26.7-32.4)a | 31.4 (29.4-32.2)b | 26.9 (23.6-30.7) |
| GA at delivery (wk) | 34.9 (32.0-38.1) | 31.0 (27.1-33.0)a | 34.1 (31.8-36.5)b | 27.9 (24.3-32.1) |
| Preterm delivery (83.3%; n=140/168) | 64.2% (34/53) | 93.3% (24/26)a | 76.7% (23/30)b | 100% (59/59) |
| Spontaneous preterm delivery within 48 h of amniocentesis (36.3%; n=61/168) | 20.8% (11/53) | 61.5% (16/26) | 20.0% (6/30)b | 47.5% (28/59) |
| Spontaneous preterm delivery before 34 wk of gestation (50.6%; n=85/168) | 30.2% (16/53) | 65.4% (17/26) | 26.7% (8/30)b | 74.6% (44/59) |
| Birthweight (g) | 2485 (1900-2941) | 1557 (1008-2119)a | 2210 (1701-2743)b | 1155 (600-1700) |
| Placental pathology | ||||
| No acute/chronic chorioamnionitis (41.7%; n=70/168) | 67.9% (36/53) | 50% (13/26)a | 36.7% (11/30)b | 16.9% (10/59) |
| Acute chorioamnionitis ≥stage 2 (31.5%; n=53/168) | 13.2% (7/53) | 42.3% (11/26) | 13.3% (4/30)b | 52.5% (31/59) |
| Chronic chorioamnionitis (23.2%; n=39/168) | 18.9% (10/53) | 7.7% (2/26) | 46.7% (14/30)b | 22.0% (13/59) |
| Acute (≥stage 2) and chronic chorioamnionitis (3.6%; n=6/168) | 0% (0/53) | 0% (0/26) | 3.3% (1/30) | 8.5% (5/59) |
| Acute funisitis (33.3%; n=56/168) | 17% (9/53) | 34.6% (9/26) | 20% (6/30)b | 54.2% (32/59) |
- AF, amniotic fluid; CXCL, C-X-C motif chemokine; GA, gestational age; IL, interleukin; acute chorioamnionitis, the presence of acute chorioamnionitis ≥stage 2 in the absence of chronic chorioamnionitis; chronic chorioamnionitis, the presence of chronic chorioamnionitis in the absence of acute chorioamnionitis ≥stage 2.
- Data presented as % (n) or median (interquartile). Normal AF IL-6 and CXCL10 concentrations: IL-6 <2.6 ng/mL and CXCL10 <2.2 ng/mL; isolated increase in AF IL-6 concentrations: IL-6 ≥2.6 ng/mL; isolated increase in AF CXCL10 concentrations: CXCL10 ≥2.2 ng/mL; increase in both AF IL-6 and CXCL10 concentrations: IL-6 ≥2.6 ng/mL and CXCL10 ≥2.2 ng/mL.
- a P<.05 for the comparison between the group of isolated increase in amniotic fluid IL-6 concentration and the group of increase in both amniotic fluid IL-6 and CXCL10 concentrations.
- b P<.05 for the comparison between the group of isolated increase in amniotic fluid CXCL10 concentration and the group of increase in both amniotic fluid IL-6 and CXCL10 concentrations.
An elevation in the concentration of both amniotic fluid CXCL10 (≥2.2 ng/mL) and amniotic fluid IL-6 (≥2.6 ng/mL) was observed in 35% (59/168) of the patients, whereas 18% (30/168) had an isolated elevation in the concentration of amniotic fluid CXCL10, 15% (26/168) had an isolated elevation in the concentration of amniotic fluid IL-6, and 32% (53/168) did not have an elevation in amniotic fluid concentrations of either CXCL10 or IL-6. All patients with elevated concentrations of both amniotic fluid CXCL10 and IL-6 delivered before 37 weeks of gestation. By contrast, 93% (24/26) of patients with an isolated elevation in the concentration of amniotic fluid IL-6 and 77% (23/30) of those with an isolated elevation in the concentration of amniotic fluid CXCL10 delivered preterm.
3.3 Acute and chronic chorioamnionitis in relationship to amniotic fluid concentrations of CXCL10 and IL-6
The prevalence of chronic chorioamnionitis was highest in patients with an isolated elevation in amniotic fluid CXCL10 concentration (46.7%; 14/30) and was lowest in those with an isolated elevation in amniotic fluid IL-6 concentration (7.7%; 2/26) (Table 2). In contrast, the prevalence of acute chorioamnionitis ≥stage 2 was highest in patients with an isolated elevation in both amniotic fluid IL-6 and CXCL10 concentrations (52.5%; 31/59), followed by patients with an isolated elevation in amniotic fluid IL-6 concentration (42.3%; 11/26). The prevalence of such placental lesions was observed in 13% (4/30) of patients with an isolated elevation in amniotic fluid CXCL10 concentration. Interestingly, almost all patients with acute and chronic chorioamnionitis (83.3%; 5/6) had an elevation in both amniotic fluid IL-6 and CXCL10 concentrations (Table 2).
The magnitudes of association between the study groups according to amniotic fluid CXCL10 and IL-6 concentrations and the presence or absence of acute or chronic chorioamnionitis are described in Figure 3. Patients with an isolated elevation in CXCL10 concentration were significantly more likely to have chronic, but not acute, chorioamnionitis (OR: 4.8, 95% CI: 1.7-14; and OR: 2.1, 95% CI: 0.5-8.9, respectively) than those with normal amniotic fluid CXCL10 and IL-6 concentrations, after adjusting for gestational age at amniocentesis. In contrast, patients with an isolated elevation in amniotic fluid IL-6 concentration were significantly more likely to have acute (≥stage 2), but not chronic, chorioamnionitis (OR: 4.2, 95% CI: 1.3-13.7; and OR: 0.5, 95% CI: 0.1-2.8, respectively) than those with normal amniotic fluid CXCL10 and IL-6 concentrations. An elevation in amniotic fluid concentrations of both CXCL10 and IL-6 was associated with acute (≥stage 2) and chronic chorioamnionitis (OR: 9.6, 95% CI: 3.1-30; and OR: 3.8, 95% CI: 1.3-11.6, respectively). None of the patients whose placentas had both acute (≥stage 2) and chronic chorioamnionitis (n=6) had normal amniotic fluid CXCL10 and IL-6 concentrations.
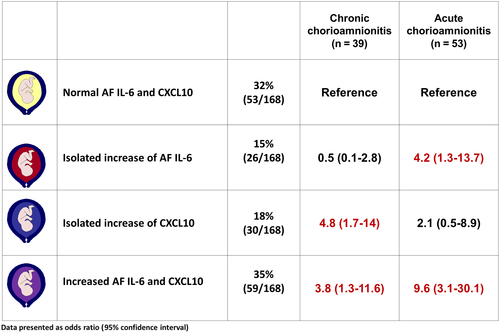
The magnitudes of association observed between the study groups are shown in Figure 4 in accord with the concentrations of both amniotic fluid CXCL10 and IL-6 as well as the placental lesions associated with maternal anti-fetal rejection. Patients with an elevation in amniotic fluid CXCL10 concentration were significantly more likely to have placental lesions associated with maternal anti-fetal rejection, but not acute chorioamnionitis (≥stage 2), than those with normal amniotic fluid CXCL10 and IL-6 concentrations, adjusting for gestational age at amniocentesis (OR: 3.7, 95% CI: 1.3-10.4; and OR: 1.6, 95% CI: 0.3-8.3, respectively).
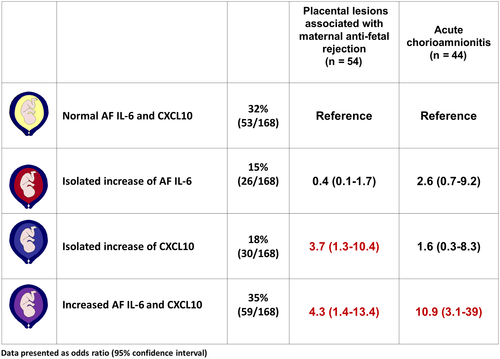
The combination of both acute chorioamnionitis (≥stage 2) and placental lesions associated with maternal anti-fetal rejection was not observed in patients without an elevation in amniotic fluid IL-6 and CXCL10 concentrations (Figure 4).
4 Discussion
4.1 Principal findings of the study
(i) An isolated elevation in amniotic fluid CXCL10 concentration is associated with chronic, but not acute (≥stage 2), histologic chorioamnionitis; (ii) in contrast, an isolated elevation in amniotic fluid IL-6 concentration was associated with acute (≥stage 2), but not chronic, histologic chorioamnionitis; (iii) similar findings were observed in relation to placental lesions associated with maternal anti-fetal rejection (chronic chorioamnionitis, VUE, and/or chronic deciduitis with plasma cells). Specifically, an isolated elevation in amniotic fluid CXCL10 concentration is associated with the subsequent delivery of placentas with lesions consistent with maternal anti-fetal rejection but not acute histologic chorioamnionitis (≥stage 2); (iv) elevation in both CXCL10 and IL-6 is associated with acute and chronic inflammatory lesions of the placenta, as well as the combination of lesions suggesting that a complex pathologic state representing a mixture of maternal anti-fetal rejection and infection may lead to early preterm delivery in these cases; and (v) all patients with elevated AF concentrations of both CXCL10 and IL-6 delivered prematurely.
4.2 Two types of intra-amniotic inflammation in preterm labor
4.2.1 Microbial-associated and sterile intra-amniotic inflammation
Preterm parturition is a syndrome caused by multiple etiologies.2, 4, 6-8, 12 Intra-amniotic infection is present in one of every three preterm deliveries and is even more frequent in cases of spontaneous preterm labor with intact membranes.221-226 Microorganisms are detected in the amniotic cavity in 25-40% of patients with preterm labor and intact membranes who deliver preterm133, 221-234 and in 50-75% of those with preterm PROM at the time of labor onset.235 The earlier the gestational age at presentation, the greater the risk of MIAC or intra-amniotic infection.6, 12, 225, 226, 233, 236-238
The current study found that 79.8% (134/168) of patients with preterm labor/PROM have no evidence of MIAC, suggesting the important role of sterile inflammation of the amniotic cavity. Using a combination of cultivation and molecular techniques, we have previously reported that only a fraction of all patients with intra-amniotic inflammation (defined as an increase in amniotic fluid IL-6 concentration) have microorganisms present in the amniotic cavity; therefore, sterile intra-amniotic fluid inflammation has emerged as an important mechanism of disease in preterm labor.124, 205-207, 209 Danger signals released during the course of cellular stress, necrosis, pyroptosis, and senescence as well as other non-microbial injury can trigger an inflammatory response in the absence of microorganisms.214, 239-256 Danger signals may also participate in the sterile inflammatory response associated with spontaneous labor at term and are probably mediated by activation of the inflammasomes.244, 257-261 Recent evidence suggests that the intra-amniotic administration of alarmins such as HMGB1 can induce preterm parturition in mice262 and that this cytokine can induce a robust immune response characterized by secretion of IL-6 and IL-1β from human fetal membranes,263 suggesting a role for the inflammasomes in the mechanisms leading to premature labor in cases of sterile inflammation.124, 214, 250 Thus, this mechanism may be involved in patients with sterile intra-amniotic inflammation characterized by elevated amniotic fluid IL-6 concentrations and acute histologic chorioamnionitis. Moreover, a fraction of patients included in this study had elevated concentrations of amniotic fluid IL-6 and CXCL10; these patients delivered preterm and had an odds ratio of 10.9 for acute histologic chorioamnionitis and 4.3 for placental lesions consistent with maternal anti-fetal rejection. The role of the interaction between the acute inflammatory processes that activate the inflammasome and involve fetal rejection is yet to be discovered.
When bacteria and other microorganisms are present in the amniotic cavity and elicit an inflammatory response, a wide range of chemokines and cytokines, such as IL-8,111-113, 131-140 IL-6,106-113, 115-121, 123-126, 128-130 monocyte chemotactic protein-1,164, 165 CXCL10 (IP-10),128, 169 macrophage inflammatory protein-1α,264, 265 growth-regulated oncogene (GRO)-α,135 and other inflammation-related proteins118, 141-163, 166-168, 170-176, 179, 266 are produced, and this can result in the chemotaxis of inflammatory cells to the chorioamniotic membranes. Among these inflammation-related proteins, IL-6 has become the key cytokine for the diagnosis of intra-amniotic inflammation because its increase in concentration has been associated with a shorter interval to delivery and an increased rate of neonatal morbidity and mortality.109, 124, 267, 268 Recently, an in-depth analysis of the chemokine network in preterm labor with and without inflammation, sterile inflammation, and intra-amniotic infection has been reported.269 Network analysis provides a greater level of insight into the biology of the process, given that the protein inflammatory process operates through a network rather than single molecules.269 Collectively, amniotic fluid IL-6 is a pragmatic marker of either microbial-associated or sterile intra-amniotic inflammation. With the development of high-fidelity assays that allow multiplex analysis of biological fluids, we anticipate that it will be possible to characterize with greater detail the biology of the immune response, timetable, response to therapy, and other important clinical characteristics.
4.2.2 A novel form of intra-amniotic inflammation characterized by CXCL10
We previously identified a form of intra-amniotic inflammation characterized by an increase in CXCL10 concentration15, 169 associated with chronic chorioamnionitis, the most common placental lesion in late spontaneous preterm delivery.22 This form of intra-amniotic inflammation is considered a manifestation of maternal anti-fetal rejection,15, 22, 26, 169 as an infectious cause has not been identified by the use of cultivation and molecular methods.
Compelling evidence suggests that CXCL10 plays an important role in the pathogenesis of graft failure and rejection in other organ systems.97-102 Overexpression of this T-cell chemokine has been observed in the serum/plasma,270, 271 urine,272, 273 and tissue biopsies99, 274-276 from patients who experienced rejection in cases of kidney,270, 277-282 heart,283-285 lung,271, 274 and vascular transplantation.286-288 Moreover, there is a significant correlation between serum/plasma CXCL10 concentrations and the timing and severity of allograft rejection.100, 270, 271, 279, 285
In chronic chorioamnionitis, which can be considered a form of allograft rejection, there is an upregulation of CXCL9, CXCL10, and CXCL11 mRNA expression in the chorioamniotic membranes.15 Upregulation of CXC chemokines for CXCR3+ (receptor for T-cell chemokines) cells in the chorioamniotic membranes is associated with a higher median amniotic fluid T-cell chemokine (CXCL10) concentration, and also chronic chorioamnionitis, presumably by stimulating amniotrophic maternal T-cell migration to the chorioamniotic membranes.15 This placental lesion represents a manifestation of maternal anti-fetal rejection as demonstrated by: (i) higher maternal anti-fetal human leukocyte antigen (HLA) sensitization18 in patients with chronic chorioamnionitis than in those without this lesion; (ii) complement deposition (C4d), a surrogate marker of antibody-mediated rejection, in the umbilical vein;16, 23, 24 and (iii) the presence of a novel form of fetal systemic inflammation (FIRS type 2) in the setting of chronic chorioamnionitis. The transcriptome of the umbilical cord blood in FIRS type 2 is different from that of FIRS type 1, indicating that this is a different condition.21 Moreover, a proteomic analysis of the amniotic fluid from patients with chronic chorioamnionitis demonstrated that these patients have lower amniotic fluid concentrations of glycodelin-A,289 a protein implicated in the maintenance of maternal tolerance against a semiallogeneic fetus.290
Interestingly, approximately 40% of placentas with chronic chorioamnionitis from patients with preterm labor or preterm PROM have concomitant VUE and chronic deciduitis with plasma cells.15 We demonstrated the systemic derangement of the chemokine concentrations that occurred in the maternal and fetal circulation systems of patients with VUE, which was distinct from that observed in the setting of acute chorioamnionitis.14 The mRNA expression of a subset of chemokines and their receptors (CXCL9, CXCL10, CXCL11, CXCL13, and CXCR3) was also higher in VUE placentas than in normal placentas.14 Moreover, the median concentrations of CXCL9, CXCL10, and CXCL11 in maternal and fetal plasma were higher in patients with VUE than in those without this lesion.14 Therefore, we also consider VUE as a manifestation of maternal anti-fetal rejection unless a microorganism can be identified.
In summary, intra-amniotic inflammation associated with maternal anti-fetal rejection differs from microbial-associated intra-amniotic inflammation; it is characterized by an elevation in T-cell chemokine concentration in the amniotic fluid and chorioamniotic membranes as well as the presence of chronic inflammatory lesions of the placenta.
4.3 CXCL10: a biomarker for chronic placental inflammatory lesions
The results of the study herein support the view that CXCL10 is a marker for chronic inflammatory lesions of the placenta. Our findings are consistent with those of Gervasi et al.,169 who reported that mid-trimester amniotic fluid CXCL10 concentrations >502 pg/mL were associated with late (>32 weeks) spontaneous preterm delivery (OR: 3.9; 95% CI: 1.6-9.9), whereas elevated amniotic fluid IL-6 concentrations (>1740 pg/mL) were associated with a higher risk of spontaneous preterm delivery prior to 32 weeks of gestation (OR: 9.5; 95% CI: 2.9-31.1). Our study differs in that we examined the relationship between the isolated elevation in either amniotic fluid CXCL10 or amniotic fluid IL-6 concentration and the association with both acute and chronic histologic chorioamnionitis.
4.4 What is the significance of an elevation in the concentrations of amniotic fluid IL-6 and CXCL10?
Thirty-five percent (59/168) of patients in this study had elevated amniotic fluid concentrations of both IL-6 and CXCL10. All of them had spontaneous preterm delivery <37 weeks and <34 weeks of gestation, respectively, suggesting a severe inflammatory process associated with preterm delivery. Moreover, patients with an elevation in both amniotic fluid IL-6 and CXCL10 concentrations had a significantly higher frequency of spontaneous preterm delivery within 48 hours of amniocentesis than those with an isolated elevation in amniotic fluid CXCL10 concentration (Table 2). Indeed, a systemic fetal inflammatory response (defined as the presence of funisitis or chorionic vasculitis)197 was detected in 54.2% (32/59) of patients with an elevation in both amniotic fluid CXCL10 and IL-6 concentrations, but in only 34.6% (9/26) and 20% (6/30) of patients with an isolated elevation in amniotic fluid IL-6 or CXCL10 concentration, respectively (Table 2). One interpretation proposes that patients with a combination of increased amniotic fluid IL-6 and CXCL10 concentrations had a more severe form of intra-amniotic inflammation than those with an isolated elevation in either CXCL10 or IL-6 concentration, in whom the clinical course leading to preterm delivery may be more indolent in nature. This could explain the trend toward a more frequent involvement of the fetus in patients with an elevation in both amniotic fluid CXCL10 and IL-6 concentrations.
CXCL10 has been implicated in the pathophysiology of sepsis by recruiting neutrophils, macrophages, and T cells.291, 292 Previous studies demonstrated that there is an upregulation of CXCL10 leading to subsequent activation of its receptor (CXCR3) during infection and inflammation.293, 294 In an experimental model of septic shock induced by cecal ligation and puncture, it has been shown that plasma and peritoneal fluid CXCL10 concentrations increase.295 Additionally, CXCL10 knockout mice and wild-type mice treated with anti-CXCL10 IgG antibody had less cytokine production and increased survival.296 Similar observations were found for the role of CXCR3 during sepsis; it regulates NK- and T-cell trafficking. Moreover, in a septic shock model for mice, the blockade of CXCR3 decreases systemic inflammation and improves survival.296, 297 CXCL10 and CXCR3 also play a role in human sepsis, and plasma CXCL10 is a predictor of septic shock.298-300 Collectively, these data suggest that CXCL10 is an inflammatory mediator involved in the response to microorganisms and bacterial products; therefore, some cases of advanced infections could have elevated concentrations of both IL-6 and CXCL10. An elevated amniotic fluid concentration of CXCL10 would be more meaningful to identify the patient at risk of chronic inflammatory lesions of the placenta if the amniotic fluid concentration of IL-6 is not elevated.
4.5 Strengths and limitations
The major strengths of this study are as follows: pathologists were blinded to the obstetrical diagnoses and outcomes; standardized protocols were utilized for placental examinations; and the consideration of isolated rather than any increase in the concentration of either amniotic fluid CXCL10 or amniotic fluid IL-6. Limitations were related to the small sample size. Further studies are required to characterize the temporal relationship between exposure to microbial products or other insults and the amniotic fluid changes in cytokines and chemokines. Moreover, large studies are necessary to determine the diagnostic indices of CXCL10 elevation to identify the patient at risk of chronic placental inflammation.
5 Conclusion
An isolated elevation in amniotic fluid CXCL10 concentration (without a concomitant elevation in IL-6 concentration) is associated with the delivery of a placenta with histologic chronic chorioamnionitis or lesions consistent with maternal anti-fetal rejection, whereas an isolated increase in amniotic fluid IL-6 concentration is associated with the delivery of a placenta with acute histologic chorioamnionitis.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Conflict of Interest
The authors declare no conflict of interests.




