Development and Real-World Evaluation of a Statewide Mainstream Model of Germline Genetic Testing for BRCA1/2 and Mismatch Repair Gene Variants (Lynch Syndrome)
Funding: The authors received no specific funding for this work.
ABSTRACT
Purpose
To develop and evaluate an evidence-based mainstream germline genetic testing model to support cancer treatment including the BRCA1/BRCA2 and mismatch repair (MMR) genes across South Australia.
Methods
Participatory action research (PAR) and implementation science principles were used to guide the development of the statewide mainstream pathway. To support the implementation of the mainstream pathway, genetic testing packages for clinicians and consumer support materials have been developed, and an education program has been delivered to clinicians. This quality improvement study used an independent sample t-test to compare the average number of monthly tests completed via mainstream and traditional pathways during two nonconsecutive 6-month periods. Acceptability among patients and clinicians and clinician knowledge, confidence, and experience measures were assessed.
Results
The total number of BRCA1/2 tests did not increase from pre- to post-pathway implementation. However, there was a significant increase in both the number of tests ordered through the mainstream pathway (pre: mean 3.5, SD 2.07; post: mean 7, SD 2.53) and the proportion of total tests ordered via mainstreaming (pre: mean 14%, SD 9.25%; post: mean 25.0%, SD 5.48%). There were no changes in MMR gene testing patterns, with no mainstream tests ordered. Among clinicians (n = 20) who responded to the post-implementation survey, positive levels of acceptability were reported.
Conclusion
This study showed that the implementation of a statewide mainstream genetic testing pathway in a public health system improved the uptake of mainstream testing for BRCA1/2. Further understanding of the barriers to uptake across settings is needed to support effective utilization.
1 Introduction
Genetic testing, including germline testing, plays a crucial role in guiding cancer treatment decisions and improving patient outcomes [1-5], with multiple studies demonstrating significant survival benefits when patients receive genomically matched therapies [1, 6]. The availability and affordability of genetic testing have also increased in recent years, partly due to technological advancements and improved funding in the public healthcare system [7]. However, lengthy wait times, often experienced in traditional models of genetic counseling and testing, can hinder timely access to genetic profile testing required for oncological treatment decision-making. This has seen the emergence of mainstream testing as a potential solution, both within Australia and internationally [8, 9].
The traditional model of genetic testing involves multiple in-person contacts with a genetic healthcare professional after referral by their treating clinician [10, 11], which may pose a significant barrier to accessing germline genetic testing for oncology patients, particularly among underserved and vulnerable populations [10-12]. In the traditional model, long wait times for appointments, testing, and results are also major barriers [13, 14], which can delay or deprive eligible patients access to the most appropriate treatment, subsequently impacting their quality of life, cancer outcomes, and healthcare costs [15]. While still valuable for a subset of patients. This traditional model is also becoming increasingly unsustainable owing to the limited number of specialist genetics services and the growing demand for genetic testing across medical disciplines, including oncology [13-16]. This highlights the need for more streamlined genetic testing models, particularly in the oncology setting, to address such disparities.
Mainstream genetic testing offers a promising solution [13, 14, 17-19]. It integrates genetic testing into routine care for eligible cancer patients, such as oncology or surgical outpatient clinics, thus providing a streamlined experience for the patient, enabling clinicians to make more efficient treatment-related decisions while simultaneously reducing workload and wait times for specialist genetic counseling services [10, 13, 19]. However, mainstream models require nongenetic healthcare professionals to provide treatment-focused information to patients and obtain consent for testing, which requires the upskilling of nongenetic healthcare professionals [13, 19]. These models are associated with a shift from a genetics specialist-led pre-test genetic counseling and informed consent model to predominately posttest counseling models for the subset of patients who require dedicated genetics support. As the demand for precision oncology continues to grow, there is a need for alternative pathways to access genetic testing, including mainstream models.
Mainstream models implemented across various cancer types have demonstrated efficiency, cost-effectiveness, increased uptake of testing, and faster turnaround times [13, 14, 19-23]. The successful implementation of mainstream models is influenced by a number of barriers and enablers including inadequate knowledge and confidence among nongenetic healthcare professionals and time constraints, while enablers include training, access to information and resources, and structured processes and protocols to support mainstream testing [19, 24]. However, their implementation has primarily focused on specific cancer types (e.g., ovarian, breast, prostate cancer), and they have not been implemented in a cross-cancer or cross-service setting [19, 25]. Evaluating the effectiveness of a system-wide approach to mainstream germline genetic testing within a statewide context addresses this gap in the existing literature. This evaluation is crucial for understanding how these models can be broadly and effectively implemented to provide efficient, consistent, and optimal care.
South Australia provides an ideal opportunity to implement and evaluate a standardized statewide approach to mainstream genetic testing, as its existing decentralized healthcare system can result in variations in patient care pathways, access, and processes [26, 27]. A standardized approach across the health system could facilitate a reduction in variation and any subsequent inequity, establish consistency of guidelines and protocols, and ultimately improve cancer care delivery.
This paper describes the systematic and pragmatic process of developing and evaluating a statewide pathway to support mainstream germline testing for cancer treatment through an evidence-based, theory-driven quality improvement initiative.
2 Objective
The project aimed to develop, implement, and evaluate a statewide pathway using an evidence-based mainstream approach to support germline genetic testing for cancer treatment. This pathway specifically focuses on BRCA1/2 in breast, ovarian, and prostate cancers, as well as mismatch repair (MMR) gene testing for colorectal and endometrial cancers following MMR immunohistochemical screening (Lynch syndrome) in adult populations. The cancer types and genetic tests were selected as those with the most clinically actionable outcomes in terms of oncological treatment planning.
3 Methods
3.1 Study Design and Setting
The project ran from April 2022 to December 2023, whereby a multistage approach was used to design, implement, and evaluate the quality improvement initiative throughout all public hospitals across South Australia, including three metropolitan and six regional Local Health Networks (LHNs). The evaluation included a retrospective clinical audit, pre- and post-implementation clinician surveys, and a post-implementation consumer survey. A mixed-method approach, such as that used in the current study, enables greater understanding of implementation within primary healthcare contexts [28, 29]. Sustainability planning was integral to the project design. From the outset, it was agreed that, once the mainstreaming pathway was implemented, responsibility for ongoing support, education, and maintenance of resources would transition to the statewide Adult Genetics Unit (AGU) as part of business-as-usual operations.
The study has followed the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines [30]. An ethics exemption was approved for the evaluation of this project as a low or negligible risk quality assurance project.
3.2 Conceptual Framework Approach
We applied a multistep approach similar to what has been used in theory-based implementation research [31]. The statewide approach followed an established project lifecycle [32] process aligned with Knowledge Translation and Implementation Science (KTIS) frameworks [33, 34] (Figure 1), specifically the Knowledge to Action Framework (KTA) [35]. The project lifecycle emphasizes co-creation and participatory action research (PAR) [36] to engage stakeholders (consumers, clinicians, decision-makers) in adapting and evaluating evidence-driven strategies for practice [32]. KTIS aims to bridge the gap between research knowledge and clinical practice by embedding evidence for effective and sustainable care, ultimately strengthening the healthcare system [37].
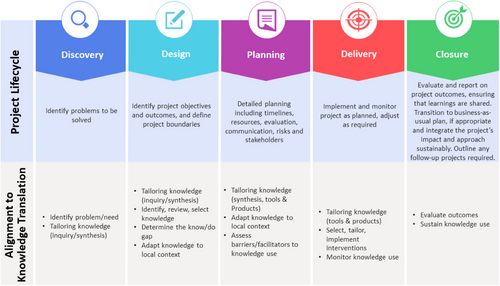
An evidence-based mainstream model from the Royal Marsden Hospital provided the theoretical foundation for the statewide pathway [14]. A key aspect of the Royal Marsden model involved upskilling cancer specialists to consent, order, interpret, and deliver genetic test results for ovarian cancer patients through online learning and algorithms. Based on this, a statewide model was developed throughout the collaborative process described below.
3.3 Approach
Throughout all stages, an expert working group, which included a multisite, multidisciplinary team (MDT) of stakeholders from across South Australia, provided guidance and advice to support the co-creation of the approach using participatory methodologies [36, 38, 39]. The working group included 14 members and met monthly for six meetings between July 2022 and January 2023. Members included clinicians (oncology, colorectal surgery, gynecology–oncology, nursing, pharmacy) and were led by a clinical geneticist, as well as consumers, researchers, a representative from a relevant nongovernment organization (Pink Hope), the commercial genomics company Illumina and the statewide public pathology service (SA Pathology).
In addition to the working group, interviews with key stakeholders were conducted between May 23 and July 1, 2022, to understand both the current LHN processes and identify barriers and facilitators to germline genetic testing. A mix of in-person and online interviews was conducted. Participants were recruited using purposeful and snowball sampling, ensuring that stakeholders across metropolitan and regional LHNs, as well as representatives from clinical genetics and genetic pathology, were included. Eighteen people participated in the interviews.
Information was reviewed and summarized to understand both the current processes at each LHN as well as theming across the health system. Themes identified through interviews and existing evidence were linked to the Theoretical Domains Framework (TDF) [40, 41] and Capabilities-Opportunities-Motivation-Behavior (COM-B) Change Wheel [42] to guide the strategy development (Table SA1). Final strategies for implementation were identified through consensus by the working group, with only those considered feasible and acceptable, and supportive of an evidence-based approach selected for adoption (Table 1). For example, while an online consent process was discussed, it was not implemented due to feasibility constraints.
| Strategy number | Implementation strategy |
|---|---|
| 1 | Use of evidence-based approach (mainstreaming) to develop standardized pathway, that is, applicable within a local context |
| 2 | Development of genetic testing packages for clinicians including standardized information and forms |
| 3 | Packages housed on website to allow ease of access to clinicians |
| 4 | Working group members identified to advocate for and champion mainstreaming germline testing |
| 5 | Delivery of germline genetic testing education to clinicians |
Implementation strategies included developing a standardized pathway for mainstream germline genetic testing, development of genetic testing packages for clinicians, dissemination of packages on an easily accessible website (AGU), engaging working group members to act as advocates for and to champion the approach locally, clinician education to support mainstreaming (process, identification, consent, and counseling), and upskilling clinicians involved in germline genetic testing.
3.4 Implementation
Mainstream packages (Table 2) were made available on the AGU website in February 2023 [43], with educational sessions provided from December 2022 to March 2023. The most current versions of the mainstream packs can be accessed on the AGU website [43]. To support clinician uptake of mainstreaming, education was delivered by a dual-trained Medical Oncologist and Clinical Cancer Geneticist (from the AGU) to 13 cancer-focused MDT meetings or similar across all LNHs in South Australia with over 300 registered clinicians/staff.
| Packages developed | Package content |
|---|---|
|
Breast and ovarian cancers Lynch syndrome cancers Prostate cancer |
General clinician information sheets Clinician considerations for ordering mainstream cancer genetic testing leaflet Cancer genetics mainstream testing flowchart Consent form for mainstream cancer genetic testing Genetic testing criteria guide Mainstream genetic testing checklist Manchester scoring system sheet (breast cancer only) Pathology request form (with pre-filled information)a Consumer information sheets (supplemented by educational video) [44] Supporting Information including additional consumer information sheets and information about life insurance and genetic testing provided separately. |
- a Cancer-type specific.
3.5 Participants
The participants in the evaluation component of the quality improvement initiative included both clinicians and consumers. The inclusion criteria for the clinician survey included all clinicians who were invited to attend the education sessions in clinical unit meetings and/or tumor-streamed MDT meetings across LNHs. The consumer survey inclusion criteria were patients aged 18 years or older who had undergone mainstream germline genetic testing via the SA public hospital system. As the survey was conducted in English, participants who did not speak English were excluded from completing the survey.
3.6 Measures and Data Collection
Data related to the number of germline genetic tests ordered for the BRCA1/BRCA2 and MMR (Lynch syndrome) genes by a requestor (genetics specialist vs. nongenetic healthcare specialist) and LHN were used for the clinical audit. Data were used for two nonconsecutive 6-month periods from July to December 2021 (pre-implementation) and March to August 2023 (post-implementation).
Clinician surveys measured clinician acceptability, knowledge, and confidence in germline genetic testing for cancer treatment, adapted from similar questionnaires [14, 17, 21]. Pre-implementation surveys were sent from November 2022 through March 2023 to align with the education sessions, with follow-up surveys sent 6 months following the initial survey. In total, 36 clinicians completed the pre-education survey, and 20 completed the post-education survey. Surveys were distributed via email to individuals associated with the MDT meetings, with a link to the online survey (SurveyMonkey). In some instances, the survey link was sent to meeting coordinators who then forwarded it to their respective groups. Because of this indirect method of distribution, response rates could not be calculated. For each survey period, reminder emails were sent 5 days after the initial invite email.
The consumer survey was sent via letter in October 2023, with the option of completing and returning a paper-based version using a reply paid envelope or completing the survey online (SurveyMonkey). The Royal Marsden Satisfaction Questionnaire was modified and used as the basis for the survey [14, 17]. The modified survey assessed patient acceptability, experience, and understanding of the process, their results, and the additional support and information available to them. Four of the 22 consumers who were sent the survey returned the completed survey (response rate of 18.18%). Given the very small sample size, limited information can be drawn from the consumer survey data is not included in the results.
The primary outcome measure was the number of tests conducted through mainstreaming compared to the traditional pathway. Acceptability among clinicians was secondary, along with acceptability, knowledge, and confidence.
3.7 Statistical Analysis
The number of genetic tests completed via mainstream and traditional pathways during two nonconsecutive 6-month periods prior to and following implementation was summarized per month. An independent sample t-test was conducted to compare the number of pre- versus post-implementation tests ordered per month (p < 0.05). The proportion of total tests ordered through mainstreaming was also calculated (the number ordered via mainstreaming/monthly total tests). All other data were summarized using descriptive statistics.
For the survey data, Likert scale responses of strongly agree and agree were combined.
4 Results
4.1 Adoption of Mainstream Genetic Testing
Only BRCA1/2 genetic testing data were provided. No eligible patients underwent Lynch syndrome mainstream testing during the data collection period.
While there was no significant difference from the pre- (mean 25, standard deviation [SD] 3.9) to post-implementation (mean 28.33, SD 8.91) for the total number of tests ordered per month, there was a significant increase in the number of tests ordered through the mainstream pathway before- (mean 3.5, SD 2.07) compared to post-implementation (mean 7.0, SD 2.53; p = 0.03; Table 3, Table SA2). Using the proportion of total tests ordered through mainstreaming, a significant increase from pre- (mean 14%, SD 9.25%) to post-implementation (mean 25%, SD 5.48%; p = 0.04) was also identified.
| Pre | Post | t | df | p | Cohen's d | |||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | |||||
| Traditional | 21.5 | 4.76 | 21.33 | 6.95 | 0.48 | 10 | 0.96 | 5.96 |
| Mainstream | 3.5 | 2.07 | 7 | 2.53 | 2.62 | 10 | 0.03 | 2.31 |
| Total combined | 25 | 3.9 | 28.33 | 9.91 | 0.84 | 6.85 | 0.43 | 6.88 |
| % of total mainstreamed | 14% | 9.25% | 25% | 5.48% | 2.43 | 8.125 | 0.04 | 7.6 |
- Abbreviations: M, mean; SD, standard deviation.
4.2 Clinician Acceptability
Clinicians' roles and locations varied throughout South Australia (Table 4), representing all relevant nongenetic healthcare professions and health networks. Clinician acceptability, confidence, and knowledge varied. For example, post-implementation, 72% of clinicians reported sufficient knowledge to both identify patients and know when mainstreaming genetic testing was appropriate, and 75% felt confident interpreting test results. However, only 44% were confident providing genetic counseling, and 29% reported that they are able to conduct the referral and counseling within a standard appointment time and only 19% reported that it was easy to know when genetic testing results were ready. Detailed survey results are provided in Tables SA3 and Figure SA1.
| Analysis population | ||
|---|---|---|
| Parameter | Pre (%) | Post (%) |
| Role | 36 | 20 |
| Medical oncologist | 13 (36.1) | 5 (25) |
| Surgeon | 8 (22.2) | 5 (25) |
| Nurse consultant/Coordinator/Practitioner | 8 (22.2) | 9 (45) |
| Other | 7 (19.5) | 1 (5) |
| Health networksa | 35 | 20 |
| North | 5 (14.3) | 4 (20) |
| South | 9 (25.7) | 6 (30) |
| Central | 17 (48.6) | 8 (40) |
| Rural | 4 (11.4) | 2 (10) |
| Cancer types treated (multiple selections allowed) | 36 | 20 |
| Breast | 18 (50) | 10 (50) |
| Ovarian | 34 (94.44) | 6 (30) |
| Prostate | 13 (36) | 6 (30) |
| Colorectal | 12 (33.3) | 11 (55) |
| Endometrial | 15 (41.7) | 6 (30) |
|
Self-reported mainstream uptake % Yes |
(n = 34) 21 (61.8) |
(n = 18) 13 (72.2) |
- Note: Data presented as No. (%).
- a North, South, and Central refer to the metropolitan-based Local Health Networks in Adelaide, South Australia; Rural refers to regional networks outside of the metropolitan area.
5 Discussion
This project successfully implemented an evidence-based mainstream pathway across the South Australian public health system to support germline genetic testing for cancer treatment. Although similar mainstream models have demonstrated effectiveness in ovarian [13, 14], breast [10, 18], and prostate [17, 21] cancer, the model has yet to be tested in a cross-cancer or cross-service setting [19, 25]. Using implementation science and other methodologies, this study demonstrated mixed results, with an increased adoption of mainstream genetic testing for the BRCA1/2 genes but no evidence of uptake of mainstreaming for MMR gene testing. These findings partially align with those of previous studies, highlighting the complexities of implementing genetic testing in clinical practice.
The results of BRCA1/2 genetic testing demonstrated a shift toward the use of mainstreaming, rather than an overall increase in germline genetic testing. This is an important finding in the context of the growing demand for genetic testing and increasing pressure on genetic specialist resources [16, 45]. Although mainstreaming pathways for genetic testing were available prior to the study, they were underutilized and lacked standardized processes or supporting resources. Increased use of mainstream pathways enables a reduced reliance on genetic specialists, focusing on their involvement where expertise is most needed. This approach can decrease wait times for genetic testing results and subsequent treatment decisions, while also allowing specialist resources to be allocated more efficiently, benefiting the healthcare system [19].
Within the literature there are limited studies that have implemented mainstream pathways for MMR gene testing in for colorectal and endometrial cancer. A recent systematic review [25] found only one known study exploring colorectal specialists’ attitudes toward mainstream consent pathways [46]. To the best of our knowledge, no studies have implemented or tested a mainstream pathway among colorectal and endometrial cancers. This demonstrates a significant gap in the literature, and future research is needed, particularly implementation trials to test the translation of mainstream pathways in these cancers. Despite the inclusion of colorectal and endometrial cancer specialists as stakeholders throughout the process, the reasons for the lack of effective uptake of this pathway are unclear. However, this discrepancy may be related to the difference in requirements for publicly funded access to precision oncology treatments in Australia, and the identification of a BRCA1 or BRCA2 germline variant is required for access to poly (ADP-ribose) polymerase (PARP) inhibitors in several tumor streams, including breast and prostate cancer, thus creating a more urgent clinical need for germline genetic testing. However, an abnormality in MMR immunohistochemistry alone, without confirmation of a germline mechanism, is sufficient for access to immunotherapy in endometrial and colorectal cancers. This difference in drug access requirements may result in less clinical urgency for germline genetic testing in Lynch syndrome, and subsequently, less perceived clinical utility in the mainstream pathway. In addition, targeting both BRCA1/2 and MMR genes is potentially too broad for an initial implementation project, and more targeted implementation efforts and resources are needed for the MMR pathway. As follow-up data were only obtained 6 months post-implementation, formal feedback from clinicians regarding the lack of MMR gene testing was not specifically collected as part of this study. The use of a single clinician education session in the more complex clinical eligibility landscape of Lynch syndrome testing may also have limited uptake; further clinician education and support may have been beneficial. Future studies should include structured follow-up with clinicians to better understand barriers to Lynch syndrome mainstreaming.
Clinicians reported high ratings of acceptability and increased confidence and knowledge post-implementation compared with pre-implementation. This is consistent with previous research, which has demonstrated that the introduction of mainstream pathways is supported and well-received by clinicians [13, 14, 17, 18, 47]. This early positive response suggests that a mainstream approach has the potential for wider implementation. However, barriers to adoption remained, including time constraints, indicating that further work is required to support the sustainability and future scalability of a mainstream approach to genetic testing pathways in routine clinical practice.
Preliminary investigations of pre-implementation processes have highlighted significant variation in practice both within and between health networks in relation to germline genetic testing. Major barriers included lack of uniform information, process complexity, and limited clinician confidence and knowledge in identifying eligible patients, as well as in the consent and counseling process. Although improvements were observed across these areas, several systemic barriers remained post-implementation. This included appointment and clinician time constraints, continued clinician support/education, and limitations with tracking results, including access to centralized data repositories. These barriers are deeply embedded in health systems and require long-term structural changes to be addressed effectively. Addressing these barriers was beyond the scope and timeframe of the current project but critical for the sustainability of mainstream pathways. A potential solution could be to further integrate the roles of Nurse Practitioners and Cancer Care Coordinators to support patients and clinicians in the utilization of mainstream pathways. This may alleviate some time constraints, assist in advocating for continued clinician education, and support the efficiency of test tracking and result communication. Previous research has highlighted the importance of mainstream champions at implementation sites, and Nurse Practitioners and/or Cancer Care Coordinators may be best placed to take on this role [10].
The participatory approach, involving diverse stakeholders, enhances the framework's relevance and applicability to our specific setting, translating research findings into practical applications, which is a key challenge highlighted in implementation science [34, 48]. Our findings contribute to the growing body of literature on pragmatic implementation of evidence into practice within healthcare settings, demonstrating how to navigate the complexities of implementation science theories and frameworks in a real-world context [49]. The mixed results, which show increased adoption of the BRCA1/2 pathway but limited uptake for MMR mainstream genetic testing pathways, highlight the need to consider implementation strategies that can be applied across different contexts and settings while recognizing that tailored implementation strategies may be necessary for varying clinical contexts.
This project was conducted as a quality improvement initiative, utilizing a KTIS approach. The strength of this methodology lies in its rigorous yet pragmatic approach, which bridges the well-documented research-to-practice gap in healthcare [48]. Our framework leverages an evidence-based mainstream model, adapted to local context and needs through a KTA and co-design principle. This strategy allowed us to balance methodological rigor with practical healthcare system requirements, creating a flexible approach that meets the needs of both academic and healthcare audiences [50].
While the implementation science approach was a strength of this study, it also imposed certain limitations. This included balancing scientific rigor with real-world and stakeholder needs. The nature of the quality improvement approach means that we were restricted in terms of data access, preventing detailed analysis of factors such as wait times and the ability to match pre- and post-surveys for clinicians. The small sample size for the clinician and consumer survey limits the generalizability of the findings; however, the results provide valuable insights to inform future research and program development. The results are also difficult to generalize to other contexts and settings, as evidenced by the difference in the uptake of BRCA1/2 and MMR mainstream testing. In addition, we were unable to account for external factors that may have influenced the uptake of BRCA1/2 and MMR mainstream testing during the study period, including underlying secular trends or incremental changes unrelated to implementation of the mainstream pathway. In addition, we did not measure outcomes specific to implementation strategies but rather the pathway as a whole.
Despite the above, this is the first study to explore the implementation of mainstream genetic testing across multiple cancer types and settings. The results of this study are promising and warrant further investigation. Future research should build on these findings with more rigorous methodologies, while balancing their approach with implementation science principles to bridge the gap between research and practice.
6 Conclusion
The mainstream framework was established to provide consistent and standardized guidelines and pathways for efficient treatment-focused germline genetic testing in patients with cancer. While challenges remain, particularly in the adoption of MMR mainstream testing, the framework has shown promise in increasing the utilization of the mainstream pathway, reducing pressure on specialist genetics services, and facilitating timely treatment decisions for cancer patients.
Acknowledgments
We acknowledge and thank all those involved in the development, implementation, and evaluation of the mainstream genetic testing pathway. We thank all patients, clinicians, and stakeholders who provided input and suggestions throughout the project. Thank you to the Cancer Statewide Clinical Network and the sub-working group, who gave up their time and provided important expertise throughout the project. Finally, we would like to acknowledge SA Pathology for its support in providing essential data for the quality improvement evaluation.
Ethics Statement
This quality assurance study was reviewed by the Central Adelaide Local Health Network Human Research Ethics Committee (CALHN HREC) and was deemed low/negligible risk under institutional and NHMRC guidelines (Reference No. 17404).
Consent
Participation in the survey was voluntary and completion of the survey was taken as implied consent. All survey responses were anonymous. For the analysis of health system data, only de-identified information collected as part of routine clinical practice was used, and no individual patient was identifiable.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




