Real-World Survival Outcomes of Patients With High PD-L1 Advanced NSCLC Who Received Chemoimmunotherapy Versus Immunotherapy
ABSTRACT
Background
Randomized studies have demonstrated superior overall survival (OS) of pembrolizumab (pembro), both alone and in combination with chemotherapy (chemo) over chemo alone in patients with programmed cell death ligand-1 (PD-L1) ≥ 50% advanced non–small cell lung cancer (NSCLC). We reviewed the real-world outcomes of patients who received pembro-chemo versus pembro only.
Methods
This Australian-based retrospective cohort study used data from patients with advanced NSCLC PD-L1 ≥ 50%, diagnosed between January 2016 and July 2021 and had received first-line pembro-chemo or pembro only. Patients with an EGFR/ALK/ROS1 sensitizing mutation were excluded. Cox proportional-hazards model and Kaplan–Meier methods were used to estimate OS and progression-free survival (PFS).
Results
Of 111 eligible patients, 25 received pembro-chemo and 86 received pembro only. After a median follow-up of 15.7 months, median (95% CI) OS was not reached in the pembro-chemo group versus 15.6 (9.5–21.7) months in the pembro-only group (HR 0.57, 95% CI 0.28–1.16, p = 0.12). Median PFS was 12.4 (6.5–18.3) versus 9.5 (6.3–12.6) months in pembro-chemo versus pembro-only groups, respectively (HR 0.62, 95% CI 0.32–1.18, p = 0.18). Objective response rate (ORR) was higher in the pembro-chemo group (60% vs. 30.3%). There were more hospitalizations in the pembro-chemo group versus pembro-only group, 28% versus 18.6%, but immune-related adverse events were similar (32% vs. 32.6%).
Conclusion
In patients with PD-L1 ≥ 50% advanced NSCLC, addition of chemo to first-line pembro yielded a higher ORR but no additional benefit in PFS or OS, supporting a shared-decision approach. However, higher rates of hospitalizations seen in the pembro-chemo group should warrant caution in use.
1 Background
Immune checkpoint inhibitors (ICIs) are highly effective therapies in patients with advanced non–small cell lung cancer (NSCLC), significantly extending the survival of these patients and rapidly changing clinical practice [1, 2]. However, its benefit varies depending on the status of a predictive biomarker, programmed cell death ligand-1 (PD-L1) [3]. Objective response rates (ORRs) to anti-PD-1/PD-L1 antibodies are 15%–20% in unselected patients compared to 45% in selected patients [3]. Keynote 001, a Phase 1b trial, first reported the early efficacy and safety of pembrolizumab (pembro), a PD-1 inhibitor, in advanced NSCLC [4, 5]. The highest ORRs were observed in patients whose tumor cells expressed PD-L1 tumor proportion score (TPS) of at least 50% [4, 5]. Prevalence of PD-L1 expression in the Keynote 001 substudy showed 33% were TPS < 1%, 38% were TPS 1%–49%, and 28% were TPS ≥ 50% [6].
In advanced NSCLC patients with high PD-L1 expression, that is, PD-L1 ≥ 50%, first-line single agent ICI [7-10] and combination of ICI and chemotherapy (chemo) [11, 12] have both shown superior efficacy in overall survival (OS) compared to chemo alone. Single (pembro, cemiplimab, and atezolizumab) [13-15] agent ICIs showed an estimated OS difference of 7.2, 13.0, and 10.4 months, respectively, compared to chemo, whereas, doublet (ipilimumab with nivolumab) [16-18] ICIs showed an estimated OS difference of 6.7 months. These data have led to approval of these agents by the US Food and Drug Administration [19-23].
In Australia, options for treatment are restricted by the Therapeutic Goods Administration (TGA) approval and Pharmaceutical Benefits Scheme (PBS). Currently, single agent pembro and chemo-pembro are both PBS-reimbursed for NSCLC patients with PD-L1 ≥ 50%. Hence, choice of therapy is largely dependent on physician's recommendation. These choices are often made based on patients’ age, choice of having chemo, volume of disease, and toxicity of treatments.
To date, there is no head-to-head comparison of efficacy of adding chemo to immunotherapy versus immunotherapy monotherapy in this group of patients. We performed a retrospective review of outcomes of NSCLC patients with PD-L1 ≥ 50% who received upfront ICI. This study aimed to evaluate if there is a survival benefit in adding chemo to pembro compared with pembro monotherapy in an Australian cohort of patients.
2 Methods
2.1 Patient Selection
Pembro was initially approved for first-line treatment of NSCLC by TGA in March 2017 [24] and subsidized by PBS in November 2018. Therefore, we have included patients with advanced NSCLC were selected between March 2017 and July 2021 (over 4 years) from electronic medical records. Patients were eligible if the tumor PD-L1 expression were ≥ 50% and received either first-line pembro and chemo or pembro only. Patients were excluded if they had a sensitizing EGFR/ALK/ROS1 mutation or if PD-L1 status was unavailable. Baseline characteristics of patients including age at diagnosis, gender, smoking status, de novo metastatic disease, metastatic site(s) at diagnosis, histology, Eastern Cooperative Oncology Group (ECOG) at diagnosis, and type of treatment were collected.
2.2 Data Analysis
The primary outcome of the study was OS. Secondary outcomes were progression-free survival (PFS), ORR, and adverse events (AEs).
Statistical analyses were conducted using IBM SPSS Statistics 26. Baseline characteristics were examined using descriptive analyses. Hazard ratios of OS and PFS and associated 95% confidence intervals were calculated using the Cox proportional-hazards model. The Kaplan–Meier method was used to estimate survival curves and landmark survival at 12 and 24 months. Subgroup analyses of OS and PFS were performed on clinically relevant characteristic to test for prognostic factors.
3 Results
3.1 Baseline Characteristics
We screened 314 patients with advanced NSCLC and 111 patients were eligible for this study (Figure 1). Of these, 25 patients received first-line combination of pembro and chemo and 86 received pembro only. At data collection cutoff (December 1, 2021), median follow-up was 15.7 months. Overall, median (range) number of cycles of pembro received was 8 (1–47) cycles and of chemo was 4 (0–6) cycles.
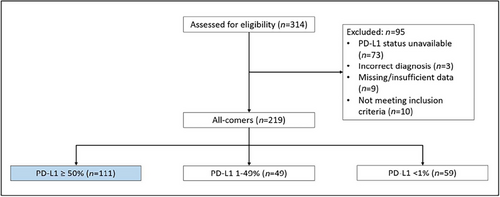
Table 1 summarizes the baseline characteristics of these two groups. Overall, the majority were males (77%), either current or ex-smokers (92%), of de novo metastatic disease (87%) and nonsquamous pathology (83%). More patients with the ECOG performance status of zero received pembro-chemo combination treatment.
| Pembro-chemo | Pembro only | Overall | |
|---|---|---|---|
| Characteristics | N, n = 25 (%) | N, n = 86 (%) | N, n = 111 (%) |
| Age (years) at diagnosis, median (range) | 64.8 (34.8–79.4) | 70.9 (44.8–87.1) | 69.2 (34.8–87.1) |
| Gender | |||
| Male | 19 (76.0) | 66 (76.7) | 85 (76.6) |
| Female | 6 (24.0) | 20 (23.3) | 26 (23.4) |
| Smoking status | |||
| Never smoked | 4 (12.0) | 5 (5.8) | 9 (8.1) |
| Ex-smoker | 15 (60.0) | 56 (65.1) | 71 (64.0) |
| Current smoker | 6 (24.0) | 25 (29.1) | 31 (27.9) |
| ECOG at diagnosis | |||
| 0 | 17 (68.0) | 39 (45.3) | 56 (50.5) |
| 1 | 6 (24.0) | 32 (37.2) | 38 (34.2) |
| 2 | 1 (4.0) | 11 (12.8) | 12 (10.8) |
| Unknown | 1 (4.0) | 4 (4.7) | 5 (4.5) |
| De novo metastatic disease | |||
| Yes | 23 (92.0) | 74 (86.0) | 96 (87.4) |
| No | 2 (8.0) | 12 (14.0) | 14 (12.6) |
| Baseline metastatic disease | |||
| Lung | 5 (20.0) | 26 (30.2) | 31 (27.9) |
| Liver | 6 (24.0) | 8 (9.3) | 14 (12.6) |
| Bone | 10 (40.0) | 32 (37.2) | 42 (37.8) |
| Brain | 6 (24.0) | 18 (20.9) | 24 (21.6) |
| Pleural effusion | 3 (12.0) | 15 (17.4) | 18 (16.2) |
| Other | 14 (56.0) | 37 (43.0) | 51 (45.9) |
| Single site | 12 (48.0) | 52 (60.5) | 64 (57.7) |
| Multiple sites | 13 (52.0) | 34 (39.5) | 47 (42.3) |
| Histology | |||
| Squamous | 3 (12.0) | 16 (18.6) | 19 (17.1) |
| Nonsquamous | 22 (88.0) | 70 (81.4) | 92 (82.9) |
3.2 Overall Survival and Progression-Free Survival
At time of data cutoff, 9 patients (36.0%) in the pembro-chemo group and 57 patients (67.0%) in the pembro-only group had died. Though OS favored combination group, there was no significant OS difference between pembro-chemo and pembro-only groups (HR OS 0.57, 95% CI 0.28–1.16, p = 0.12; Figure 2A). Median OS (95% CI) was not reached in the combination group and was 15.6 months (9.5–21.7) in the pembro-only group. The 12- and 24-month OS rates were 60.6% versus 56.2% and 55.1% versus 35.5%, in the pembro-chemo versus pembro-only groups, respectively.
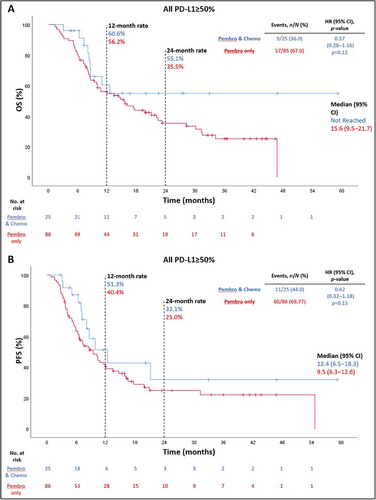
Median PFS rates were 12.4 months (95% CI 6.5–18.3) and 9.5 months (95% CI 6.3–12.6) in the pembro-chemo and pembro-only groups, respectively (HR PFS 0.62, 95% CI 0.32–1.18, p = 0.15; Figure 2B). The estimated 12-month PFS rates were 51.3% and 40.4% in the pembro-chemo and pembro-only groups, respectively. Estimated 24-month PFS rates were 32.1% and 25.0% in the pembro-chemo and pembro-only groups, respectively.
3.3 Subgroup Analyses
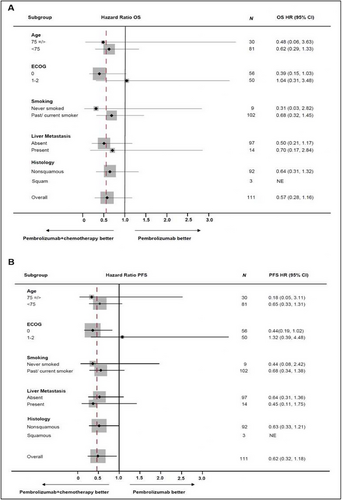
Subgroup analyses, including age < 75 and ≥ 75, ECOG 0 versus 1–2, never and ever smokers, with and without liver metastases, single versus multiple metastatic sites at diagnosis, and squamous and nonsquamous histology were assessed (Figure 3, Figure S1). Although OS favored pembro-chemo group in general, but there was no statistically significant difference in OS compared to pembro-only group. In patients with ECOG 0, the pembro-chemo group showed a PFS benefit (Figure 3). The addition of chemo to first-line pembro also did not appear to provide any additional OS benefit in both squamous and nonsquamous subgroups (Figures S2 and S3).
3.4 Objective Response Rate
ORR occurred in 15 of 25 patients (60.0%, all partial response [PR]) in the pembro-chemo group and 26 of 86 patients (30.3%) in the pembro-only group (complete response [CR], n = 1; PR, n = 25; Table 2). Median durations of response (DORs) were 4.1 months (0.7–16.0) and 11.6 (1.6–41.5) months in the pembro-chemo and pembro-only groups, respectively. A selected group of patients, 16% pembro-chemo group and 20% patients in the pembro-only group, had a DOR of 12 months or greater. While ORRs were significantly higher in the pembro-chemo group, DOR appeared to be longer in the pembro-only group.
| Treatment outcome | Pembro + chemon = 25 | Pembro onlyn = 86 | All PD-L1 ≥ 50%n = 111 |
|---|---|---|---|
| Best overall response | |||
| CR | 0 (0) | 1 (1.2) | 1 (0.9) |
| PR | 15 (60.0) | 25 (29.1) | 40 (36.0) |
| SD | 5 (20.0) | 12 (14.0) | 17 (15.3) |
| PD | 5 (20.0) | 48 (55.8) | 53 (47.7) |
| ORR | 60% | 30.3% | 36.9% |
| Median DOR, months (range) | 4.1 (0.7–16.0) | 11.6 (1.6–41.5) | 8.3 (0.7–41.5) |
- Note: Data are no. (%) unless otherwise indicated.
- Abbreviations: chemo, chemotherapy; CR, complete response; DOR, duration of response; PD, progressive disease; pembro, pembrolizumab; PR, partial response; SD, stable disease.
3.5 Adverse Effects
Immune-related AEs (irAEs) were reported in 32% of patients in the pembro-chemo group and 36% in the pembro-only group. More hospitalizations during treatment occurred in the pembro-chemo group (28% vs. 18.6%). The main reasons for hospitalizations in the pembro-chemo group were pneumonitis and febrile neutropenia secondary to treatment. In the pembro-only group, the main reasons for hospitalizations were pneumonitis and irAEs (nephritis, pancreatitis). There was no ICU admission in the pembro-chemo group compared to 5.8% in the pembro-only group. The main reason for ICU admission in this group was for management of pancreatitis/nephritis. Discontinuation of therapy due to AEs (either immunotherapy, chemo, or both) occurred in 44% of the pembro-chemo group and 19.8% of the pembro-only group (Table S1).
Pneumonitis and nausea/vomiting were the most common AEs in the pembro-chemo group, whereas immune-related diabetes, pancreatitis, and colitis were the most common in the pembro-only group. Steroids were given for AEs in 16% of the combination therapy group and 22.1% of the immunotherapy group (Table S1).
4 Discussion
In this retrospective cohort study, addition of chemo to first-line pembro in advanced NSCLC with PD-L1 ≥ 50% did not appear to improve OS or PFS. However, a higher ORR was noted in the combination therapy group compared with the monotherapy group. A higher proportion of hospitalizations and additional chemo toxicities were observed in the combination group.
There was a noted lack of clear OS and PFS benefit by adding chemo to pembro in this patient cohort. Sequential treatments of immunotherapy followed by chemo may explain the lack of OS difference between the groups. PR was higher in the combination group compared to the pembro-only group (60% vs. 29.1%). CR was only observed in one patient in this study, from the pembro-only group. The median DOR was, however, longer in the pembro-only group in comparison with the pembro-chemo group (11.6 vs. 4.1 months). A higher proportion of hospitalizations was observed in the pembro-chemo group (28% vs. 18% in the pembro-only group), but no ICU admissions were observed. This could be explained by increasing experience of oncologists in management of irAEs with early steroid introduction in the combination group, or due to patient choice to opt for conservative ward-based management due to having advanced disease.
While there is not prospective randomized data directly comparing chemo-IO and IO-only regimens in high PD-L1 expressing metastatic NSCLC, one could only extract data from the chemo-IO arms from Phase III trials such as Keynote 189, 407, and 024 [7, 9, 10, 13, 25-28]. The results from these trials all favor an OS and PFS benefit in the chemo-IO group regardless of squamous or nonsquamous subtype [25, 27-29]. In comparison to this study, median OS of the chemo-IO group was similarly not reached in the Keynote 189 and 024 studies [25, 28, 29]. In terms of PFS, the median PFS in the chemo-IO arms of the Keynote studies ranged from 6.4 to 10.3 months, which was slightly less than the median PFS of the pembro-chemo group of our study (12.4 months) [25, 27-29]. Our study showed the favorable PFS benefit of pembro-chemo.
The oncologist choice of regimen was reflected through this real-world data. We observed that younger patients with liver metastases received combination of pembro-chemo, likely with the assumption of more likelihood to reduce burden of disease and symptoms rapidly. In the 2022 FDA pool analysis of RCTs of NSCLC patients with PD-L1 > 50%, no significant OS and PFS difference was observed between patients receiving chemo-IO and IO-only regimens, including subgroups of all age groups (< 65, 65–75, and > 75) as well as ECOG (0 vs. 1+) [30]. These findings are further supported by later systematic reviews and meta-analyses of results from RCTs of NSCLC patients with PD-L1 > 50% [31-34]. Similar findings of chemo-IO not conferring increased survival advantage are also reported in other retrospective studies from 2022 to 2024 comparing outcomes of patients who received IO only versus chemo-IO, including subgroups of all age groups, tumor histology, smoking status, and ECOG [35-37].
The extended PFS benefit of pembro-chemo observed in our study could be contributed by bias from the retrospective nature of the study. It is limited mainly by selection bias and results being hypothesis-generating only. Only patients with formal testing proving high PD-L1 and complete data were selected. There was likely also performance bias as patients were not randomly assigned to treatment groups. The unblinded nature of our study led to performance bias of physicians giving younger and fitter patients pembro-chemo over the pembro-only treatment. This study also had a small sample size (111 patients overall with imbalanced patient numbers between the groups), leading to lower statistical power to observe differences in OS between the groups. Though we reported AEs during treatment, this did not directly reflect the quality of life of these patients, which is best assessed in a prospective randomized trial. Furthermore, data on subsequent lines of treatment were not collected. This may also have an impact on OS. Further study of outcomes with evaluation of intermediate endpoints such as PFS2 (time from start of first-line treatment to progression on second-line treatment) should be considered.
In conclusion, in patients with advanced NSCLC without sensitizing mutations and PD-L1 ≥ 50%, adding chemo to first-line pembro yielded a higher ORR but no additional benefit in OS or PFS. Apart from ECOG status, similar findings were observed in the subgroup analyses. However, higher rates of hospitalizations were demonstrated in the pembro-chemo group should warrant caution in use. These results support a patient–physician shared decision approach in selecting first-line therapy for advanced NSCLC with PD-L1 ≥ 50%.
Acknowledgments
Open access publishing facilitated by Western Sydney University, as part of the Wiley - Western Sydney University agreement via the Council of Australian University Librarians.
Ethics Statement
The authors have nothing to report.




