Prophylactic cranial irradiation in patients with resected small-cell lung cancer: A systematic review and meta-analysis
Haoning Peng and Jianqi Hao contributed equally to this work.
Abstract
Prophylactic cranial irradiation (PCI) was recommended for limited-stage small-cell lung cancer (SCLC) patients with complete or partial response to primary chemoradiotherapy. But it is still controversial regarding its role in SCLC patients who have had radical resection. This meta-analysis aims to evaluate the efficacy of PCI in resected SCLC patients. We searched PubMed, EMBASE, Web of Science, CENTRAl, and ClinicalTrials for controlled trials and cohort studies regarding PCI in postoperative SCLC patients. The correlation between PCI and post-operative outcomes in SCLC patients, including survival and brain metastasis rate (BMR), was examined using hazard ratios (HRs) and risk ratios with corresponding 95% confidence intervals. Quality of studies was assessed by the Newcastle–Ottawa Scale (NOS), and publication bias was assessed by Begg's test. Meta-analysis of eight studies with 2688 patients in total showed PCI was associated with improved overall survival (OS) for resected SCLC (HR: 0.65, 95% CI: 0.57–0.75, p < 0.01). In addition, subgroup analysis on three studies including 923 patients confirmed the protective role of postoperative PCI in N0 SCLC patients (HR: 0.79, 95% CI: 0.61–0.97, p < 0.05). There was also a significant reduction in BMR in the PCI group pooled from six studies (HR: 0.58, 95% CI: 0.40–0.85, p < 0.01). The use of PCI delayed brain recurrence and improved OS in patients with resected, stage I-III SCLC. Importantly, patients with N0 SCLC can also benefit from postoperative PCI. In future studies, PCI's role in patients with resected N0 SCLC at different T stage may need to be explored.
INTRODUCTION
The latest global cancer burden statistics indicate that small-cell lung cancer (SCLC) accounts for 9% of new lung cancer cases in men and 11% in women.1 At the time of diagnosis, two-thirds of SCLC patients have distant metastasis and about 50% patients develop brain metastases in the course of the disease.2, 3 Prophylactic cranial irradiation (PCI) was found to reduce the incidence of brain metastasis and improved overall survival (OS) for both limited and extensive stage SCLC in the previous research4-7 However, it is still controversial whether patients with early-stage SCLC should undergo PCI since brain metastases are relatively uncommon in these patients8-10 and the adverse effect of PCI cannot be ignored.11, 12
The results of multiple retrospective studies indicated surgery followed by adjuvant chemotherapy is superior to chemoradiotherapy for limited, especially early-stage SCLC.13-17 A growing number of SCLC patients are undergoing surgery in a real-world setting, but the role of PCI in surgically resected SCLC patients is still unclear.18 Considering the limited patient population, it is difficult to conduct randomized controlled trials to determine the effectiveness of PCI in postoperative SCLC. A meta-analysis carried out by Y Yang et al. in 2018 demonstrated that resected SCLC patients may benefit from PCI except for p-stage I patients.19 However, the illustration of these conclusions should be cautious due to the limited number of studies enrolled and small sample sizes in subgroup analyses. Herein, we performed a comprehensive meta-analysis assessing survival outcomes and brain metastasis rate (BMR) of SCLC patients with or without postoperative PCI based on more recent studies. In addition, subgroup analysis for N0 patients was conducted to facilitate clinical decision making.
METHOD
Study design
This systematic review and meta-analysis was preregistered in INPALSY, with registration number INPLASY202480054. The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and no individual patient data were used.
Search strategy
The literature search was performed in PubMed, EMBASE, Cochrane library (CENTRAL), Web of Science, and ClinicalTrials by two independent reviewers. A combination of Medical Subject Headings (MeSH) terms and keywords in title and abstract were used to search for “small cell lung cancer,” “cranial irradiation,” and “surgery.” No special restrictions were applied to any of the available studies published up to July 2024. The complete search strategy is demonstrated in the supplementary materials S1. Saturation of potentially eligible studies were ensured by scanning the reference lists of identified review articles.
Selection criteria
Literatures were firstly screened for duplication based on title and abstract by two researchers. Case reports, conference abstracts, letters, editorials, commentaries, meta-analysis, and review articles were excluded. The remaining articles were selected through the following criteria: population: small-cell lung cancer patients with radical resection; intervention: postoperative PCI; comparison: patients without PCI; outcomes: OS, BMR; study design: randomized controlled trials (RCT) or cohort studies. Literatures written in languages other than English were excluded. Disagreement over included studies were solved through discussion.
Data extraction and quality assessment
Two authors (H. Peng and J. Hao) extracted data independently using a predetermined extract table including author, year of publication, nationality, accrual years, TNM stage of patients, PCI regime, sample size in both PCI and non-PCI group, median follow-up time, proportion of patients received adjuvant chemotherapy and thoracic radiotherapy (TRT), hazard ratio (HR) and 95% confidence interval (CI) for OS, and BMR. Any conflict was resolved by discussion or consensus with a third reviewer.
Studies included in the review were assessed using the Newcastle–Ottawa Scale (NOS). A study that receives a score of >6 was considered high quality based on the NOS rating scale.
Statistical analysis
The impact of PCI on survival of postoperative SCLC patients were pooled with HR. A subgroup analysis for N0 patients was conducted for OS. Statistical heterogeneity across studies were assessed by Q statistics and I2 test. Random effect model was adopted for data analysis when the I2 value exceeded 50%, indicating significant heterogeneity. Otherwise, fixed effect model was used. Sensitivity analysis was performed for identifying possible origin of heterogeneity. The publication bias was assessed using Egger's tests and funnel plots, and trim-and-fill method was performed for evaluating pooled HR after adjusting potential bias. All statistical analyses were conducted in STATA 15.0.
RESULTS
Search results
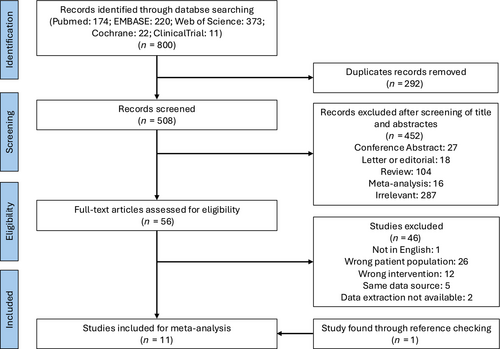
The process of literature screening and studies selection are depicted in Figure 1. Database retrieval initially yielded 800 records. After removal of 292 duplicated studies, 508 records were screened based on title and abstract and 452 among them were excluded due to irrelevant topics or incorrect article types. A total of 56 studies underwent full-text review, and 11 articles were finally enrolled for meta-analysis.
Basic characteristics of included studies
The basic characteristics of all eligible studies were showed in Table 1. All literatures included in meta-analysis were retrospective cohort studies. Though not reported in few studies, most patients enrolled in this study received adjuvant chemotherapy, while proportion of patients received adjuvant TRT was various between PCI and non-PCI group among different studies. An independent review by two authors showed good consistency in evaluating the quality of the included studies. All studies in this review received a score of 7 or more according to NOS quality assessment, indicating that they were of high quality (Table 2).
| Study | Year of publication | Country | Accrual years | Total number of patients | PCI | Non-PCI | PCI regime | TNM stage | Median follow-up (months) | Adjuvant chemotherapy (PCI/non-PCI or overall proportion) | Adjuvant TRT (PCI/non-PCI or overall proportion) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang* et al.26 | 2021 | China | 2006–2014 | 664 | 124 | 540 | NR | I-III | NR | 89.5%/68.9% | 73.4%/39.3% | OS |
| Zhou et al.29 | 2021 | USA | 1986–2019 | 164 | 43 | 121 | 25 Gy/10 fr | I-III | NR | 86%/62% | 26% | OS and BMR |
| Guo et al.30 | 2020 | China | 2005–2016 | 251 | 59 | 192 | NR | I-III | 46.8 | 87.30% | 31.50% | OS |
| Resio et al.25 | 2019 | USA | 2004–2015 | 859 | 202 | 657 | NR | I-III | NR | NR | 0% | OS |
| Chen et al.21 | 2018 | China | 2003–2015 | 52 | 19 | 33 | 25 Gy/10 fr | I-III | NR | 100%/75.8% | 42.1%/18.2% | OS and BMR |
| Xu et al.9 | 2016 | China | 2006–2014 | 349 | 115 | 234 | NR | I-III | NR | 93.0%/91.5% | 70.4%/63.2% | OS and BMR |
| Yokouchi et al.31 | 2015 | Japan | 2003–2013 | 156 | 13 | 143 | 25 Gy/10 fr or 30 Gy/15 fr | I-IV | 25.5 | 64.10% | NR | OS and BMR |
| Zhu et al.23 | 2014 | China | 2003–2009 | 193 | 67 | 126 | 25 Gy/10 fr | I-III | 39.4 | 100% | 58.2%/43.7% | OS and BMR |
| Yang* et al.26 | 2021 | China | 2006–2014 | 111 | 37 | 74 | NR | T1-4N0M0 | NR | 75.70% | 73.90% | OS for N0 subgroup |
| Lou et al.20 | 2020 | China | 2006–2017 | 146 | 46 | 100 | NR | T1-4N0M0 | 27.5 | 89.1%/89% | NR | OS for N0 subgroup |
| Uprety et al.18 | 2019 | USA | 2004–2013 | 666 | 180 | 486 | NR | T1-2N0M0 | 31.8 | 100% | 0% | OS for N0 subgroup |
| Bischof et al.32 | 2007 | German | 1995–2006 | 39 | 21 | 18 | 30 Gy/15 fr | T1-2N0-1M0 | 29 | 90% | 41% | BMR |
- Note: *This study was included in the meta-analysis of OS for patients with resected stage I-III SCLC as well as for the No subgroup. The characteristics of the different patients group are presented seperately.
- Abbreviations: BMR, brain metastasis rate; NR, not reported; OS, overall survival; PCI, prophylactic cranial irradiation; TRT, thoracic radiotherapy.
| Study | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | ||
| Bischof (2007) | * | * | * | * | * | * | * | 7 | |
| Chen (2018) | * | * | * | * | * | * | * | 7 | |
| Guo (2020) | * | * | * | * | * | * | * | 7 | |
| Lou (2020) | * | * | * | * | * | * | * | 7 | |
| Resio (2019) | * | * | * | * | * | * | * | 7 | |
| Uprety (2019) | * | * | * | * | * | * | * | 7 | |
| Xu (2016) | * | * | * | * | * | * | * | * | 8 |
| Yang (2021) | * | * | * | * | * | * | * | 7 | |
| Yokouchi (2015) | * | * | * | * | * | * | * | 7 | |
| Zhou (2021) | * | * | * | * | * | * | * | * | 8 |
| Zhu (2014) | * | * | * | * | * | * | * | 7 | |
- Note: *The study meets assessment criteria.
Survival outcomes
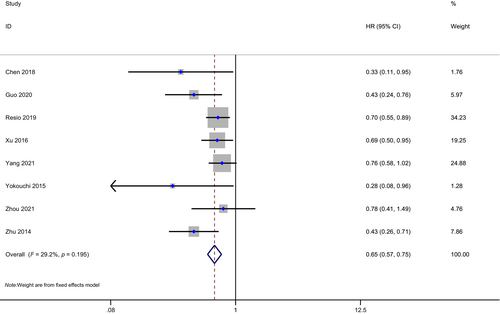
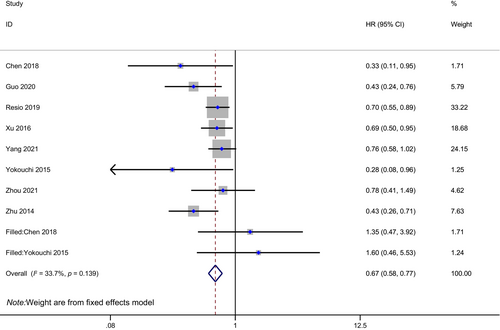
Eight studies with 2688 patients in total, 642 in the PCI group, and 2046 in the non-PCI group were included in the meta-analysis of OS for resected SCLC staged I to III (one study including few stage IV patients). SCLC patients with postoperative PCI had longer OS compared to those who did not undergo PCI (HR: 0.65, 95% CI: 0.57–0.75, p < 0.01; Figure 2). There was little heterogeneity between studies (I2 = 29.2%, H = 1.19).
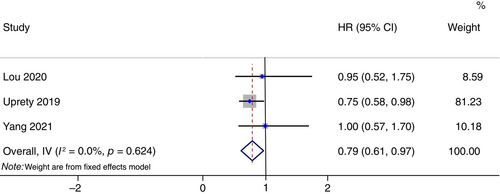
Three studies reported OS data for N0 patients. Totally, 923 patients with 263 in PCI group and 660 in non-PCI group were enrolled in N0 subgroup meta-analysis. Similarly, PCI was associated with improved OS in the N0 subgroup of resected SCLC (HR: 0.79, 95% CI: 0.61–0.97, p < 0.05; Figure 3). There was a high degree of homogeneity among studies (I2 = 0.00%, H = 1.00).
BMR
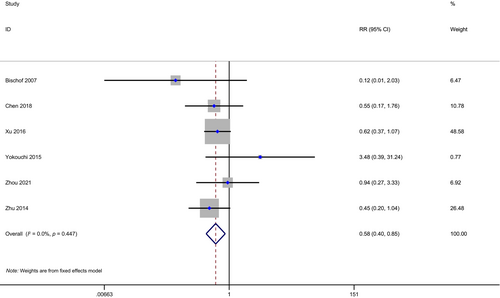
A meta-analysis of BMR was conducted on six studies with 953 patients, 278 in the PCI group, and 675 in the non-PCI group. With little heterogeneity (I2 = 0.00%, H = 1.06), BMR was found to be significantly lower in the PCI group (HR: 0.58, 95% CI: 0.40–0.85, p < 0.01; Figure 4).
For patients with N0 SCLC, Lou et al. reported 10.9% (5/46) of patients in the PCI cohort and 10.0% (10/100) of those in the non-PCI cohort had brain metastases 2 years after surgery.20 But in Chen et al.'s research, the incidence of brain metastasis in patients with p-stage I disease was 0% (0/5) and 16.6% (2/12) in the PCI and non-PCI groups, respectively.21
Sensitivity analysis and publication bias
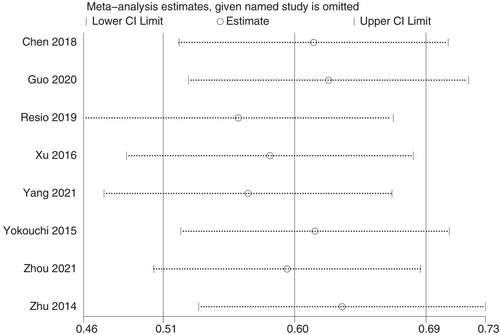
We performed sensitivity analysis for eight included studies for meta-analysis of OS (Figure 5). A single study removed from the analysis did not significantly affect the pooled HR, indicating the robustness of pooled results.
As can be seen in the funnel plot of the Begg's test, the result was asymmetric, and Egger's test showed a significant publication bias (p < 0.05). We then evaluated the reliability of integrated HRs for OS using the trim-and-fill method (Figure 6). Although two studies were filled to the funnel plot, the pooled HR showed limited changes, suggesting that publication bias did not significantly affect reliability of HR in OS (Figure 7).
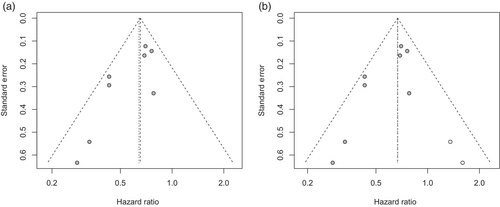
Due to the small number of eligible studies reporting BMR and OS for N0 patients, sensitivity analysis, Egger's test, and funnel plots were not performed.
DISCUSSION
This study found PCI can significantly improve OS and reduce BMR for patients with resected SCLC. The results of this study are consistent with previous systematic reviews and retrospective studies comparing the PCI group and non-PCI group among limited-stage SCLC patients receiving either surgery or chemoradiation as their primary treatment.5, 6, 19, 22
The current NCCN guidelines for small-cell lung cancer recommend surgery should be considered for patients with T1-T2N0M0. But due to the relatively low incidence of brain metastasis for stage I SCLC patients, it is still unclear whether PCI is beneficial for patients with resected SCLC at an early stage.10 In order to investigate the role of PCI in early-stage SCLC, we performed subgroup analysis of OS for N0 patients with radical resection. To our knowledge, this is the first meta-analysis indicating postoperative PCI was associated with survival benefit for N0 SCLC patients. A previous meta-analysis showed that patients with stage I, resected SCLC were not benefit from PCI.19 However, several limitations of meta-analysis for pooled HR of OS in resected, stage I SCLC patients in their study should be noticed. First, HR was calculated from Kaplan–Meier curve instead of cox proportional hazard regression from study of Zhu et al.23 Second, the control group in study of Yang et al.24 did not receive chemotherapy, while the PCI group did. Since the different definition of T stage between the 6th or 7th AJCC and the 8th or 9th AJCC staging system, few studies were eligible for stage I subgroup analysis according to the latest edition of TNM classification. Therefore, we performed meta-analysis on N0 subgroup instead. Resio et al.25 reported that patients with N0 SCLC numerically benefited from PCI, but the lack of information about chemotherapy made this study ineligible for the meta-analysis. Instead, we included data from Uprety et al.18 with more detailed clinical information based on the same database. Data from Yang et al.26 covered data from Xu et al.,9 so only the former study was considered for N0 subgroup analysis. Of noticed, studies conducted by Yang et al.26 included patients with T1-4N0M0, while study of Uprety et al.18 and Lou et al.20 contained patients with T1-3N0M0 when converting to the 9th edition of AJCC. Although we found that postoperative PCI may benefit patients with N0 SCLC, whether N0 patients with different T stage according to the latest AJCC classification would benefit from PCI remains to be studied. Uprety et al.18 reported survival benefits with postoperative PCI in clinical T1 patients (HR, 0.72; 95% CI, 0.53–0.98; P = 0.03). Controversially, Xu et al.9 found PCI failed to significantly extend OS for patients with p-stage I disease (HR 1.61, 95% CI: 0.68–3.83; P = 0.282). Future perspective studies are needed to solve this issue.
In the context of general low incidence of brain metastasis and possible risk of neurotoxicity, PCI's role in early-stage SCLC was controversial. Due to small sample sizes, BMRs of N0 patients undergoing radical resection in both PCI and non-PCI group were various among studies. In addition, data about PCI related acute adverse effect and neurocognitive side effect in patients with resected SCLC were not available in most studies. A multicenter randomized clinical trial showed that fatigue, headache, vomiting, or nausea were most common acute toxic events of PCI in patients with limited-stage SCLC in complete remission after chemoradiotherapy.12 Another phase III randomized controlled trial including patients with extensive-disease SCLC after chemotherapy treatment found PCI had very limited impact on role functioning, cognitive functioning, etc.27 Moreover, several randomized clinical trials found the neurotoxicity and acute side effects were milder for patients assigned to standard 25 Gy compared to higher 36 Gy PCI total dose.12, 28 The neurologic side effects of postoperative PCI could be controlled to a low level as long as PCI was not given concurrently with adjuvant chemotherapy and given at a low dose (25 Gy in 10 daily fraction).
Several limitations need to be identified in this study. First, covariables including adjuvant chemotherapy and TRT were hard to be unified among different studies. Though multivariable cox was used for HR calculation, these covariates may still confound differences between PCI and non-PCI group. In addition, few studies use propensity score matching to reduce the impact of selection bias and potential confounders. And there is not enough data for us to do the subgroup analysis based on histology type, adjuvant therapy, tumor size, etc. Of noticed, few patients who received neoadjuvant chemotherapy were included in this study. As neoadjuvant are increasingly used in SCLC, survival benefits of PCI may need further validation in these subgroups. Second, many studies did not report exact PCI regime, which may cause various treatment effect among different studies. Third, more than half of studies enrolled for OS and BMR meta-analysis were from east Asia, leading to a selection bias which cannot be ignored. Forth, all 11 studies include in this meta-analysis were retrospective. Perspective cohort studies or randomized controlled trials may need to confirm the effectiveness of PCI in resected SCLC patients in a real-world setting. Fifth, few studies compared postoperative PCI with non-PCI in resected, N0 SCLC patients, and there are even fewer studies comparing these two groups in T1-2N0M0 patients. Though more than 10 studies reported PCI's role in resected SCLC, many of them used patients' data from the same database, such as national cancer database from USA and Shanghai Chest hospital Database from China. To reduce duplicate counting and ensure the authenticity of the research, we only selected one representative study from each database for pooled analysis. Therefore, limited number of studies were included for the N0 subgroup analysis, and our findings needs more external validation.
CONCLUSION
Our findings suggest PCI can significantly reduce BMR and improve OS for SCLC patients after radical resection. Postoperative PCI also showed survival benefits for N0 patients. More retrospective or prospective studies are needed to investigate whether postoperative PCI is effective in improving long-term survival for patients with N0 SCLC at different T stages.
AUTHOR CONTRIBUTIONS
(I) Conception and design: H. Peng, J. Hao, L. Liu; (II) literature screening and selection: H. Peng, Z. Li, C. Chen; (III) data extraction: H. Peng, J. Hao, M. Chen; (IV) statistical analyses: J. Hao, Z. Li, B. Dong; (V) manuscript writing: H. Peng, J. Hao, L. Liu; (VI) final approval of manuscript: all authors.
ACKNOWLEDGMENTS
This research was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC21002 to Lunxu Liu).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its supplementary material. Further inquiries can be directed to the corresponding author.




