MicroRNA-1307-3p contributes to breast cancer progression through PRM2
Abstract
Background
Despite advances in screening and therapy, breast cancer (BC) remains the predominant cancer in women globally. Dysregulation of microRNAs (miRNAs) is pivotal in carcinogenesis across various cancers, including BC. Evidence indicates that miR-1307-3p is upregulated in BC tumors, yet its target genes are not fully elucidated. This study aimed to explore how miR-1307-3p regulates BC proliferation, migration, invasion, and angiogenesis and to identify potential target genes.
Methods
Basal miR-1307-3p levels were quantified in BC cell lines MDA-MB-231 and MCF-7, as well as MCF-10A using quantitative real-time reverse transcription-PCR (RT-qPCR). The impact of miR-1307-3p inhibition on BC cell proliferation, migration, invasion, and angiogenesis was assessed. Nine miRNA-target prediction databases identified potential miR-1307-3p targets. Target expression was validated using RT-qPCR, Western blot, and dual-luciferase reporter assays. MiR-1307-3p was overexpressed in MDA-MB-231 and MCF-7 compared to MCF-10A.
Results
Inhibiting miR-1307-3p significantly reduced BC cell proliferation, migration, invasion, and angiogenesis. Bioinformatics analysis identified 17 potential miR-1307-3p targets, with protamine 2 (PRM2) overexpression confirmed via Western blot and dual-luciferase assays.
Conclusion
MiR-1307-3p overexpression in BC promotes proliferation, migration, invasion, and angiogenesis. PRM2 emerges as a novel miR-1307-3p target in BC.
INTRODUCTION
Despite advancements in screening techniques and treatments, breast cancer (BC) remains the leading type of cancer among women worldwide.1 In 2020 alone, over 2 000 000 new cases of BC and 600 000 BC-related deaths were reported.2 Current treatment options for BC include chemotherapy, radiotherapy, surgery, and multidrug therapies. However, there is an urgent need for improved therapies to reduce BC mortality rates.3, 4 Personalized and targeted treatments, especially those focusing on miRNAs, have become increasingly relevant.5, 6
MiRNAs are small non-coding RNAs (21–25 nucleotides) that regulate gene expression by primarily interacting with the 3′UTR region of their target mRNA through base pairing.7, 8 Evidence indicates that miRNA expression is dysregulated in various types of cancer, including BC.9, 10 Due to their ability to target multiple genes, deregulation of a single miRNA can contribute to BC development.11, 12
In BC, numerous deregulated miRNAs, including miR-1307-3p, have been documented. Shimomura et al. reported increased levels of miR-1307-3p in the serum of BC patients.13 Sathipati and Ho employed bioinformatic techniques to identify a miRNA signature predictive of BC stage, implicating miR-1307-3p.14 Han et al. demonstrated that miR-1307-3p promotes BC progression by interacting with the methyltransferase SMYD4, validated through protein expression measurements and luciferase assays.15 These findings suggest that miR-1307-3p has oncogenic activity and promotes BC progression; however, the processes and targets involved remain poorly understood.
In this study, we observed higher expression of miR-1307-3p in BC cell lines compared to breast epithelial cells. Elevated levels of miR-1307-3p correlate with enhanced proliferation, migration, invasion, and angiogenesis. Additionally, we identified human protamine 2 (PRM2) as a direct target of miR-1307-3p in BC.
MATERIALS AND METHODS
Cell culture
The human breast epithelial cell line MCF-10A, the human breast cancer cell lines MDA-MB-231 and MCF-7, and the human umbilical vein endothelial cells (HUVEC) were purchased from the American Type Culture Collection (ATCC). Except for MCF-10A cells, all cell lines were maintained in Dulbecco's modified Eagle medium (DMEM)/nutrient mixture F-12 Ham's medium (Sigma-Aldrich, Merck, USA) supplemented with 10% FBS (GenClone, USA) and 1% antibiotics (Gibco, USA). MCF-10A cells were maintained in mammary epithelial cell growth medium (MEBM) supplemented with bovine pituitary extract (BPE), human epidermal growth factor (hEGF), insulin, hydrocortisone, and gentamicin sulfate amphotericin B (GA-1000) (Lonza, Switzerland). For the experiments, all the cells were maintained in 5% CO2/95% air at 37°C. Experiments were performed at 60% to 80% confluency.
RNA isolation for miRNA expression profiles
Total RNA was extracted from cells using the miRNeasy Mini Kit (Qiagen, Germany) according to the manufacturer's protocol. Briefly, 1.0 × 107 MDA-MB-231, MCF-7, or MCF-10A cells were lysed with 700 μL of QIAzol Lysis Reagent, followed by addition of 140 μL of chloroform, vigorous shaking, and centrifugation. The aqueous phase (350 μL) containing RNA was mixed with ethanol, applied to an RNeasy Mini spin column, and washed with Buffer RWT and Buffer RPE. After elution with RNase-free water, RNA concentration was measured using a Thermo Scientific NanoDrop spectrophotometer.
Quantitative real-time reverse transcription-PCR (RT-qPCR) of miR-1307-3p
Total RNA containing miRNAs with an optical density A260/280 ratio of 1.8–2.1 was used for RT-qPCR with the MystiCq™ MicroRNA™ Quantitation System (Sigma-Aldrich, Merck). cDNA synthesis used the MystiCq™ microRNA cDNA Synthesis Mix Kit. RT-qPCR included 10 μM MystiCq Universal PCR Primer (Sigma-Aldrich, Merck) and either 10 μM miR-1307-3p primer (5′ TGGCGTCGGTCGTGA 3′) (IDT, USA) or SNORD 44 primer (5′ GCAAATGCTGACTGAACATGAA 3′) (IDT), which was performed in duplicate on a Roche Light Cycler Nano with manufacturer-recommended parameters. Expression was analyzed using the 2−ΔΔCT method16 with SNORD44 as the endogenous control. Results show the fold change in miRNA levels compared to MCF-10A cells.
Cell transfection
For transfection, 9.0 × 104 cells (MDA-MB-231 or HUVEC) or 3.0 × 105 cells (MCF-7) were seeded in six-well plates. After 24 h, cells were treated with 100 nM miR-1307-3p inhibitor or negative control inhibitor (Invitrogen) using Hiperfect (Qiagen) at a 1:3 ratio (v/v) in Opti-MEM (Gibco). The mixture was incubated for 20 min at room temperature; then, the cell culture medium was replaced with the transfection mixture. Cells were incubated with this mixture for 24 h before collecting the cell pellet for experiments including clonogenic, migration, invasion, angiogenesis assays, RT-qPCR, and Western blot analysis.
Clonogenic assay
Transfected BC cells were plated in six-well plates. Due to different proliferation rates, 800 MCF-7 cells and 400 MDA-MB-231 cells were seeded. Colony formation was assessed at 10 days for MDA-MB-231 and 18 days for MCF-7 cells. Colonies were stained with 0.5% crystal violet in methanol, and those with at least 50 cells were counted using a Zeiss Primovert inverted microscope at 10× magnification. Clonogenicity percentages were calculated relative to the untransfected control group.
Transwell migration and invasion assays
A transwell plate with 8 μM pores (Corning, USA) assessed cell migration and invasion. A total of 2.1 × 104 MDA-MB-231 and 1.0 × 105 MCF-7 transfected cells in 200 μL of serum-free media were seeded onto transwell chambers precoated with gelatin for migration assays and Cultrex™ Basement Membrane Extract (BME) for invasion assays. The lower compartment was filled with 700 μL of complete culture medium. Migration assays were conducted for 72 h for MCF-7 cells and 24 h for MDA-MB-231 cells. Invasion assays were conducted for 72 h for MCF-7 cells and 48 h for MDA-MB-231 cells. The upper chambers with residual cells were removed, and the cells beneath the surface were fixed using the Fisher HealthCare™ PROTOCOL™ Hema 3™ Manual Staining System (Fisher Scientific, USA). Eight random fields were counted under a Leica CME microscope (40× magnification), and the percentage of migrated and invaded cells was calculated relative to the untransfected control group.
Tube formation assay
Transfected HUVEC were used to evaluate endothelial cell tube formation. The cells (2.5 × 104 cells) were seeded in a 96-well plate precoated with Cultrex™ BME (R&D Systems) (60 μL). The plate was then incubated for 24 h, and photographs of four fields were captured using a Zeiss Primovert inverted cell culture microscope (10× magnification) to observe loop formation.
Mir-1307-3p target prediction
We searched nine databases (TargetScan, miRDB, RNA Central, RNA22, miSTAR, DIANA, miRmap, miRabel, and MicroT) to identify potential targets of miR-1307-3p. From each database, we selected the top 100 targets based on their scores. Subsequently, we filtered the targets that were present in at least three databases. This approach increases the likelihood of selecting genuine targets, as each database employs its own algorithms. The selected targets were then used to design a custom 384-well qPCR plate (Bio-Rad, USA).
SYBR Green–based RT-qPCR
A custom-made 384-well plate equipped with predesigned forward and reverse primers was acquired from Bio-Rad. Using the GenElute Mammalian Total RNA Mini Kit by MilliporeSigma, total RNA was extracted from MDA-MB-231 cells in accordance with the manufacturer's guidelines. Subsequently, the RNA was reverse transcribed using the iScript Reverse Transcription Supermix for RT-qPCR provided by Bio-Rad. SYBR Green-based qPCR was then carried out using the SsoAdvanced™ Universal SYBR® Green Supermix from Bio-Rad, in conjunction with a CFX384 Touch Real-Time PCR detection system. The fold changes and cycle threshold (Ct) values were computed utilizing the internal software of the instrument, relative to MDA-MB-231 cells transfected with either 200 nM of the miR-1307-3p inhibitor or the negative control and normalized against β-actin. This normalization was performed alongside controls for gDNA, PCR, RT, and RNA quality.
To depict gene expression changes, we used the R environment (version 4.2.2) and the ggplot2 package. The process began with preprocessing the qPCR data to calculate 2−∆∆CT fold changes. We established fold change thresholds of 0.15 for upregulation and −0.15 for downregulation to classify genes accordingly. This methodological shift underscores a refined approach to visualizing gene expression alterations, with fold change serving as the primary metric for assessing variation in gene activity.
Western blot analysis
Cells were transfected using the previously described protocol with 200 nM miR-1307-3p inhibitor or negative control, as used in the prior high-throughput RT-qPCR assay. BC cells were detached with 0.25% trypsin–EDTA at 37°C and washed with PBS containing protease inhibitor cocktail (Sigma-Aldrich, Merck). Cell lysates were prepared using ice-cold lysis buffer (1% Triton-X, 150 mM NaCl, 25 mM Tris HCl) and 1% protease inhibitor cocktail, incubated on ice for 30 min, and vortexed periodically. Lysates were centrifuged, supernatants collected, and total protein concentration determined with Bradford reagent (Sigma-Aldrich, Merck). Equal protein amounts (40 μg per well) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, blocked with 5% non-fat dry milk (Santa Cruz Biotechnology) for 1 h, rinsed, and probed with primary antibody against PRM2 (Anti-Protamine 2 [EPR15738], Abcam) at 1/10 000 dilution for 24 h at 4°C. Membranes were rinsed and incubated with goat anti-rabbit IgG (H + L) secondary antibody, HRP (31 460, Invitrogen) at 1/1000 dilution, and rinsed again. Protein bands were detected using Luminol Reagent Immunocruz (Santa Cruz Biotechnology). GAPDH (sc-47 724) and β-actin (sc-47 778) (Santa Cruz Biotechnology) antibodies were used as loading controls. Band densities were analyzed by ImageJ software.17
Dual-luciferase reporter assays
A total of 7.0 × 103 MDA-MB-231 cells were seeded in a 96-well plate. After 24 h, 1.5 μg of miRNA 3′UTR target expression clone for human PRM2 (NM_002762.3) (HmiT107027-MT06) (GeneCopoeia) or miRNA target clone control vector for pEZX-MT06 (CmiT000001-MT06) (GeneCopoeia) was transfected using Hiperfect at a 1:3 ratio (v/v) in Opti-MEM media. Both vectors, purchased commercially, were predesigned with the 3′UTR sequence of PRM2. The control vector lacked the PRM2 3′UTR site (Supplementary Figure S1). Six hours post-transfection, a second transfection with 200 nM of the miR-1307-3p inhibitor was performed using the previously described methodology for 24 h. The second transfection mixture was then replaced with complete DMEM/F-12 medium. After 48 h, firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System Kit (Promega) following the manufacturer's instructions. Luminescence measurements were conducted using a Synergy™ HT plate reader (BioTek® Instruments). Firefly luciferase activity was normalized to Renilla control, and relative luciferase activity was plotted relative to the negative 3′UTR control vector.
Potential signaling pathways of miR-1307-3p predicted by ingenuity pathway analysis
The targets identified through bioinformatic analysis in different miRNA expression databases were subjected to analysis using ingenuity pathway analysis (IPA) (QIAGEN) to identify potential interactions between miR-1307-3p and its candidate targets, aiming to predict signaling pathways.
Correlations between target expression, overall survival, and relapse-free survival among individuals diagnosed with BC
To perform the Kaplan–Meier survival analysis, we used the KM plotter tool (www.kmplot.com), which utilizes expression datasets from various public databases. We examined the expression of miR-1307-3p and PRM2 mRNA in BC patients, correlating both parameters with overall survival (OS) and relapse-free survival (RFS), respectively. A KM survival plot was generated for OS and RFS for all BC patients, without restrictions based on stage, grade, or age. p-values <0.05 were considered statistically significant.
Statistical analysis
All experiments were conducted in three biological replicates unless otherwise indicated. Statistical analyses and construction of graphs were performed using GraphPad Prism software (GraphPad Software Inc., USA). p-values were calculated using parametric tests (t-test or analysis of variance [ANOVA]) as determined by normality tests. p-values <0.05 were considered statistically significant.
RESULTS
Increased expression levels of miR-1307-3p in BC
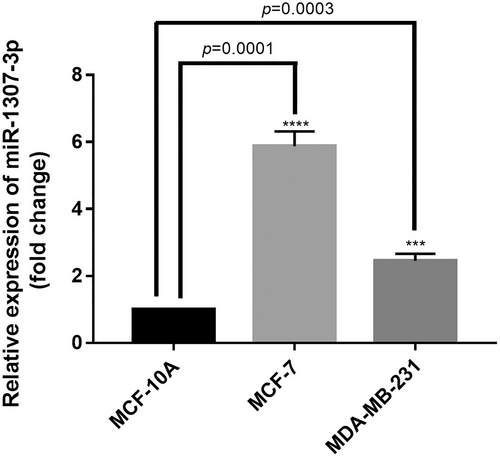
We evaluated miR-1307-3p expression levels, previously identified as overexpressed in the tumor tissue of women with BC compared to their healthy tissue using The Cancer Genome Atlas database (unpublished data) (Supplementary Figure S2), in the BC cell lines MCF-7 and MDA-MB-231, using the non-tumorigenic MCF-10A cell line as a control. MiR-1307-3p expression was significantly higher in both MCF-7 (p = 0.0001) and MDA-MB-231 (p = 0.0003) BC cell lines than in the control cells (Figure 1).
Reducing the expression of miR-1307-3p decreases proliferation
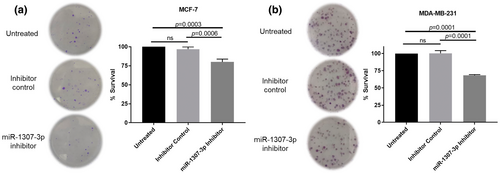
To determine the effect of targeting miR-1307-3p on proliferation, both cell lines were transfected with a miRNA inhibitor. The inhibitor's efficiency and its impact on cell survival were evaluated dose-dependently (Supplementary Figure S3A–D). Proliferation was assessed using a colony formation assay. Transfection with the miR-1307-3p inhibitor resulted in a 16.92% reduction in MCF-7 colony formation compared to the negative control (p = 0.0006) (Figure 2a) and a 32.02% decrease in MDA-MB-231 cells (p = 0.0001) (Figure 2b; Supplementary Figure S4A,B). These findings indicate that reduced miR-1307-3p expression decreases BC cell proliferation.
MiR-1307-3p facilitates the migration and invasion of BC cells
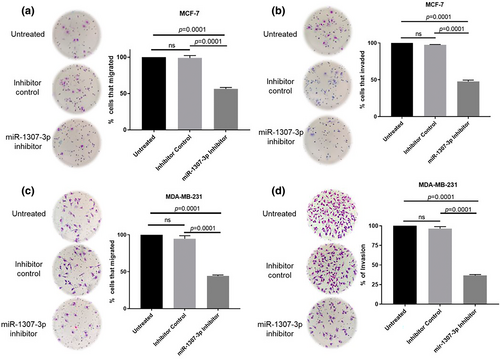
Next, we assessed the effect of targeting miR-1307-3p on migration and invasion, which are linked to metastatic potential.18 MCF-7 cells showed a 42.31% reduction in migration (p = 0.0001) (Figure 3a) and a 49.88% reduction in invasion (p = 0.0001) (Figure 3b). MDA-MB-231 cells exhibited a 50.51% decrease in migration (p = 0.0001) (Figure 3c) and a 59.91% decrease in invasion (p = 0.0001) (Figure 3d). These findings indicate that miR-1307-3p promotes migration and invasion in both cell lines.
MiR-1307-3p promotes angiogenesis in human umbilical vein endothelial cells
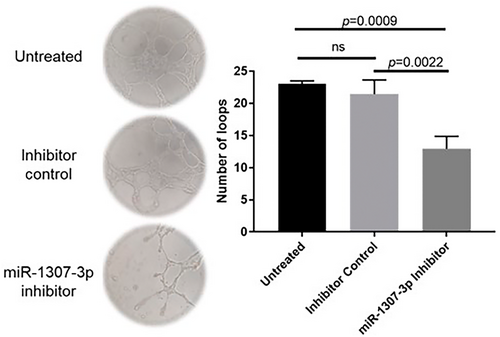
Another crucial process in BC progression is the formation of new blood vessels, essential in the tumor microenvironment (MTE) for supplying nutrients and sustaining growth.19 We assessed the effect of targeting miR-1307-3p on angiogenesis and observed a significant decrease (p = 0.0022) in the ability of HUVEC to form loops following transfection with the miR-1307-3p inhibitor compared to the group treated with the negative control inhibitor (Figure 4). This evidence supports the involvement of miR-1307-3p in angiogenesis.
MiR-1307-3p target prediction
To gain deeper insights into the impact of miR-1307-3p on BC progression, we investigated its potential target genes across specialized miRNA databases, selecting the top 100 targets from each. Genes were further refined based on their presence in at least three databases to enhance reliability amidst diverse selection criteria. Subsequently, we experimentally validated these targets using RT-qPCR, identifying 17 potential targets (Table 1). Results indicated one upregulated target, 11 unchanged targets, and five downregulated targets (Figure 5), employing 2−∆∆CT fold changes. Notably, PRM2 emerged as a candidate warranting further evaluation due to its increased expression following miR-1307-3p inhibition and its role as a reported or suggested tumor suppressor, aligning with the miR-1307-3p oncomiR profile. PRM2 was thus selected for subsequent protein expression analysis via Western blotting.
| Gene | Name |
|---|---|
| RASSF2 | Ras association (RalGDS/AF-6) domain family member 2 |
| NADSYN1 | NAD synthetase 1 |
| BCL2 | B-cell CLL/lymphoma 2 |
| SPERT | Spermatid associated |
| TMEM158 | Transmembrane protein 158 |
| BAIAP3 | BAI1-associated protein 3 |
| BRD3 | Bromodomain containing 3 |
| RP9 | Retinitis pigmentosa 9 |
| VKORC1L1 | Vitamin K epoxide reductase complex. subunit 1-like 1 |
| TAGLN3 | Transgelin 3 |
| VPS37C | Vacuolar protein sorting 37 homolog C |
| PRKCZ | Protein kinase C zeta |
| AGAP1 | ArfGAP with GTPase domain. ankyrin repeat and PH domain 1 |
| PRM2 | Protamine 2 |
| TNPO2 | Transportin 2 |
| ISM1 | Isthmin 1 |
| NUCKS1 | Nuclear casein lkinase and cyclin-dependent kinase substrate 1 |
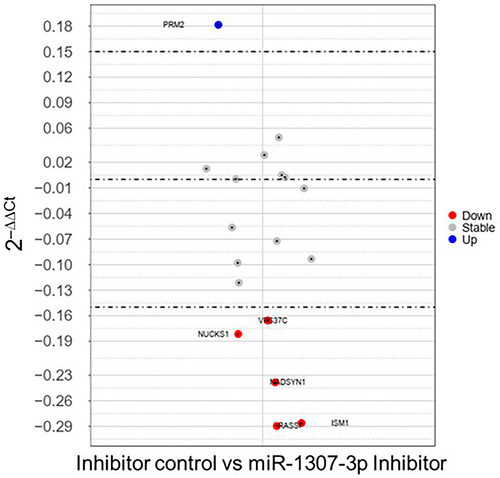
PRM2 protein expression in BC cells
In MDA-MB-231 cells compared to MCF-7 cells, PRM2 shows increased expression (Figure 6a). After transfection with the miR-1307-3p inhibitor, Western blotting confirmed a 14% increase (p = 0.0009) in PRM2 expression compared to the negative control (Figure 6b; Supplementary Figure S5A), indicating miR-1307-3p targets PRM2. Expression of PRM2 varied among BC cell lines (Supplementary Figure S5B,C), demonstrating differential expression levels.
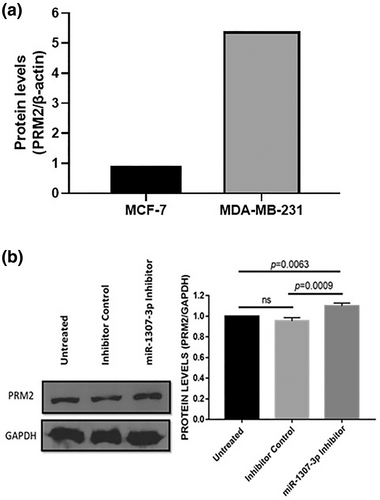
Validation of the interaction between miR-1307-3p and the 3′UTR of PRM2 mRNA
To validate the miR-1307-3p interaction with PRM2 mRNA's 3′UTR, a dual-luciferase reporter assay was conducted. Predicted binding by TargetScan platform (www.targetscan.org)20, 21 (Figure 7a) supported this interaction. In MDA-MB-231 cells, transfection with the miR-1307-3p inhibitor significantly increased luciferase activity (p = 0.0067), confirming direct binding to PRM2 mRNA's 3′UTR (Figure 7b). These findings establish PRM2 as a direct target of miR-1307-3p in BC cells.
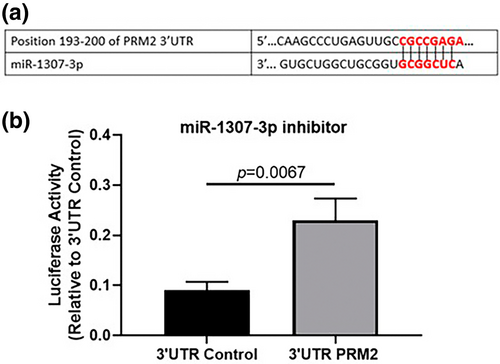
Signaling pathways associated with miR-1307-3p and PRM2ç
Using IPA software, we explored molecular interactions involving PRM2 and identified connections with RABL6, a member of the RAS oncogene family, which is implicated in various cancers, including BC22-24 (Figure 8a). Additionally, IPA helped construct a hypothetical signaling pathway illustrating the interplay between miR-1307-3p and PRM2 in BC cells (Figure 8b). These findings provide a foundational basis for future experimental investigations.
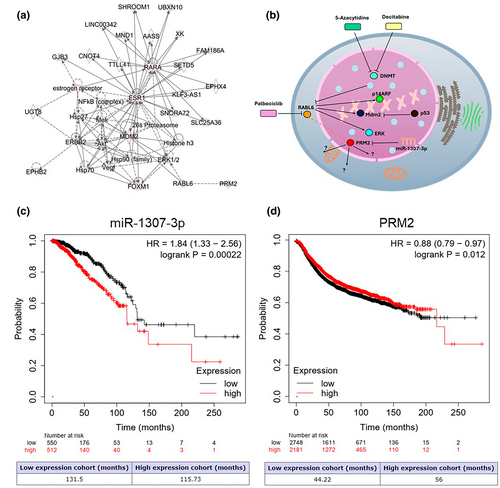
Associations of the expression of miR-1307-3p and PRM2 with overall survival and relapse-free survival in BC patients
We conducted Kaplan–Meier analysis using the KM-plotter patient database to assess the clinical relevance of miR-1307-3p and PRM2 expression in BC. Higher miR-1307-3p expression correlated with lower overall survival (OS) (p = 0.00022) (Figure 8c), while higher PRM2 expression correlated with longer relapse-free survival (RFS) (p = 0.012) (Figure 8d). These results indicate that the regulation of PRM2 by miR-1307-3p is associated with the OS and RFS of BC patients.
DISCUSSION
We observed that miR-1307-3p plays an oncogenic role in BC, as decreasing miR-1307-3p reduced the proliferation, migration, invasion, and angiogenesis of BC cells15, 25 Furthermore, we identified a novel target of miR-1307-3p, PRM2, in BC cells, which was not previously reported. We observed upregulation of miR-1307-3p in the MCF-7 and MDA-MB-231 cell lines, consistent with the findings reported by Han et al. in 2019. Nevertheless, they reported higher miRNA expression in the MDA-MB-231 cell line.15 However, we detected increased expression in MCF-7 cells. These discrepancies might stem from the use of SNORD44 as an endogenous control rather than U6, which was used by Han et al.15 It has been documented that U6 exhibits expression fluctuations across various cell lines, rendering it unreliable for miRNA expression analyses.26, 27 Shimomura et al. reported an increase in miR-1307-3p levels in the serum of patients in the early stages of BC, using miR-149-3p as an endogenous control, suggesting an oncomiR effect of miR-1307-3p.13 However, the comparability of the data obtained is not always assured, possibly due to variations in sample nature or the use of different endogenous controls for data normalization.28, 29 Our data confirm the overexpression of miR-1307-3p in BC cells, aligning with previous findings and providing evidence of its oncogenic role. Furthermore, there is a report describing a contrasting role of miR-1307-3p in BC, namely a tumor-suppressive effect, as its elevated expression leads to sensitization of BC cells to cisplatin therapy by downregulating MDM4.25 This counteractive effect of miR-1307-3p could be attributed to the metabolic changes induced by prolonged exposure to cisplatin during the process of establishing BC cell lines resistant to this drug.30 These contrasting effects highlight the need for further in-depth study of the effect of miR-1307-3p to better understand its role in BC progression.
One limitation of our study is that the basal expression levels of miR-1307-3p were only analyzed in two BC cell lines. Therefore, it is imperative to assess the expression of this miRNA in a larger array of cell lines, employing SNORD44 as an endogenous control, to gain a more comprehensive understanding of miR-1307-3p expression in BC.
There are few reports on the functional effect of miR-1307-3p on BC progression. Our findings show that targeting miR-1307-3p significantly reduces BC cell proliferation. Han et al. also reported similar results, demonstrating decreased proliferation and growth inhibition in soft agar upon inhibiting miR-1307-3p using a lentiviral vector.15 Both studies highlighted the role of miR-1307-3p in proliferation, despite the use of different experimental methods and transfection techniques.
Targeting miR-1307-3p diminished the migratory and invasive capabilities of BC cells. Our study represents the sole report evaluating the role of miR-1307-3p in migration and invasion processes in BC. However, there are studies examining its effects on other cancer types. In 2019, Chen et al. discovered that a reduction in miR-1307-3p levels leads to decreased migration and invasion in the MHCC97H and HCCLM3 hepatocellular carcinoma cell lines.31, 32 On the other hand, in 2022, Guo et al. showed that the inhibition of miR-1307-3p via the circular RNA circDIDO1 significantly affects the migration and invasion of MGC-803 and HCG-27 gastric cancer cells.33 In 2020, Jiao et al. reported that the circular RNA circRNA_101308 can reduce migration and invasion in SiHa, CaSki, and HeLa cervical cancer cell lines by affecting miR-26a-5p, miR-196a-5p, miR-196b-5p, miR-355-3p, and miR-1307-3p.34 Notably, miRNAs can exhibit different functions depending on their cellular localization.35, 36 The compartmentalization of these molecules can determine whether they act as oncomiRs or tsmiRs.37 Nevertheless, all reports regarding the role of miR-1307-3p in migration and invasion align with its oncogenic behavior, regardless of the cancer type studied. An interesting approach for future studies will be to evaluate whether transfecting MCF-10A cells with the miR-1307-3p inhibitor grants them the ability to migrate and invade.
Our data showed for the first time that the inhibition of miR-1307-3p significantly reduces the ability of HUVEC to form new vasculature, which is crucial for tumor maintenance, and that miRNAs have been reported to play key roles in the regulation of this process.38 García-Donas et al. demonstrated using bioinformatics and sequencing that miR-1307-3p could influence the response to antiangiogenic drugs like tyrosine kinase inhibitors in metastatic renal cell carcinoma cells.39 The above approach could be applied to predict the effect of miR-1307-3p on other types of cancer, such as BC.31, 32
Despite reports on miR-1307-3p and its oncogenic effect on BC, little is known about its specific targets. RT-qPCR on a custom plate identified PRM2 as a potential target, confirmed by Western blot and dual-luciferase reporter assays. PRM2 expression varied across BC cell lines and showed an inverse correlation with miR-1307-3p levels: higher miR-1307-3p in MCF-7 cells corresponded to lower PRM2 protein expression and conversely in MDA-MB-231 cells. This inverse relationship reflects the expected interaction between an oncomiR and its target.
Humans express two types of protamines: PRM1 and PRM2, both of which play roles in chromatin condensation.40 Protamines are characterized by their arginine- and cysteine-rich nuclei, enabling them to interact with the negatively charged DNA backbone, thus leading to transcriptional repression.41 Additionally, their induction in somatic cells has been shown to suppress cell division in tumor cells. A study demonstrated that in Escherichia coli and HeLa cervical cancer cells, protamine expression decreases cell proliferation. However, its expression has primarily been associated with testis tissue and male fertility problems.40, 42
For this reason, it was surprising to detect expression of the PRM2 protein in the MDA-MB-231 cell line, despite multiple databases indicating that it is a potential target of miR-1307-3p. To our knowledge, this is the first study to report the presence of the target gene PRM2 in BC cells, which presents a significant opportunity to further investigate its role and gain insights into the biology of this protein in breast tissue and its implications.
Overall, targeting miR-1307-3p reduces the progression of BC by inducing proliferation, migration, invasion, and angiogenesis. The biological effects associated with increased levels of miR-1307-3p observed in BC could occur through PRM2. Hence, the molecular mechanism by which PRM2 is regulated by miR-1307-3p in BC cells should be further investigated. The biological effect of deleting or decreasing PRM2 expression on the processes involved in BC progression should be examined. In this regard, the use of molecules such as siRNAs could be a suitable approach, as could scaling up to an in vivo model. Utilizing miR-1307-3p inhibitors in conjunction with existing treatments could represent a novel approach for BC treatment.
AUTHOR CONTRIBUTIONS
José Roberto Estupiñan-Jiménez conducted the experiments, analyzed the data, and wrote the manuscript. Valeria Villarreal-García conducted the experiments and analyzed the data. Vianey Gonzalez-Villasana and Pablo E. Vivas-Mejia conceptualized, funded, and supervised the project. Jose Manuel Vazquez-Guillen analyzed the experiments and reviewed the manuscript. Patricio Adrián Zapata-Morin analyzed the data and reviewed the manuscript. Marienid Flores-Colón conducted the experiments and analyzed the data. Claudia Altamirano-Torres conducted the experiments. Cristina Ivan performed the bioinformatics analysis. Mohammed H. Rashed and Recep Bayraktar conducted the experiments, and Cristina Rodríguez-Padilla reviewed the final version of the manuscript. Gabriel Lopez-Berestein conceptualized the project. Diana Resendez-Perez provided conceptualization. All authors have read and approved the manuscript.
ACKNOWLEDGMENTS
We express our gratitude for the support provided by Arianna López-Sauceda, Patrick A. Del Real-Villa, and Fabiola A. Castañeda-Lugo in reviewing the databases. We also extend our thanks to Ricardo Noriega-Rivera and Victor G. Reyes-Burgos for their valuable assistance in conducting the experiments, along with Alejandra Arreola-Triana for her support in editing this manuscript.
FUNDING INFORMATION
This work was supported by the Programa Presupuestario F003, CB2017-2018, CONAHCYT of the Consejo Nacional de Humanidades Ciencias y Tecnologıas (CONAHCYT) (grant number A1-S-45974, VG-V), the National Institute of General Medical Sciences (NIGMS) Support for Research Excellence (SuRE) Program (R16) grant, 5R16GM145558-02 (PEVM), institutional seed funds from the University of Puerto Rico Comprehensive Cancer Center (PEVM), and the National Institute of General Medical Sciences—Research Training Initiative for Student Enhancement (NIGMS-RISE) Program R25-GM061838 (MFC). In addition, financial support was provided by CONACYT as a scholarship (CVU) 893151 in the Ph.D. program in science with a focus on immunobiology.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [VGV], upon reasonable request.




