The efficacy and safety of a novel PD-1/CTLA-4 bispecific antibody cadonilimab (AK104) in advanced non-small cell lung cancer: A multicenter retrospective observational study
Hongxin Li and Wen Zhao contributed equally to this work. They are listed as the co-first authors.
Abstract
Background
For patients with advanced non-small cell lung cancer (NSCLC) who have received frontline immunochemotherapy, subsequent treatment options are limited. As the first dual programmed cell death-1 (PD-1)/cytotoxic T lymphocyte-associated antigen-4 bispecific antibody approved globally, cadonilimab demonstrated potential antitumor activity in advanced NSCLC patients resistant to anti-PD-1/PD-L1 antibodies.
Methods
We retrospectively collected efficacy and safety data from advanced NSCLC patients treated with cadonilimab-based regimens in later therapy lines.
Results
A total of 41 advanced NSCLC patients refractory to anti-PD-1/PD-L1 therapy were enrolled. More than half of the patients received cadonilimab-based regimen as a fourth or later line of treatment. At the data cutoff date, treatment efficacy could be evaluated in 23 patients. One patient (4.3%) achieved partial response, eight patients (34.8%) experienced stable disease, and 14 patients (60.9%) progressed. The objective response rate and disease control rate were 4.3% and 39.1%, respectively. The median progression-free survival for all evaluated patients was 108.0 days. Due to the short follow-up period, the median overall survival has not yet been reached. Treatment-related adverse events (TRAEs) and immune-related AEs occurred in 63.4% and 22% patients, respectively. The most common TRAEs included gamma-glutamyl transferase elevation (17.1%), coughing (14.6%), and fatigue (12.2%). Five patients (12.2%) experienced grade ≥3 TRAEs.
Conclusions
In this heavily pretreated cohort of advanced NSCLC patients, cadonilimab-based regimens showed moderate antitumor efficacy with a generally tolerable and manageable safety profile. However, more evidence is needed to support the administration of cadonilimab in NSCLC patients refractory to previous anti-PD-1/PD-L1 therapy.
INTRODUCTION
Worldwide, lung cancer remains the leading cause of cancer-related deaths, with over 1.2 million deaths expected globally in 2023.1 Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases, and its treatment has undergone significant changes in recent years.2 For the majority of NSCLC patients without an identifiable targeted therapy option, chemotherapy has been the mainstay for more than 40 years, with a median overall survival (OS) of less than 24 months for patients at advanced stages.3 Immune checkpoint inhibitors (ICIs) targeting programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) have revolutionized the treatment of solid cancer including NSCLC. In 2017, the addition of pembrolizumab (anti-PD-1) to platinum-based frontline chemotherapy provided a significant OS benefit in advanced NSCLC and heralded the age of immunochemotherapy combination treatment.4, 5 Recent studies have shown that ICI combination regimens also improve survival in patients with driver mutations progressing from targeted therapy.6 However, for patients who experienced disease progression after anti-PD-1/L1 therapy, treatments options are limited, and there is still no standard of care.
Preclinical studies have shown that co-inhibition of PD-1 and CTLA-4 synergistically transformed the tumor immune microenvironment into an antitumor phenotype.7 Clinical evidence suggested that CTLA-4 inhibitor combined with PD-1 or PD-L1 inhibitors had complementary action.8, 9 As the world's first approved dual-specific ICI, cadonilimab (AK104) simultaneously blocks the immunosuppressive response of PD-1 and CTLA-4 signaling pathways, exerting synergistic antitumor efficacy.10 On June 29, 2022, cadonilimab received approval in China for the treatment of recurrent or metastatic cervical cancer that had progressed following platinum-based chemotherapy.11 In a phase Ib/II trial, cadonilimab in combination with anlotinib achieved an objective response rate (ORR) of 62.5% and disease control rate (DCR) of 100% in eight evaluable advanced treatment-naive NSCLC patients.12 And the ORR reached 80% in a subgroup of five patients with nonsquamous NSCLC. Besides, this combination demonstrated a favorable safety profile in advanced NSCLC patients.12
However, it remains unclear whether advanced NSCLC patients who have progressed after frontline immuno-chemotherapy could benefit from cadonilimab therapy, and immune-related adverse events (irAEs) associated with cadonilimab in real-world settings also need further clarification. The current retrospective study thus aims to evaluate the efficacy and safety of cadonilimab in heavily pretreated advanced NSCLC patients.
METHODS
Patients and ethnic statement
This retrospective study was conducted in three cancer centers in Shandong Province, China, to investigate the efficacy and safety of cadonilimab in patients with advanced NSCLC. The inclusion criteria were (i) confirmed stage IV or recurrent NSCLC treated with cadonilimab or cadonilimab-based regimens and (ii) at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The exclusion criteria were (i) radiation therapy or other local treatments within 4 weeks prior to receiving cadonilimab for the target lesions used for assessing efficacy and (ii) long-term treatment with corticosteroids or immunosuppressants required due to accompanying diseases. Cadonilimab was administered intravenously at a dose of 6 mg/kg approximately every 2 weeks, until disease progression or the appearance of intolerable severe toxicity. The dosage and administration of other drugs were determined according to their specific instructions. Patients who were lost to follow-up but had records of adverse events were also analyzed for the safety profile. In total, 59 patients treated with cadonilimab were screened, and finally, 41 patients were enrolled for subsequent analysis.
This study complied with the Ethical Guidelines for Medical and Health Research Involving Human Subjects (KYLL-202309-062). The study protocol received approval from the Ethical Review Boards and Institutional Review Boards of all participating institutions. In China, cadonilimab has been officially approved for the treatment of patients with recurrent or metastatic cervical cancer who have experienced disease progression following platinum-based chemotherapy. The utilization of cadonilimab in our study was considered as an off-label application. Physicians obtained informed consent from patients prior to prescribing cadonilimab. Given the retrospective nature of our study, further informed consent for the analysis was not required, and the exposure of personal information was strictly avoided.
Data collection
The following information was retrospectively collected through electronic medical records: (i) demographic information: age, sex, body mass index (BMI), smoking history, and Eastern Cooperative Oncology Group performance status (ECOG PS) score; (ii) disease information: stage, histological type, PD-L1 expression status (tumor cell proportion score [TPS]), genetic mutations, metastatic sites, and treatment history; (iii) efficacy information: DCR, ORR, PFS, and OS; (iv) TRAEs: hematological toxicity, hypertension, hand-foot skin reactions, liver function damage, thyroid function abnormalities, decreased appetite, and rash.
Outcome assessments and endpoints
Treatment response was evaluated according to RECIST version 1.1 based on CT imaging. Adverse events during cadonilimab treatment were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. The primary endpoints were ORR, DCR, PFS, and safety; secondary endpoints included OS. The ORR was defined as the percentage of patients exhibiting either a complete or partial response to treatment, while the DCR was defined as the percentage of patients with a complete response, partial response, or stable disease. PFS and OS were assessed from the date of initial administration of cadonilimab. PFS was followed up until the first occurrence of tumor progression or death from any cause, whichever occurred first. OS was recorded until death from any cause or censored at the date of the latest available survival information.
Statistical analysis
All statistical analyses in this study were performed using SPSS version 27 (IBM, Chicago, IL, USA) and GraphPad Prism version 9.5.1 (San Diego, CA, USA). Demographic and clinical characteristics, as well as the incidence of adverse events, were summarized using descriptive statistics. Intergroup comparisons of DCR were conducted using the Pearson chi-squared test or Fisher's exact test to calculate 95% confidence intervals (CIs). The Kaplan–Meier method was used to estimate the median PFS and OS with 95% CIs. The median PFS between different subgroups was compared using the log-rank test at a 5% significance level. Hazard ratios and 95% CIs for different treatment strategies and clinicopathological characteristics were computed using the univariate Cox proportional hazards model. Variables with a p value of less than 0.05 (considered statistically significant) in the univariate analysis were included in the multivariate Cox proportional hazards analysis.
RESULTS
Patient characteristics
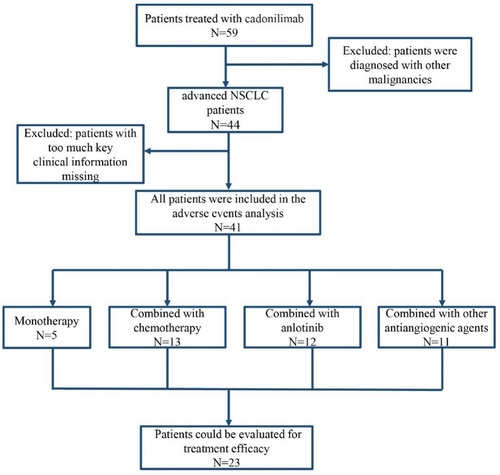
As shown in Figure 1, a total of 59 patients treated with cadonilimab were screened. Forty-one patients with advanced NSCLC meeting the study criteria were included for subsequent analysis, of which 23 patients could be evaluated for therapeutic efficacy.
Demographics, clinicopathological characteristics, and treatment information are presented in Table 1. The median age at diagnosis was 58 years old (range 35–73), and 28 patients (68.3%) were male. Notably, most patients with NSCLC had an ECOG-PS score of 0 or 1 (92.7%) at the start of the treatment, whereas the remaining 7.3% had an ECOG-PS score of 2. Seventeen patients (41.5%) had a history of smoking. The proportion of adenocarcinoma and squamous cell carcinoma was 63.4% and 36.6%, respectively. PD-L1 TPS was <1% in 29.3% of patients, ≥1% in 24.4% of patients, and unknown in 46.3% of patients. Regarding driver gene mutations, 14 (34.1%) patients had EGFR mutation, and 2 (4.9%) patients had ALK mutation. Nine patients (22%) did not undergo detection of driver gene mutation, and all of them were diagnosed with advanced lung squamous cell carcinoma (LUSC). The proportion of brain metastasis, bone metastasis, liver metastasis, and adrenal glands metastasis was 41.5%, 51.2%, 31.7%, and 17.1%, respectively.
| Characteristics, N (%) | Treatment modality of cadonilimab | All patients | |||
|---|---|---|---|---|---|
| Single agent | Combined with chemotherapy | Combined with anlotinib | Combined with other antiangiogenic agents | ||
| Patients | 5 (12.2) | 13 (31.7) | 12 (29.3) | 11 (26.8) | 41 (100) |
| Male | 2 (7.1) | 10 (35.7) | 9 (32.1) | 7 (25) | 28 (68.3) |
| Female | 3 (23.1) | 3 (23.1) | 3 (23.1) | 4 (30.8) | 13 (31.7) |
| Age (years), median (range) | 63 (35–70) | 59 (38–73) | 56 (40–71) | 57 (40–69) | 58 (35–73) |
| Smoking history | |||||
| Ever | 1 (5.9) | 7 (41.2) | 3 (17.6) | 6 (35.3) | 17 (41.5) |
| Never | 4 (16.7) | 6 (25) | 9 (37.5) | 5 (20.8) | 24 (58.5) |
| Histological type | |||||
| Squamous cell carcinoma | 3 (20) | 6 (40) | 3 (20) | 3 (20) | 15 (36.6) |
| Adenocarcinoma | 2 (7.7) | 7 (26.9) | 9 (34.6) | 8 (30.8) | 26 (63.4) |
| Stage | |||||
| IIIA | 1 (100) | - | - | - | 1 (2.4) |
| IIIC | - | 2 (100) | 2 (4.9) | ||
| IV | 4 (10.5) | 13 (34.2) | 12 (31.6) | 9 (23.7) | 38 (92.7) |
| ECOG PS | |||||
| 0–1 | 5 (13.2) | 11 (28.9) | 12 (31.6) | 10 (26.3) | 38 (92.7) |
| 2 | - | 2 (66.7) | - | 1 (33.3) | 3 (7.3) |
| PD-L1 (TPS) | |||||
| ≥1% | - | 4 (40) | 4 (40) | 2 (20) | 10 (24.4) |
| <1% | 1 (8.3) | 4 (33.3) | 2 (16.7) | 5 (41.7) | 12 (29.3) |
| Unknown | 4 (21.0) | 5 (26.3) | 6 (31.6) | 4 (21.0) | 19 (46.3) |
| Genetic mutation | |||||
| EGFR | 2 (14.3) | 3 (21.4) | 5 (35.7) | 4 (28.6) | 14 (34.1) |
| ALK | - | - | 2 | - | 2 (4.9) |
| EGFR/ALK wild type | 1 (6.25) | 6 (37.5) | 4 (25) | 5 (31.25) | 16 (39.0) |
| Unknown | 2 (22.2) | 4 (44.4) | 1 (11.1) | 2 (22.2) | 9 (22.0) |
| Metastasis locations | |||||
| Liver | 1 (7.7) | 6 (46.2) | 5 (38.5) | 1 (7.7) | 13 (31.7) |
| Brain | 1 (5.9) | 7 (41.2) | 5 (29.4) | 4 (23.5) | 17 (41.5) |
| Bone | 1 (4.8) | 8 (38.1) | 7 (33.3) | 5 (23.8) | 21 (51.2) |
| Adrenal glands | 2 (28.6) | 1 (14.3) | 4 (57.1) | - | 7 (17.1) |
| Treatment lines of cadonilimab | |||||
| 2 | - | 1 (25) | 3 (75) | - | 4 (9.8) |
| ≥ 3 | 5 (13.5) | 12 (32.4) | 12 (32.4) | 8 (21.6) | 37 (90.2) |
| ≥ 4 | 5 (17.2) | 10 (34.5) | 10 (34.5) | 4 (13.8) | 29 (70.7) |
| Previous anti-PD-1/PD-L1 antibodies | |||||
| Yes | 5 (13.2) | 13 (34.2) | 10 (26.3) | 10 (26.3) | 38 (92.7) |
| No | - | - | 2 (66.7) | 1 (33.3) | 3 (7.3) |
| Response to cadonilimab (N = 23) | |||||
| PR | - | - | 1 (100) | - | 1 (4.3) |
| SD | 2 (25) | 2 (25) | 2 (25) | 2 (25) | 8 (34.8) |
| PD | 1 (7.1) | 4 (28.6) | 2 (14.3) | 7 (50) | 14 (60.9) |
- Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; PD-L1(TPS), programmed cell death ligand-1 (tumor proportion score); PD-1, programmed cell death-1; PR, partial response; SD, stable disease; PD, progressive disease.
Thirty-eight patients (92.7%) have previously been treated with anti-PD-1/PD-L1 antibodies, and cadonilimab-containing regimens were given as third or later-line treatment in most (90.2%) cases. Of patients, 12.2% received cadonilimab as a single-agent treatment, while 31.7%, 29.3%, and 26.8% of patients received cadonilimab in combination with chemotherapy, anlotinib, and other antiangiogenic agents, respectively.
Treatment outcomes
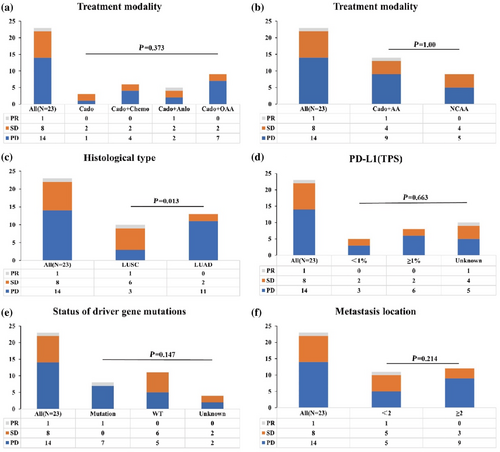
The median follow-up time was 135 days (range 16–406 days). By August 29, 2023, 23 patients could be evaluated for treatment efficacy. The ORR and DCR were 4.3% and 39.1%, respectively. Specifically, one patient (4.3%) achieved a PR, eight patients (34.8%) achieved SD, and 14 patients (60.9%) experienced PD (Figure 2a). The DCR for patients treated with cadonilimab as a single agent, cadonilimab combined with chemotherapy, cadonilimab combined with anlotinib, and cadonilimab combined with other antiangiogenic agents were 66.7%, 33.3%, 60%, and 22.2%, respectively. Patients treated with cadonilimab and antiangiogenic agents showed a lower DCR than those treated with cadonilimab-based regimens that did not include any antiangiogenic agents (35.7% vs. 44.4%, p = 0.505) (Figure 2b). Patients with LUSC achieved a higher DCR than those with LUAD (70% vs. 15.4%, p = 0.012) (Figure 2c). The DCR divided by PD-L1 expression is presented in Figure 2d. A relatively lower DCR was observed in patients carrying driver gene mutations than in those with wild-type driver genes (14.3% vs. 54.5%, p = 0.08) (Figure 2e). Patients with two or more metastatic sites achieved a DCR of 25%, while those with only one metastatic site showed a DCR as high as 54.5% (p = 0.154) (Figure 2f).
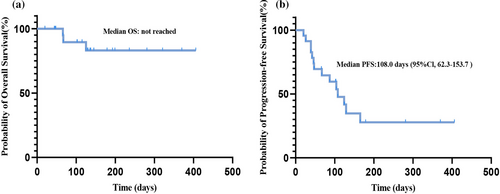
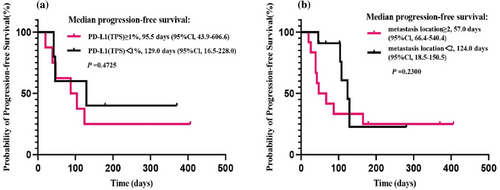
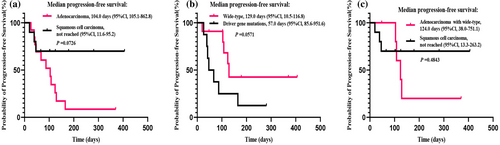
Due to the short follow-up period, median OS has not been reached yet (Figure 3a). The median PFS of all evaluated patients was 108.0 days (95% CI 62.3–153.7) (Figure 3b). There was no significant difference in the PFS according to PD-L1 expression groups (TPS ≥1% [95 days] vs. TP <1% [129 days], p = 0.4725; Figure 4a), nor based on the number of metastasis sites (<2 [124 days] vs. ≥ 2 [57 days], p = 0.23; Figure 4b). The median PFS was 104 days in LUAD patients, while the median PFS was not reached in LUSC patients (p = 0.0726, Figure 5a). Considering driver gene mutation status, patients with driver gene mutation showed shorter PFS than those with wild-type driver genes when treated with cadonilimab (57 vs. 129 days, p = 0.0571, Figure 5b). Additionally, there was no significant difference in PFS between LUAD patients with wild-type driver genes and LUSC patients (p = 0.4843, Figure 5c).
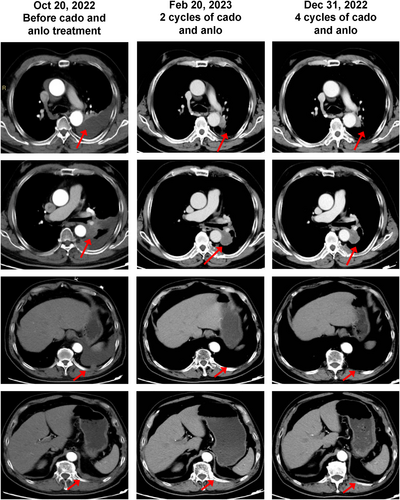
The only patient who achieved a PR is presented here (Figure 6). This male patient was diagnosed with LUSC. His disease stage at diagnosis was cT4N0M0, IIIA, based on thoracic CT, brain MRI, and PET-CT results. No driver gene mutation was detected by a 51-gene high-throughput sequencing. After five cycles of albumin-bound paclitaxel and nedaplatin treatment, he achieved stable disease, with the primary lesion in the left lung slightly enlarging. He underwent high-throughput sequencing again, which showed an EGFR 19del mutation with a 0.56% mutation allele frequency and a PD-L1 TPS of 3% by SP263 antibody. He received afatinib for 1 month and radiotherapy (77Gy/22f) simultaneously. Due to the low abundance of the EGFR 19del mutation, the multidisciplinary team believed that the benefit of targeted therapy might be limited and recommended consolidation immunotherapy. He began durvalumab consolidation on June 7, 2022, and the best response was stable disease. During durvalumab consolidation, the disease progressed on October 20, 2022, with thoracic CT showing pleural effusion on the left side, a mass in the hilus of left lung, and multiple pleural metastases. Thoracic puncture and pleural effusion drainage were performed on November 2, 2022, and intrapleural chemotherapy infusion was given with cisplatin and recombinant human endostatin. No EGFR mutation was detected in the pleural effusion. Cadonilimab and anlotinib were administered on November 19, 2022 with a best response of PR achieved (Figure 6).
Prognostic factors
Univariate and multivariate Cox proportional hazards models were used to identify prognostic factors affecting PFS in this previously heavily treated advanced NSCLC cohort (Table 2). Univariate analysis revealed that male gender and smoking history were related to favorable PFS (p < 0.05). Due to the high collinearity between gender and smoking history, a multivariate Cox proportional hazards model was not conducted.
| Factor | Univariate analysis | ||
|---|---|---|---|
| HR | 95% Cl | p value | |
Gender Male Female |
0.245 | 0.081–0.735 | 0.012* |
Age >65 ≤65 |
0.318 | 0.041–2.433 | 0.270 |
Smoking history Ever Never |
0.273 | 0.083–0.900 | 0.033* |
Histological type Squamous cell carcinoma Adenocarcinoma |
0.328 | 0.091–1.184 | 0.089 |
ECOG PS 0–1 2 |
0.601 | 0.131–2.754 | 0.512 |
PD-L1 (TPS) <1% ≥1% |
1.680 | 0.402–7.018 | 0.477 |
EGFR/ALK mutation Yes No |
2.958 | 0.920–9.506 | 0.069 |
Liver metastasis location Yes No |
2.368 | 0.728–7.700 | 0.152 |
Brain metastasis location Yes No |
1.943 | 0.676–5.583 | 0.218 |
Bone metastasis location Yes No |
2.007 | 0.669–6.022 | 0.214 |
Metastasis location ≥2 <2 |
1.951 | 0.640–5.943 | 0.240 |
Combined with antiangiogenic agents Yes No |
0.661 | 0.215–2.030 | 0.470 |
Combined with chemotherapy Yes No |
1.528 | 0.474–4.924 | 0.477 |
Combined with anlotinib Yes No |
0.522 | 0.116–2.355 | 0.398 |
Treatment lines >3 ≤3 |
2.649 | 0.799–8.783 | 0.111 |
- Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PD-L1, programmed cell death-ligand 1; EGFR/ALK, epidermal growth factor receptor/anaplastic lymphoma kinase.
Safety outcomes
TRAEs were observed in 26 patients (63.4%), with five patients (12.2%) experiencing grade 3 or higher TRAEs according to the CTCAE Version 5.0. The overall incidence rate of TRAEs was 43.8% among patients with EGFR/ALK mutations (Supplementary Table 1), which was not higher than that in the overall patient population. The most frequently reported TRAEs were elevated gamma-glutamyl transferase (17.1%), cough (14.6%), and fatigue (12.2%). A total of nine patients (22%) experienced irAEs, which included rash in four patients (9.8%), infusion reactions in two patients (4.9%), TSH elevation in one patient (2.4%), pruritus in one patient (2.4%), and myocarditis in one patient (2.4%). The incidence of TRAEs resulting in drug discontinuation was 17.1%. Medications were discontinued in six patients due to adverse events, and in one patient due to cachexia. Specifically, two patients temporarily paused their medication because of Grade 2 rash; one patient and two patients permanently discontinued due to Grade 3 ICI-related myocarditis and Grade 2 infusion reactions, respectively. It is noteworthy that the patient who discontinued cadonilimab due to dyspnea had a history of pericardial effusion and chronic obstructive pulmonary disease prior to the administration of cadonilimab. A comprehensive list of all safety outcomes is shown in Table 3.
| Adverse events, N (%) | Mono therapy | With chemotherapy | With anlotinib | With other antiangiogenic agents | All (N = 41) |
|---|---|---|---|---|---|
| TRAEs | |||||
| All TRAEs | 3 (11.5) | 10 (38.5) | 5 (19.2) | 8 (30.8) | 26 (63.4) |
| ≥G3 TRAEs | 1 (20) | 2 (40) | - | 2 (40) | 5 (12.2) |
| GGT elevation | - | - | - | 1 (100) | 1 (2.4) |
| Thrombocytopenia | - | - | - | 1 (100) | 1 (2.4) |
| ICI-related myocarditis | - | 1 (100) | - | - | 1 (2.4) |
| Anemic | - | 1 (100) | - | - | 1 (2.4) |
| Dyspnea | 1 (100) | - | - | - | 1 (2.4) |
| Reported in ≥10% of patients | |||||
| GGT elevation | 1 (14.3) | 2 (28.6) | 2 (28.6) | 2 (28.6) | 7 (17.1) |
| Cough | 2 (33.3) | 3 (50) | - | 1 (16.7) | 6 (14.6) |
| Fatigue | - | 3 (60) | - | 2 (40) | 5 (12.2) |
| Immune-related adverse events | |||||
| All irAEs | - | 4 (44.4) | - | 5 (55.6) | 9 (22) |
| Rash | - | 2 (50) | - | 2 (50) | 4 (9.8) |
| Infusion reactions | - | 1 (50) | - | 1 (50) | 2 (4.9) |
| TSH elevation | - | - | - | 1 (100) | 1 (2.4) |
| Pruritus | - | - | - | 1 (100) | 1 (2.4) |
| ICI-related myocarditis | - | 1 (100) | - | - | 1 (2.4) |
| ≥G3 irAEs | - | - | - | — | 1 (2.4) |
| ICI-related myocarditis | - | 1 (100) | - | - | 1 (2.4) |
| Leading to discontinuation | 1 (14.3) | 4 (57.1) | - | 2 (28.6) | 7 (17.1) |
| Caused by irAEs | - | 3 (60) | - | 2 (40) | 5 (12.2) |
| Caused by cachexia | - | 1 (100) | - | - | 1 (2.4) |
| Caused by dyspnea | 1 (100) | - | - | - | 1 (2.4) |
- Abbreviations: G3, grade 3; GGT, gamma-glutamyl transferase; ICI-related myocarditis, immune checkpoint inhibitor-related myocarditis; irAEs, immune-related adverse events; TRAEs, treatment-related adverse events.
DISCUSSION
To the best of our knowledge, this study represents the first real-world investigation into the efficacy and safety profile of cadonilimab in patients with advanced NSCLC. In this cohort, which had been heavily pretreated—92.7% of patients had progressed following anti-PD-1/PD-L1 therapy—cadonilimab-based regimens were administered as third-line or later treatment in the majority of cases (90.2%). These regimens exhibited moderate efficacy, with an ORR of 4.3% and a DCR of 39.1%. The safety profile was manageable, and the incidence of Grade 3 or higher TRAEs was 12.2%.
Over the past decade, ICIs have revolutionized the therapeutic landscape for solid tumors. Notably, several inhibitors targeting PD-1, PD-L1, and CTLA-4 have emerged, demonstrating promising efficacy and an acceptable safety profile across a variety of solid tumors. Among the various therapeutic strategies, the combination of two ICIs stands out as particularly promising, particularly the pairing of PD-1/PD-L1 inhibitors with CTLA-4 inhibitors. Mechanistically, CTLA-4 inhibits T cell activation during the antigen presentation phase, while PD-1/PD-L1 inhibitors act during the antigen elimination phase. This complementary action underpins the rationale for combining these agents.13 Preclinical data have demonstrated that the dual inhibition of CTLA-4 and PD-1/PD-L1 synergistically boosts T cell proliferation and suppresses Treg-mediated suppression of antitumor responses.14, 15 Clinical trials corroborate that the synergistic inhibition of PD-1/PD-L1 and CTLA-4 pathways yields favorable outcomes in urothelial carcinoma, NSCLC, and melanoma.16-18 Nivolumab in combination with ipilimumab has demonstrated efficacy and safety in treating advanced NSCLC in the CheckMate 012 trial.19 This immunotherapy combination has further demonstrated a significant survival benefit over standard chemotherapy in the subsequent CheckMate 227 trial, reinforcing its role as a valuable treatment option for this patient population.20 In 2020, the combination of nivolumab and ipilimumab was approved as a first-line treatment option for patients with metastatic NSCLC (PD-L1 ≥1%) who lack sensitizing EGFR mutations or ALK translocations.
The effectiveness of this strategy for advanced NSCLC patients who have progressed on anti-PD-1/PD-L1 therapy is still uncertain, given that PD-1/PD-L1 inhibitor-based regimens have become the standard first-line treatment approach. A recent phase II trial, however, has shed light on the potential antitumor activity of the immunotherapy combination of durvalumab and tremelimumab in patients with metastatic NSCLC who are refractory to prior PD-L1 therapy.21 However, the efficacy of this combination was found to be limited in patients with metastatic squamous NSCLC, as evidenced by the Lung-MAP S1400F study. In this study, patients who had progressed from anti-PD-L1 therapy within 24 weeks or more experienced an ORR of 0 and a median OS of 7.7 months.22 In our present study, a significant proportion of patients, 92.7%, had received PD-1 or PD-L1 inhibitors as first-line therapy. For 90.2% of these patients, cadonilimab-based regimen was administered as a third-line or later treatment. The observed ORR was 4.1%, and the DCR was 39.1%. The median PFS was 108 days, and the median OS was not reached at the time of analysis, likely due to the short follow-up period. Recently, a prospective study evaluated the efficacy and safety of cadonilimab in patients with previously treated NSCLC as a second-line therapy.23 It showed an ORR of 10% and 0 in immunotherapy-naïve group and immunotherapy-resistant group, respectively. However, the sample size of immunotherapy-resistant group was only 23 in the above study. Exploration is needed into larger-scale clinical trials and potential beneficiary populations. The VEGF family is known to play a crucial role in tumor microenvironment remodeling. A previous study has reported the effectiveness of combining cadonilimab with the VEGF inhibitor anlotinib in the treatment of advanced NSCLC.12 However, our study did not demonstrate superior efficacy for cadonilimab-based regimens that included antiangiogenic agents compared with those that did not (DCR of 35.7% vs. 44.4%, respectively).
Currently, there is a scarcity of studies aimed at identifying the specific patient populations that may derive the most benefit from PD-L1 and CTLA-4 bispecific antibodies. In the context of advanced NSCLC, where nivolumab combined with ipilimumab is used as a first-line therapy, the survival benefit observed has been consistent across various levels of PD-L1 expression.20, 24 Our findings also indicated that PD-L1 expression level did not serve as a predictive factor for PFS or DCR following treatment with cadonilimab, which was in line with previous reports.23 Our study revealed a trend suggesting that cadonilimab may exhibit a more pronounced antitumor effect in patients with advanced LUSC compared with those with LUAD, as evidenced by a higher DCR (70% vs. 15.4%) and longer PFS (129 vs. 57 days). This disparity might be attributed to the lower rate of driver gene mutations in LUSC. Indeed, we observed that patients with driver gene mutations had a shorter PFS than those with wild-type driver genes. Interestingly, no significant difference in PFS was noted between LUAD patients without driver gene mutations and those with LUSC. Further research is warranted to pinpoint molecular prognostic factors that can predict treatment response to cadonilimab in advanced NSCLC patients.
The simultaneous blockade of PD-1/PD-L1 and CTLA-4 using respective inhibitors has shown promising outcomes. However, this strategy is associated with a higher incidence of severe adverse events, and there is a lack of robust biomarkers to predict treatment response.13, 17, 18, 25, 26 In advanced solid tumors, the incidence rate of Grade 3 or higher TRAEs for combination immunotherapy with two ICIs has been reported to range from 27% to 53%.13, 17, 18, 27 Bispecific antibodies, which are designed to bind two distinct epitopes on the same or different antigens, have demonstrated the potential to enhance immune responses while aiming to minimize toxicity.28, 26 In our study, the overall incidence rates of TRAEs and irAEs were 63.4% and 22%, respectively. The most frequently reported adverse events included elevated gamma-glutamyl transferase (17.1%), cough (14.6%), and fatigue (12.2%). Notably, only 12.2% of patients experienced Grade 3 or higher TRAEs, affecting five individuals. Based on these data, the bispecific antibody cadonilimab demonstrated a favorable safety profile compared with the combination therapy involving two ICIs.
This retrospective study had several inherent limitations. Firstly, the retrospective design of our study led to a lack of standardized matching in patient characteristics across different subgroups, potentially introducing selection bias. To mitigate this in future studies, we intend to enhance our research methodology by concentrating on well-defined subpopulations, ensuring that variables affecting patient survival are matched appropriately across groups. Secondly, the modest sample size of this real-world study might limit the statistical robustness of our findings. Consequently, the results, especially those derived from subgroup analyses, should be approached with caution. Thirdly, the median follow-up period was relatively brief. As of our data cutoff, a number of patients were still alive and had not yet experienced disease progression. We remain dedicated to ongoing follow-up efforts to assess the long-term survival outcomes for these individuals.
In summary, our findings indicate that cadonilimab-based regimens demonstrate moderate antitumor efficacy in heavily pretreated patients, including those who have received anti-PD-1 or anti-PD-L1 therapy. The safety profile of these regimens is deemed acceptable, with TRAEs generally being mild to moderate in severity and manageable. Nonetheless, further research is warranted to delve deeper into the efficacy of cadonilimab in the treatment of advanced NSCLC.
FUNDING INFORMATION
This work was supported by Shandong Provincial Natural Science Foundation (ZR2020LZL018), National Natural Science Foundation of China (82303749), Hui Lan Public Welfare Foundation Project (HLZY-20231128001), Beijing Science and Technology Innovation Medical Development Foundation (KC2023-JX-0186-PZ091), and Clinical Research Fund of Shandong Medical Association-Qilu Special Project (YXH2022ZX02212).
CONFLICT OF INTEREST STATEMENT
All authors declare that the research was conducted without any commercial or financial relationships and they have on conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




