Rapidly developing intrathoracic low-grade fibromyxoid sarcoma: A case report
Abstract
Low-grade fibromyxoid sarcoma (LGFMS) is a rare mesenchymal tumor that primarily arises in the limbs and trunk of young adults, and rarely in the thoracic cavity. An 84-year-old Japanese woman presented with a right intrathoracic mass which was 8 cm in size. CT-guided needle biopsy did not provide a definitive diagnosis. Perioperatively, a mass was found in the right lower lobe of the lung and was suspected to have invaded the chest wall at the sixth–eighth ribs. A right lower lobectomy and combined chest wall resection were performed. Microscopic examination revealed that the tumor was a low-grade spindle cell tumor originating from the pleura demonstrating focal invasion of the lung. The tumor exhibited positivity for MUC4, and FUS gene translocation was confirmed through fluorescence in situ hybridization. Unfortunately, 10 months postoperatively, tumor recurrence was noted as peritoneal dissemination, and the patient passed away 13 months postoperatively. Although LGFMS may be diagnosed histologically as a low-grade tumor by needle biopsy, in this case, it was highly malignant. Postoperative long-term regular medical follow-up is recommended considering the highly malignant nature of the tumor and the high risk of local recurrence and pulmonary metastasis.
INTRODUCTION
Low-grade fibromyxoid sarcoma (LGFMS) is characterized by rare mesenchymal tumors that typically arise in the deep soft tissues of the proximal extremities and trunk.1 Intrathoracic LGFMS is rare, and only 15 such cases have been reported to date (Table 1). In this case report, we describe a patient with intrathoracic LGFMS which was removed by pulmonary lobectomy and combined chest wall resection. Unfortunately, the patient had a poor prognosis due to peritoneal dissemination.
| Case | Reference | Age/sex | Primary location | Tumor size (cm) | Treatment | MUC4 expression | FUS translocation | Follow-up | Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| #1 | Magro et al.14 | 20F | Lung | 2 | Biopsy only | Unknown | Unknown | 12 M | No |
| #2 | Takanami et al.10 | 35M | Mediastinum | 6.0*5.5 | Surgery | Unknown | Unknown | 9Y | Yes |
| #3 | Kim et al.4 | 37M | Pleura | Unknown | Surgery | Unknown | Unknown | Unknown | Unknown |
| #4 | Kim et al.15 | 50F | Lung | 7.5 | Surgery | Unknown | Positive | Unknown | Unknown |
| #5 | Jakowski et al.17 | 44F | Right heart | 12 | Surgery | Unknown | Positive | 7 M | No |
| #6 | Steiner et al.5 | 12F | Chest wall | 23.5*14.5 | Surgery | Unknown | Unknown | 12 M | No |
| #7 | Maeda et al.11 | 19F | Mediastinum | 23.5*21.5 | Surgery | Unknown | Unknown | 5Y | Yes |
| #8 | Maeda et al.11 | 50M | Mediastinum | 13*13 | Surgery | Unknown | Unknown | 5Y | No |
| #9 | Higuchi et al.6 | 20F | Chest wall | 6.0*4.5 | Surgery | Unknown | Positive | 18 M | No |
| #10 | Tominaga et al.7 | 70M | Chest wall | 18*15 | Surgery & RT | Unknown | Unknown | 30 M | No |
| #11 | Liang et al.8 | 42F | Pleura | 11*10 | Surgery | Unknown | Unknown | Unknown | Unknown |
| #12 | Perez et al.9 | 32F | Diaphragmatic pleura | 11 | Surgery | Positive | Unknown | 29 M | No |
| #13 | Sajid MI et al.13 | 26M | Mediastinum | 17*12 | Surgery | Positive | Unknown | 22 M | No |
| #14 | Williams et al.12 | 50M | Mediastinum | Unknown | Chemotherapy | Unknown | Positive | 5 M | Yes |
| #15 | Yoshimura et al.16 | 22M | Lung | 4.5*4.0 | Surgery | Positive | Negative | Unknown | Unknown |
| #16 | Current case | 84F | Chest wall | 8.0*7.7 | Surgery | Positive | Positive | 13 M | Yes |
CASE REPORT
An 84-year-old Japanese woman presented with a right intrathoracic tumor found on routine chest radiography. The patient was asymptomatic, had no family history of malignant genetic diseases but had a medical history of hypertension, stroke, and mild dementia.
Serum tumor markers showed mild increases in cytokeratin-19 fragment (6.1 ng/mL [<3.5 ng/mL]) and progastrin-releasing peptide (112.4 pg/mL [≦80.0 pg/mL]), but carcinoembryonic antigen and squamous cell carcinoma-related antigen levels were within the normal range.
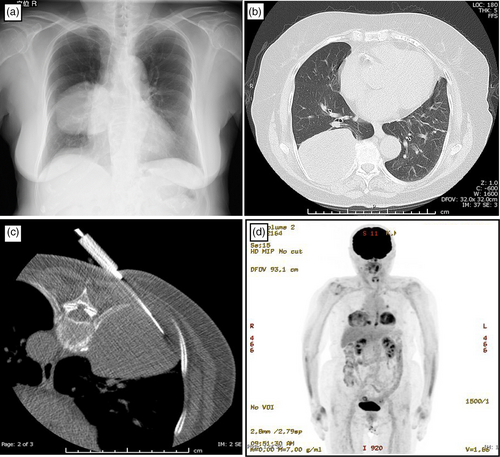
Radiological examination revealed an 8-cm mass in the right lower pulmonary lobe invading the ribs and intercostal muscles, which was suspected to be lung cancer (Figure 1a, b). The tumor was thought to have grown rapidly because a chest computed tomography (CT) scan performed 3 years prior showed no abnormalities. A CT-guided needle biopsy with a 16-gauge core needle was performed (Figure 1c), but no definitive diagnosis was obtained due to the predominantly mature, slightly glassy fibrotic tissue composition of the tumor. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) revealed an abnormal FDG uptake (maximum standardized uptake value = 7.2) at the edge of this mass (Figure 1d). No distant metastases were observed. Surgical resection was conducted for diagnosis and treatment. Assuming lung cancer, clinical stage was c-T4N0M0, c-stage IIIA.2
Intraoperatively, right lower lobectomy (Figure 2a) and combined resection of the chest wall were performed (Figure 2b). A separate daughter tumor nodule was identified in the parietal pleura near the primary tumor (Figure 2c). The tumor boundaries were visibly well-defined and the resection procedure was conducted with a negative surgical margin. The whole procedure was performed by open chest surgery (Figure 2d). The patient was discharged on the 13th postoperative day.
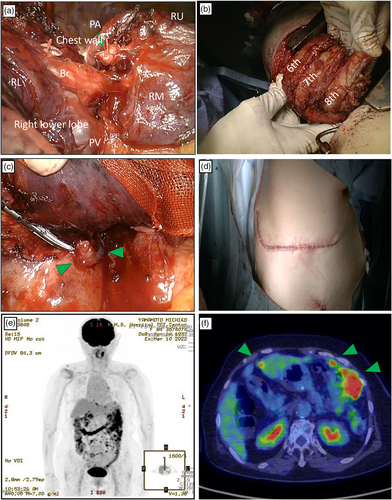
Ten months after surgery, the patient complained of acute stomach pain during an outpatient clinic visit. FDG-PET-CT examination was performed, which indicated the absence of recurrent intrathoracic tumors. However, the examination revealed disseminated recurrent tumors in the peritoneum (Figure 2e, f). Best supportive care was initiated, and she passed away 13 months later.
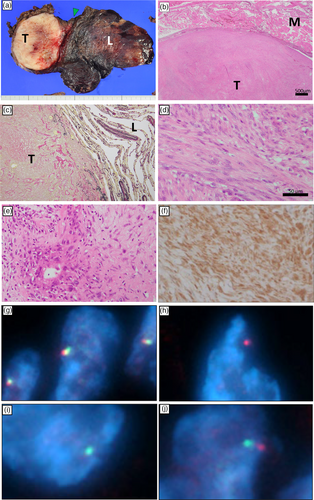
The resected mass measured 8.0 × 7.7 × 6.0 cm. The cross-section of the tumor was white, and its interior appeared to be degenerative (Figure 3a). The tumor originated from the chest wall (Figure 3b) and focally invaded the lung parenchyma (Figure 3c). The tumor consisted of bundles of bland-appearing spindle cells with mild atypia with a mixture of high and low cell density (Figure 3d, e). Surgical resection margins were negative microscopically. Immunohistochemically, the cytoplasm of tumor cells was diffusely positive for MUC4 (Figure 3f) which is a specific immunohistochemical marker of LGFMS. The tumor was focally positive for alpha-SMA, but was negative for CAM5.2, CD3, STAT6, β-catenin, and calretinin, Therefore, diagnoses of epithelial tumors, solitary fibrous tumors, desmoid fibromatosis, and mesotheliomas were excluded. The tumor was diagnosed as a LGFMS by two pathologists. Further consultation with an expert pathologist registered at the National Cancer Center Japan Institute for Cancer Control (Tokyo, Japan) supported the diagnosis. The MIB1 index was as low as 5%–10%. Later, fluorescent in situ hybridization (FISH) analysis performed at Chromosome Science Laboratory revealed the presence of a FUS gene translocation at 16p11 (Figure 3g–j).
DISCUSSION
LGFMS is known as a tumor associated with chromosomal abnormalities. The majority of LGFMS cases (95%) have a fusion of the FUS-CREB3L2.3 In fact, our case had rearrangement of the FUS gene at 16p11 (Figure 3g–j).
Only 15 cases of primary intrathoracic LGFMS have been previously reported (Table 1). Six cases originated from the chest wall,4-9 four from the mediastinum,10-13 three from the lung,14-16 and one from the epicardium.17 Only one case exhibited lung invasion,4 and ours was the second such case. Chemotherapy and radiotherapy are not effective for LGFMS because of their low nuclear grade and infrequent mitotic activity. Therefore, surgical resection is the first-line treatment option.18 Thirteen intrathoracic LGFMS were surgically resected, two of which recurred during long-term follow-up periods of 5- and 9-years. Other cases with shorter follow-up periods might have had a potential risk of recurrence.
In conclusion, we present a rare case of surgically resected intrathoracic LGFMS. The patient experienced a recurrence of peritoneal dissemination 13 months later. LGFMS was histologically low grade but exhibited aggressive clinical behavior. Long-term intensive radiological follow-up is recommended due to the high risk of recurrence.
AUTHOR CONTRIBUTIONS
Dr. Anayama led the study design, analysis, and manuscript drafting. Dr. Narukami managed data collection and literature review, with assistance from Dr. Yamamoto. Drs. Bunno, Miyazaki, and Okada provided patient care and study resources. Dr. Eguchi offered pathological diagnoses and contributed to study verification.
ACKNOWLEDGMENTS
The authors thank Dr Shinji Fukunaga, Center for Cancer Control and Information Services, National Cancer Center, Tokyo, Japan, for consultation regarding the histopathological diagnosis.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no competing interests.




