Assessment of lymph node metastasis of ≤20 mm non-small cell lung cancer originating from superior segment compared to basal segment
Abstract
Background
Segmentectomy with curative intention is occasionally performed for early non-small cell lung cancer (NSCLC). However, a major problem has been pointed out, in that the rate of locoregional recurrence is higher after segmentectomy than after lobectomy. This study aimed to investigate differences in rates of lymph node metastasis between segment 6 and basal segment NSCLC as potential candidates for segmentectomy and to explore factors associated with locoregional recurrence of segmentectomy.
Methods
We retrospectively analyzed 461 patients with lower lobe NSCLC who underwent segmentectomy or lobectomy with mediastinal lymph node dissection between 2011 and 2021. Among these, 122 patients with clinical N0 NSCLC, diameter ≤ 20 mm, and consolidation tumor ratio >0.5 were analyzed.
Results
The 122 patients were divided into a segment 6 group (n = 51) and a basal segment group (n = 71). Frequency of lymph node metastasis was significantly higher in the segment 6 group (17.7%) than in the basal segment group (4.2%; p = 0.01). Metastases to lymph node station 7 were seen in five of 122 patients (4.1%). Hilar lymph node metastasis occurred in nine of 122 patients (7.4%). Notably, metastases to station 11, 11i and 11 s lymph nodes were the most frequent patterns for hilar lymph nodes (41.7%).
Conclusions
Station 11 lymph nodes are adjacent to the remaining lung segment or pulmonary artery in S6 segmentectomy or basal segmentectomy. Part of the NSCLC in segment 6 patients may thus be considered for lobectomy owing to the difficulty of complete dissection of station 11 lymph nodes.
INTRODUCTION
Small and early-stage lung cancers are increasingly being identified due to improvements in computed tomography (CT) technologies.1 Although lobectomy with mediastinal lymph node dissection is a gold standard for stage I non-small cell lung cancer (NSCLC), curability and respiratory function show a trade-off relationship. To preserve both of these clinical factors, sublobar resections with curative intention have occasionally been performed for patients with small or early-stage NSCLC. On the other hand, no strong consensus has been reached on indications for sublobar resection of early-stage NSCLC. In 2022, The Japan Clinical Oncology Group and West Japan Oncology Group conducted a randomized trial on lobectomy versus segmentectomy for small (maximum diameter, ≤20 mm) and peripheral invasive lung cancer (JCOG0802/WJOG4607L).2 This randomized controlled trial showed the noninferiority of segmentectomy to lobectomy in terms of 5-year overall and relapse-free survivals. Segmentectomy for early-stage NSCLC is thus garnering attention around the world.
However, higher rates of locoregional recurrence after segmentectomy still constitute a major clinical problem and have been reported as 10.5%–20.7%.2, 3 The most frequent recurrences occurred in ipsilateral hilar and mediastinal lymph nodes (41.8%) according to the results of the JCOG0802/WJOG4607L trial. Although appropriate lymph node dissection during segmentectomy is essential for surgical quality of curative intent operation, an adequate range is still controversial. Moreover, tumor location potentially contributes to the rate of lymph node metastasis. Previous studies have demonstrated that segment 6 tumors tend to result in lymph node metastasis more often than basal segment tumors.4, 5 The main purpose of this study was to assess whether the segment of tumor location affects the lymph node metastasis rate and to investigate the appropriate range of lymph node dissection during segmentectomy in patients with NSCLC of the lower lobe who met the same criteria as the JCOG0802/WJOG4607L trial.
METHODS
Ethical statement
This study was a retrospective analysis in the Department of Thoracic Surgery at Iwate Medical University between 2011 and 2021. All study protocols were approved by the institutional review board (approval no. MH2021-150). The present study was conducted in accordance with the Declaration of Helsinki.
Patients
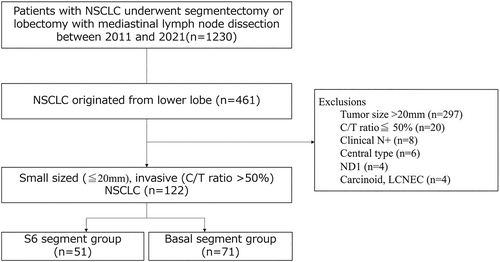
A total of 461 patients who underwent lobectomy or segmentectomy with mediastinal lymph node dissection for NSCLC of the lower lobe were included. Among these, 339 patients were excluded based on diameter > 20 mm (n = 297), consolidation tumor ratio ≤0.5 (n = 20), clinical node-positive state (n = 8), central-type tumor (n = 6), only hilar lymph node dissection (n = 4) or carcinoid tumor or large cell neuroendocrine tumor (n = 4). These patients were eligible for analysis. A total of 122 patients were divided into a segment 6 group (n = 51) and a basal segment group (n = 71) according to the segment in which the tumor was located (Figure 1). Baseline variables collected included age, sex, smoking index, clinical findings and pathological diagnosis.
Pre- and postoperative staging
Clinical staging was based on chest x-ray, contrast-enhanced CT of the chest and abdomen, brain CT or magnetic resonance imaging and positron emission tomography. All patients underwent the above examinations and evaluation of clinical staging. Postoperative pathological staging was based on the eighth edition of the American Joint Commission on Cancer TNM staging system for NSCLC.6 Histological classification was determined according to World Health Organization criteria.7 Hilar and mediastinal lymph node stations were numbered according to the International Association for the Study of Lung Cancer (IASLC) lymph node map.8
Radiological evaluations
The segment in which the tumor was located was assessed based on the involved pulmonary vein on preoperative CT. The V6c branch was defined as the border interface between segment 6 and the basal segment in this study. When the tumor involved both segment 6 and the basal segment, the segment with the greater proportion of tumor or the involved pulmonary vein was used. The consolidation component was defined as an area of increased opacity that completely obscured underlying vascular markings. The ground-glass component was defined as an area of slight, homogeneous increase in density that did not the obscure underlying vascular markings.9 The consolidation-to-tumor ratio was defined as the ratio of the maximum diameter of the consolidation component to maximum tumor diameter in this study.
Surgical procedures
Pulmonary resection was performed under general anesthesia with a double-lumen endotracheal tube for single-lung ventilation. The affected lung was deflated as soon as the pleural space was opened, and deflation was maintained throughout most of the operative course. The patient was placed in the lateral decubitus position. Our alternative surgical procedure was described previously.10 In brief, complete video-assisted thoracic surgery was performed via three ports under monitor vision only. Complete and systematic dissection of hilar and mediastinal lymph nodes was performed in all lobectomy and segmentectomy cases.
Statistical analysis
JMP version 14.1.0 statistical software (SAS Institute) was used for all statistical analyses in this study. Categorical variables were compared between groups using Pearson's chi-square test or the Wilcoxon rank-sum test. Differences between groups were considered significant for values of p < 0.05. Continuous data are expressed as mean ± standard deviation, and categorical data are expressed as count and proportion.
RESULTS
Patient characteristics
Clinical data of the 122 patients are summarized in Table 1. The proportion of male patients (68.6% vs. 31.4%; p = 0.006) and smoking index (539.0 vs. 336.9; p = 0.005) were both significantly higher in the segment 6 group. No significant differences between groups were seen in age, mean serum carcinoembryonic antigen level or mean serum cytokeratin fragment level. Total tumor diameter was 16.5 mm in the segment 6 group and 15.6 mm in the basal segment group (p = 0.11); solid component size was 15.3 mm in the segment 6 group and 14.8 mm in the basal segment group (p = 0.51). Consolidation-to-tumor ratio was 93.1% in the segment 6 group and 95.6% in the basal segment group (p = 0.15). No significant differences in radiological findings of the tumor were identified between groups.
| Variables | Superior segment (S6) | Basal segment (S8–10) | p-value |
|---|---|---|---|
| Number of patients | 51 (41.8%) | 71 (58.2%) | |
| Age (years) | 70.4 ± 7.2 | 69.4 ± 9.5 | 0.66 |
| Sex | 0.006 | ||
| Male | 35 (68.6%) | 16 (31.4%) | |
| Female | 31 (43.7%) | 40 (56.3%) | |
| Smoking index | 539.0 ± 491.7 | 336.9 ± 550.5 | 0.005 |
| CEA (ng/ml) | 4.1 ± 4.0 | 3.3 ± 2.4 | 0.39 |
| CYFRA (ng/ml) | 2.5 ± 1.5 | 2.0 ± 1.0 | 0.08 |
| Radiological findings | |||
| Total tumor size (mm) | 16.5 ± 2.9 | 15.6 ± 2.8 | 0.11 |
| Solid component size (mm) | 15.3 ± 3.7 | 14.8 ± 3.6 | 0.51 |
| Consolidation/tumor ratio (%) | 93.1 | 95.6 | 0.15 |
| Maximum standardized uptake value | 3.8 ± 3.1 | 3.0 ± 2.3 | 0.07 |
| Clinical T stage | 0.53 | ||
| T1a | 4 (7.8%) | 8 (11.3%) | |
| T1b | 47 (92.2%) | 63 (88.7%) | |
| Surgical procedure | 0.95 | ||
| Lobectomy | 48 (94.1%) | 67 (94.4%) | |
| Segmentectomy | 3 (5.9%) | 4 (5.6%) |
- Abbreviations: CEA, carcinoembryonic antigen; CYFRA, cytokeratin fragment.
Pathological diagnosis and patterns of lymph node metastasis
Pathological data of the 122 patients are summarized in Table 2. No significant differences between groups were seen in histological subtype, visceral pleural invasion or number of dissected lymph nodes. Tumor side (right or left) did not affect the rate of lymph node metastasis (8.6% vs. 11.5%; p = 0.59). The segment 6 group showed a significantly higher incidence of lymph node metastasis than the basal segment group (17.7% vs. 4.2%; p = 0.01).
| Variables | Superior segment (S6) | Basal segment (S8–10) | p-value |
|---|---|---|---|
| Pathological subtype | 0.44 | ||
| Adenocarcinoma | 39 (76.5%) | 62 (87.3%) | |
| Squamous carcinoma | 9 (17.7%) | 9 (12.7%) | |
| Others | 3 (5.9%) | 0 | |
| Visceral pleural invasion | 13 (25.5%) | 14 (19.7%) | 0.45 |
| Number of dissected lymph nodes | 17.5 ± 10.1 | 16.5 ± 7.4 | 0.86 |
| Lymph node metastasis | |||
| pN+ | 9 (17.7%) | 3 (4.2%) | 0.01 |
| pN1 | 6 (11.8%) | 1 (1.4%) | |
| pN2 | 3 (5.9%) | 2 (2.8%) |
Table 3 shows the patterns of lymph node metastasis according to the IASLC lymph node map in 12 patients diagnosed pN1 or pN2. Upper mediastinal lymph node metastasis (lymph nodes 2R, 4R, 4 L, 5 and 6) occurred in only one patient (Patient 4). On the other hand, lower mediastinal lymph node metastasis (lymph nodes 7, 8 and 9) occurred in five of 122 patients (4.1%). Hilar lymph node metastasis occurred in 9 of 122 patients (7.4%). Notably, station 11, 11i and 11 s lymph nodes were the most frequent metastasis patterns in hilar lymph nodes (41.7%).
| Patient No | Right or left | Segment | Pathological subtype | 2R | 4R | 4 L | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 11 s | 11i | 12u | 12 L | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lt | 8 | Adenocarcinoma | 0/1 | 3/4 | 0/2 | 1/6 | 0/3 | ||||||||||
| 2 | Lt | 6 | Adenocarcinoma | 0/2 | 0/5 | 0/2 | 0/3 | 0/1 | 0/4 | 2/4 | 0/6 | 1/2 | ||||||
| 3 | Rt | 6 | Adenocarcinoma | 0/2 | 0/5 | 0/5 | 0/1 | 2/3 | 0/5 | 0/2 | ||||||||
| 4 | Rt | 6 | Adenocarcinoma | 0/6 | 1/4 | 3/7 | 0/2 | 1/1 | 0/1 | 0/2 | ||||||||
| 5 | Rt | 6 | Adenocarcinoma | 0/4 | 0/3 | 3/11 | 0/1 | 0/1 | 0/1 | |||||||||
| 6 | Lt | 6 | Adenocarcinoma | 0/1 | 0/1 | 0/1 | 0/1 | 2/2 | 0/1 | 0/1 | ||||||||
| 7 | Lt | 8 | Adenocarcinoma | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | ||||||||||
| 8 | Lt | 6 | Adenosquamous carcinoma | 0/1 | 0/9 | 1/3 | 0/5 | 0/7 | ||||||||||
| 9 | Rt | 6 | Squamous cell carcinoma | 0/7 | 0/5 | 0/8 | 0/4 | 0/3 | 0/2 | 1/4 | ||||||||
| 10 | Rt | 8 | Squamous cell carcinoma | 0/5 | 0/6 | 0/5 | 0/3 | 1/2 | ||||||||||
| 11 | Lt | 6 | Adenocarcinoma | 0/3 | 0/2 | 0/2 | 0/1 | 0/4 | 1/1 | |||||||||
| 12 | Rt | 6 | Adenocarcinoma | 0/5 | 0/4 | 1/4 | 0/3 | 0/1 |
DISCUSSION
In the present study, we hypothesized that tumor location would affect the rate of lymph node metastasis in patients with small peripheral NSCLC originating in the lower lobe. Indeed, we demonstrated a significantly higher incidence of lymph node metastasis in the segment 6 group than in the basal segment group (17.7% vs. 4.2%; p = 0.01). Furthermore, station 11 lymph nodes were considered to represent key lymph nodes in radical operations for lower lobe NSCLC. Although recent studies have pointed out the higher incidence of locoregional recurrence after segmentectomy (10.5%–20.7%) than after lobectomy (5.4%–8.2%), detailed analyses have rarely been reported.2, 3, 11 Our findings suggest that the segment in which the tumor is located may represent a new prognostic factor, which may open up reconsiderations for surgical indication for sublobar resection of small peripheral NSCLC.
The lower lobe occupies half of the hemithorax, which is formed of five segments on the right and four segments on the left. Segment 6 is the most apical of the segments in the lower lobe, posterior to the upper lobe aspect of the oblique fissure and apicoposterior segment of the upper lobe. Watanabe et al. and Handa et al. reported that tumor originating from the superior segment showed a significantly higher incidence of lymph node metastasis than the basal segment group.4, 5 One reason may have been the anatomically shorter distance to hilar lymph nodes in segment 6 compared with the basal segment. However, the present study included only small (≤20 mm) peripheral tumors matching the JCOG0802/WJOG4607L study, which has not previously been described in similar studies. The present study suggested that station 11, 11 s and 11i lymph nodes should be dissected in segmentectomy, as these lymph nodes were the most frequent recipients of metastasis in patients with small-sized NSCLC originating from the lower lobe. However, some hilar lymph nodes are distant from the intersegmental plane or pulmonary fissure, and are not operated on in usual surgical procedures for segmentectomy. By way of example, in S6 segmentectomy, station 11i lymph nodes may be difficult to dissect without transecting the interlobar fissure. Insufficient hilar lymph node dissection could potentially lead to higher rates of locoregional recurrence after segmentectomy. Although segmentectomy + hilar and mediastinal lymph node dissection is performed, the practical range of hilar lymph node dissection is unclear. We therefore suggest that complete hilar lymph node dissection or qualitative diagnosis are essential for curative segmentectomy even for small (≤20 mm) peripheral NSCLC in the lower lobe. In particular, segment 6 tumor merits careful attention when deciding surgical procedures. Intraoperative rapid diagnosis of lymph nodes may help surgeons to select the surgical procedures.
This study showed several limitations that warrant consideration. First, this study was retrospective in nature and included only patients from a single institute. Our findings may thus not be generalizable to other institutions and a larger, multicenter study is necessary to confirm the present findings. Second, the present study analyzed only pathological data, so we could not assess the prognosis of patients with S6 NSCLC compared with basal segment NSCLC. Particularly, the relationship between radical no.11 lymph node dissection and survival rate was unclear in the present study. Regarding the prognosis of small-sized NSCLC originating in the lower lobe, a large study with long-term postoperative follow-up is required.
In conclusion, we suggest that complete hilar lymph node dissection or qualitative diagnosis be performed with curative segmentectomy. In particular, in segment 6 patients with sufficient respiratory function, part of the NSCLC may be a candidate for lobectomy because of the difficulty of complete dissection of station 11 lymph nodes.
AUTHOR CONTRIBUTIONS
Conception and design: Ryuichi Yoshimura, Collection and assembly of data: Ryuichi Yoshimura, Hiroyuki Deguchi, Makoto Tomoyasu, Wataru Shigeeda, Yuka Kaneko and Hidenobu Iwai. Data analysis and interpretation: Ryuichi Yoshimura and Hajime Saito. Manuscript writing: All authors. Final approval manuscript: All authors.
ACKNOWLEDGMENT
The authors wish to thank Tatsuo Tanita for his valuable suggestions.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.




