Circulating microRNAs as indicators in the prediction of neoadjuvant chemotherapy response in luminal B breast cancer
Funding information: Beijing Natural Science Foundation, Grant/Award Number: 7171012; National Key R&D Program of China, Grant/Award Number: 2016YFC0904900; National Natural Science Foundation of China, Grant/Award Numbers: 81872940, 81973395, 82073935; National Science and Technology Major Projects for Major New Drugs Innovation and Development, Grant/Award Numbers: 2017ZX09101001, 2017ZX09304028, 2018ZX09201014
Abstract
Purpose
Circulating microRNAs (miRNAs) have been indicated as predictive biomarker for the response to neoadjuvant chemotherapy (NAC) and the prognosis of breast cancer (BC); however, to date the conclusions have been controversial. The biological characteristics of BC were affected by molecular subtypes. Hence, we aimed to investigate the predictive effect of miRNAs on NAC response in luminal B BC patients.
Methods
Thirty-seven luminal B BC patient under NAC were prospectively enrolled in this study. Based on their clinical, pathological, and comprehensive response, the patients were defined as responder or non-responders, respectively. Circulating miRNAs were isolated from blood samples before and at the middle of NAC, and candidate miRNAs (miR-34a-5p, miR-125b-5p, miR-210, miR-222, miR-375, miR-718, miR-4516, and let-7g) were analyzed by quantitative real-time polymerase chain reaction (PCR). In addition, the association between miRNAs and disease-free survival (DFS) was examined.
Results
miR-718, miR-4516, miR-210, and miR-125b-5p were found to be specific miRNAs associated with chemo-sensitivity of luminal B HER2 negative patients (n = 24). In the luminal B HER2 positive cohort (n = 13), dynamics of miR-222 and let-7g correlated with pathological response. Treatment-induced increase in miR-34a-5p in the responders except who reached pathologic complete response (pCR) was consistently identified across all luminal B patients and its two subgroups. Finally, after adjustments for Neo-Bioscore, patients with increased levels of miR-125b-5p during NAC had a worse DFS than those with decreased levels (HR = 5.86, 95% CI = 1.39–24.62, p = 0.016).
Conclusion
Specific circulating miRNAs were identified as predictive markers for NAC response and prognosis in luminal B BC. The underlying mechanism needs further studies.
INTRODUCTION
Among women, breast cancer (BC) is the most common invasive cancer and the leading cause of cancer-related death in the world.1 As a heterogeneous disease, the subtypes of BC are associated with the different clinicopathological characteristics, treatment strategies, and prognosis. According to the estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki67, we can categorize BC into four molecular subtypes: luminal A, luminal B (including HER2 positive [HER2+] and HER2 negative [HER2−]), HER2 enriched, and basal-like.2 Among them, luminal BC is the most common type in women diagnosed with early-stage BC.3
Neoadjuvant chemotherapy (NAC) has gained a paramount role in the treatment of early/locally advanced BC and enables an objective evaluation of treatment efficacy.4, 5 Pathologic complete response (pCR), usually used as the primary endpoint, has been proposed as a surrogate endpoint of survival, such as disease-free survival (DFS) and overall survival (OS).6, 7 Improving pCR rates became the goal of NAC with the expectation of improved patient survival. However, pCR rates varies among the different BC subgroups: 0%–8% in luminal A, ~15% in luminal B HER2−, 22%–48% in luminal B HER2+ with trastuzumab combined in NAC, and higher in HER2-enriched and triple negative BC (TNBC).8, 9 Hence, it is of significance to predict the response of NAC to avoid side effects and poor outcomes from the ineffective treatment. Unfortunately, at present there is no method that definitely predicts chemotherapeutic responders from non-responders. However, the good thing is that obtaining tumor specimens or blood samples from the patient before, during, and after NAC is convenient, enabling to find prognostic and predictive biomarkers, such as microRNAs (miRNAs).
Several previous studies have reported an association between miRNAs aberrant expression, chemotherapy response, and resistance in BC.10, 11 Given the stable, non-invasive, real-time, and easily recurring sampling characteristics, circulating miRNAs represent an important avenue in the search for a biomarker for NAC response prediction. Circulating miR-34a, miR-122, miR-125b, miR-451, miR-663, and other miRNAs were found to be associated with NAC outcomes,12-17 but without BC subtypes classified. NEOCENT study found an increase in plasma let-7a was seen at surgery for luminal patients with objective radiological response to NAC.18 As for luminal A BC, miR-19a and miR-205 in the serum may predict the chemosensitivity.19 Some other studies20-25 focused on the HER2+, TNBC, or hormone receptor positive/HER2− subtypes, but the relationship between circulating miRNAs and NAC response in luminal B BC has not been specifically studied. Moreover, most studies12, 18, 26-28 defined complete response (CR) and partial response (PR) as responders and stable disease (SD) and progression disease (PD) as non-responders based on response evaluation criteria in solid tumors (RECIST) 1.1,29 and some19, 21, 22, 30, 31 assessed pathologic response according to different standards. The inconsistency of evaluation of the NAC efficacy deserves equally attention.
Therefore, this study aimed to determine the predictive value of circulating miRNAs regarding treatment response and survival in luminal B BC patients receiving NAC.
METHODS
Patients
Based on the definitions of intrinsic subtypes of BC from St. Gallen consensus 2013,32 we recruited 37 luminal B BC patients undergoing NAC at Peking University First Hospital from December 2015 to February 2018. This study was reviewed and approved by Ethical Review Board of the Peking University First Hospital (2015 [999]). All the patients received first-line taxane and/or anthracycline based regimen, and trastuzumab was combined with chemotherapy for some HER2+ cases. Other interventions as standard procedures were described previously.33 Neo-Bioscore,34 a clinical-pathologic staging system incorporating ER, the nuclear grade, and HER2 status, was used to help assess the prognosis stratification. All of the patients were followed-up until June 2021. The study was performed in accordance with reporting recommendations for tumor marker prognostic studies (REMARK) criteria.35
To assess the agreement of miRNAs' predictive efficacy between different standards, we evaluated the NAC responses of patients according to clinical, pathological, and comprehensive outcomes. Clinical response was divided into two groups (CR/PR and SD/PD) based on RECIST 1.1, as noted above. The Miller–Payne (MP) system,36 including 5 grades from grade (G) 1 to G5, was used to evaluate the pathological response by comparing the specimens taken from surgery and before NAC. We grouped patients with G1–2 as non-responders, and G3–5 as responders. Considering the significance of pCR in NAC response evaluation, we also stratified patients into pCR and non-pCR in pathological evaluation. pCR was defined as the histopathological complete absence of invasive lesions in both breast and axillary lymph node specimens. Pathological response was assessed by two independent pathologists.
Based on clinical and pathological examination, comprehensive evaluation was established. Patients who achieved PD or SD in clinical evaluation and G1 or G2 in pathological evaluation were stratified into non-responders, and the others were defined as responders.
Serum collection and RNA extraction
Given that early determination of poor response offers the chance to alter treatment regimens and drugs with the goal of achieving an optimized response, blood samples were collected at baseline (point A) and after two/four cycles of NAC (point B) for screening candidate miRNAs whose fluctuations might reflect response. For each patient, 4 mL of peripheral blood was collected at each time point. Within 4 hours of blood drawl, samples were centrifuged at 1500g for 10 minutes. The serum supernatant was quickly removed and immediately stored at −80°C until further use. Total RNA was extracted and purified by using the miRNeasy Serum/Plasma Advanced Kit (Qiagen) following the manufacturer's instructions.
Quantitative real-time polymerase chain reaction
We focused on eight candidate miRNAs, including miR-34a-5p, miR-125b-5p, miR-210, miR-222, miR-375, miR-718, miR-4516, and let-7g, which are considered to be closely related with chemosensitivity from literatures. Complementary DNA (cDNA) templates were prepared using a TaqMan advanced miRNA cDNA synthesis kit (ThermoFisher Scientific) following the manufacturer's guidelines. A quantitative real-time polymerase chain reaction (qRT-PCR) reaction was subsequently performed in triplicate using the predesigned TaqMan advanced miRNA assays (ThermoFisher Scientific) on the StepOne Plus Real-Time PCR System (Applied Biosystems). miR-16-5p was used as endogenous reference and relative expression of miRNA was determined using the comparative cycle threshold (CT) method and reported as 2-ΔCT.
Bioinformatics analysis
Target prediction algorithm miRWalk (http://mirwalk.umm.uni-heidelberg.de/)37 was used to identify putative targets of miRNAs. This online website covers two other miRNA-target prediction data-sets (Targetscan and miRDB) and one validated interaction data from miRTarBase. Therefore, for each miRNA, only those target genes predicted by all available algorithms in miRWalk were included in the followed analysis. Genes targeted by at least two miRNAs were screened as target nodes, whereas the corresponding miRNAs were considered source nodes. Cytoscape 3.8.238 was used for visualization of the miRNA–mRNA regulatory network. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed using the web-based tool Metascape39 (http://metascape.org/gp/index.html#/main/step1), and the cut-off criterion used the default value.
Statistical analysis
Demographic and clinicopathological characteristics of study population were analyzed using statistical description method. Fisher's exact χ2 test was used for categorical patient variables. Difference in miRNA levels between groups was evaluated using the Mann–Whitney unpaired test when two groups were compared. To compare more than two groups, a non-parametric Kruskal-Wallis test was used instead. For before/after comparison within one group, Wilcoxon signed-rank test was used. A univariate logistic regression analysis was also performed presenting with the odd ratio (OR) and the 95% confidence interval (CI). Area under the curves (AUC) of receiver operating characteristic (ROC) was used to assess the predictive accuracy of miRNAs. DFS was defined as the interval between initiation of NAC and the date of disease relapse or death from any cause. Cases without relapse or death events were censored at the date of last follow-up. Cox proportional hazards model were performed to evaluate the prognostic values of selected miRNAs. Statistical analysis and graphics were performed using the R 4.0.3 and GraphPad Prism 9. Two-sided tests with p < 0.05 were considered statistically significant.
RESULTS
Patient characteristics and response to NAC
In all, 37 luminal B BC patients were included in this study, with 13 HER2+ and 24 HER2− (Table 1). The patients ranged in age from 27 to 65 and the median age was 54 years old, among which 48.6% were pre-menopausal status. All cases are ER positive. The majority was diagnosed with clinical stage II and pathological stage III. The rate of responders was 70.3%, 78.4%, 18.9%, and 81.1% based on clinical response, pathological response (MP), pathological response (pCR) and comprehensive evaluation, respectively. As shown in Table 1, except for Neo-Bioscore, there was no significant differences were observed in the characteristics between the luminal B HER2+ and HER2− group. Patients with HER2− had more advanced stagings of Neo-Bioscore (p = 0.012).
| Characteristics | Luminal B n = 37 | HER2+ n = 13 | HER2− n = 24 | p (between subgroups) |
|---|---|---|---|---|
| Age (y), median (range) | 54 (27–65) | 54 (30–64) | 53 (27–65) | 0.730 |
| ≤54 | 22 | 7 | 15 | |
| >54 | 15 | 6 | 9 | |
| Menopause | 1 | |||
| Premenopausal | 18 | 6 | 12 | |
| Postmenopausal | 19 | 7 | 12 | |
| Grade | 0.734 | |||
| I/II | 24 | 9 | 15 | |
| III | 13 | 4 | 9 | |
| Clinical staging | 0.288 | |||
| I | 2 | 1 | 1 | |
| II | 27 | 8 | 19 | |
| III | 8 | 4 | 4 | |
| Pathological staging | 0.190 | |||
| 0 | 7 | 5 | 2 | |
| I | 10 | 2 | 8 | |
| II | 9 | 4 | 5 | |
| III | 11 | 2 | 9 | |
| Neo-Bioscore | 0.012 | |||
| 0 | 3 | 3 | 0 | |
| 1 | 5 | 2 | 3 | |
| 2 | 11 | 6 | 5 | |
| 3 | 13 | 2 | 11 | |
| 4 | 5 | 0 | 5 | |
| Clinical response | 0.464 | |||
| CR/PR | 26 | 8 | 18 | |
| SD/PD | 11 | 5 | 6 | |
| Pathological response (MP) | 1 | |||
| G3/4/5 | 29 | 10 | 19 | |
| G1/2 | 8 | 3 | 5 | |
| Pathological response (pCR) | 0.072 | |||
| pCR | 7 | 5 | 2 | |
| Non-pCR | 30 | 8 | 22 | |
| Comprehensive evaluation | 0.678 | |||
| Response | 30 | 10 | 20 | |
| Non-response | 7 | 3 | 4 |
- Abbreviations: CR, complete response; HER2, human epidermal growth factor receptor 2; MP, Miller–Payne system; pCR, pathologic complete response; PD, progression disease; PR, partial response; SD, stable disease.
Baseline miRNA expression and clinicopathological characteristics
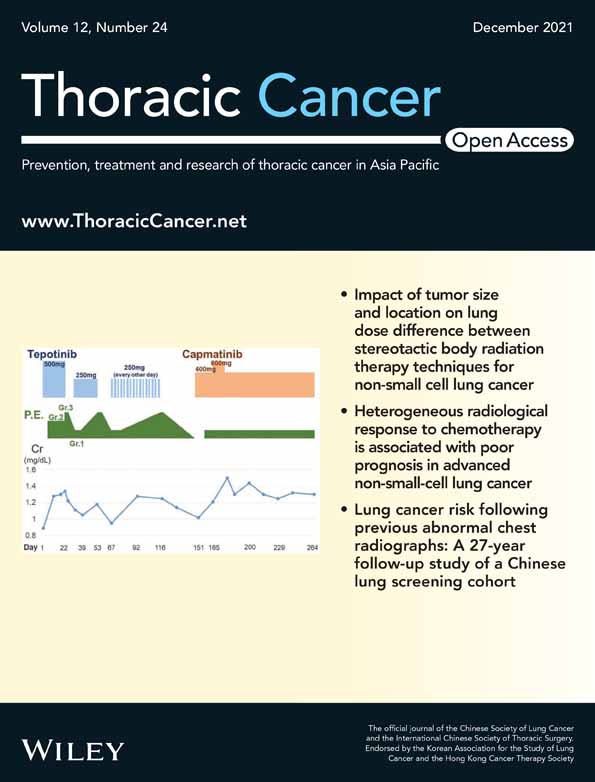
We correlated the baseline expression of eight miRNAs with the clinicopathological characteristics, and the results showed that miR-125b-5p upregulation was related to higher pathological grade (p = 0.005) (Figure 1(a)). In addition, miR-375 (p = 0.049), miR-718 (p = 0.001), and miR-4516 (p < 0.001) were significantly associated with the different HER2 status: low expression in HER2− tumors versus HER2+ tumors (Figure 1(b)–(d)). Non-significant differences were found between other miRNAs and clinicopathological characteristics.
Relationship between baseline miRNA expression and NAC response
The significant associations between baseline miRNAs expression and the evaluations of NAC response analyzed by nonparametric Mann–Whitney test were summarized in Tables 2, in which HER2+ and HER2− were analyzed respectively. The AUC of individual miRNA ranged from 0.727 to 1, suggesting moderate to high indicative value for these markers.
| Evaluation | Luminal B | p | Luminal B HER2+ | p | Luminal B HER2− | p |
|---|---|---|---|---|---|---|
| Clinical response | ||||||
| CR/PR vs. SD/PD | miR-718↓ | 0.031 | – | – | miR-4516↓ | 0.022 |
| miR-4516↓ | 0.016 | |||||
| Pathological response (MP) | ||||||
| G3/4/5 vs. G1/2 | miR-210↑ | 0.017 | – | – | miR-210↑ | 0.019 |
| miR-4516↓ | 0.036 | |||||
| Pathological response (pCR) | ||||||
| pCR vs. non-pCR | – | – | – | – | miR-210↑ | 0.007 |
| miR-375↓ | 0.043 | |||||
| miR-718↑ | 0.029 | |||||
| Comprehensive evaluation | ||||||
| Response vs. non-response | – | – | – | – | miR-375↓ | 0.025 |
- Note: ↑: indicating this miRNA expression was significantly higher in responders than non-responders; ↓: indicating this miRNA expression was significantly lower in responders than non-responders; −: none.
- Abbreviations: CR, complete response; HER2, human epidermal growth factor receptor 2; MP, Miller–Payne system; pCR, pathologic complete response; PD, progression disease; PR, partial response; SD, stable disease.
At baseline, miR-718 (p = 0.031) and miR-4516 (p = 0.016) expression were lower in clinical responders than non-responders, and miR-210 (MP: p = 0.017) expression was elevated in the pathological responders compared with that in the non-responders in luminal B BC. In the subgroup analysis stratified by HER2 status, the results showed no significant differences in miRNAs expression between responders and non-responders based on those four evaluations in luminal B HER2+ patients at point A. As for luminal B HER2− patients, the low expression of miR-4516 was not only in clinical responders (p = 0.022) like all subjects, but also in patients with G3–5 (p = 0.036). The associations between miR-210 upregulation and pathological response (MP: p = 0.019; pCR: p = 0.007) were also found. Besides the above miRNAs, miR-375 had a relatively low expression in HER2− patients achieving pCR (p = 0.043) and comprehensive response (p = 0.023). With respect to miR-718, its expression was significantly higher in pCR group than non-pCR (p = 0.029).
To evaluate the value of miRNAs in predicting response to NAC, these potential miRNAs in Table 2 were analyzed by resorting to a univariate logistic regression model on their continuous original scale. Unfortunately, probably because of compromised sample size and small number of events, none of them were independent predictors of response. Further, we used ROC curves to derive the optimal cut-off value for those significant miRNAs. Only miR-210, grouped into low or high expression based on baseline relative expression (cut-off value 0.0013), was demonstrated to be indicative of pathological response (MP) to NAC in all patients (OR = 0.07, 95% CI = 0.01–0.45, p = 0.01) and HER2− group (OR = 0.05, 95% CI = 0–0.58, p = 0.02).
Relationship between miRNA dynamics and NAC response
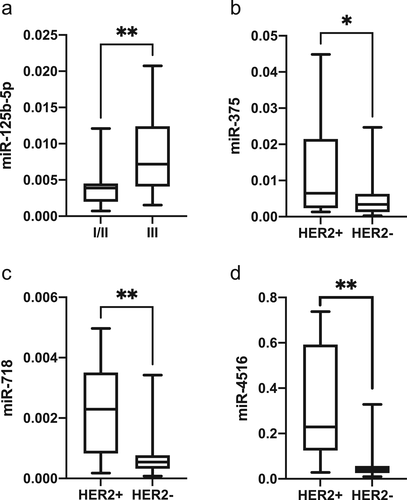
The changes in the eight miRNAs expression from point A to B were analyzed. The results showed that only changes in miR-34a-5p expression during the treatment were significantly associated with NAC in all patients regardless of HER2 status (Figure 2(a)). Treatment-induced noteworthy upregulation of miR-210 in all subjects, downregulation of miR-222 in HER2+ group and upregulation of miR-375 in HER2− patients were also detected (Figure 2(b)–(d), respectively). The significant associations between miRNAs dynamics during the NAC and the evaluations of NAC response analyzed by Wilcoxon signed-rank test were summarized in Table 3.
| Evaluation | Point B vs point A | |||||
|---|---|---|---|---|---|---|
| Luminal B | p | Luminal B HER2+ | p | Luminal B HER2− | p | |
| Clinical response | ||||||
| CR/PR | miR-34a-5p↑ | <0.001 | miR-34a-5p↑ | 0.039 | miR-34a-5p↑ miR-375↑ miR-4516↑ |
0.018 0.024 0.003 |
| SD/PD | miR-210↑ | 0.024 | – | – | miR-125b-5p↓ | 0.031 |
| Pathological response (MP) | ||||||
| G3/4/5 | miR-34a-5p↑ | <0.001 | miR-34a-5p↑ | 0.010 | miR-34a-5p↑ miR-375↑ miR-4516↑ |
0.014 0.011 0.040 |
| G1/2 | miR-210↑ | 0.008 | – | – | – | – |
| Pathological response (pCR) | ||||||
| pCR | – | – | – | – | – | – |
| Non-pCR | miR-34a-5p↑ miR-210↑ miR-375↑ |
<0.001 0.004 0.045 |
miR-34a-5p↑ miR-222↓ let-7g↓ |
0.016 0.039 0.039 |
miR-34a-5p↑ miR-210↑ miR-375↑ miR-4516↑ |
0.036 0.014 0.006 0.042 |
| Comprehensive evaluation | ||||||
| Response | miR-34a-5p↑ | <0.001 | miR-34a-5p↑ | 0.010 | miR-34a-5p↑ miR-375↑ miR-4516↑ |
0.009 0.006 0.027 |
| Non-response | miR-210↑ | 0.016 | – | – | – | |
- Note: ↑: indicating this miRNA expression in this group was significantly higher at point B than point A; ↓: indicating this miRNA expression in this group was significantly lower at point B than point A; −: none.
- Abbreviations: CR, complete response; HER2, human epidermal growth factor receptor 2; MP: Miller–Payne system; pCR, pathologic complete response; PD, progression disease; PR, partial response; SD, stable disease.
In all luminal B patients, fluctuation patterns for two candidate miRNAs were associated with NAC response. In non-responders, regardless of the evaluation standards, markedly elevated expression of plasma miR-210 was detected after treatment, but in the response group no significant change in the level of miR-210 was detected. With respect to plasma miR-34a-5p, as displayed in Table 3, upregulation of this miRNA (point B vs. point A) was observed in the responders rather than the non-response group, except for those who were evaluated based whether achieved pCR. Treatment induced significant upregulation of miR-34a and miR-375 in the non-pCR group, whereas in the pCR group the levels of these two miRNAs remained relatively stable.
In the luminal B HER2+ cohort, the dynamic change of miR-34a-5p after NAC was same like that in the all subjects. Chemotherapy also induced significant downregulation of miR-222 and let-7g in the non-pCR group, whereas the levels of these two miRNAs remained relatively stable in the pCR group.
In the luminal B HER2− cohort, we also found the same significant changes of miR-34a-5p after treatment like all patients and HER2+ group. Noteworthy upregulation of miR-375 and miR-4516 was observed after treatment in the same group like miR-34a-5p. With respect to miR-125b-5p, markedly decreased expression was detected after chemotherapy in non-response group only based on clinical outcomes, and the levels of miR-125b-5p remained relatively stable in CR/PR group. Significantly elevated expression of plasma miR-210 was observed in non-pCR group rather than patients who achieved pCR.
To evaluate the dynamics of the miRNAs in predicting response to NAC, the fold change (point B compared with point A) was calculated by 2-ΔΔCT method and incorporated into the logistic regression models. As a continuous variable, the fold change of miR-210 was found to be independent predictors of pathological response (MP) (OR = 1.63, 95% CI = 1.07–2.48, p = 0.023) in all patients. The fold change of miR-210 was grouped into up- or down-regulated based on the cut-off value 2.33 from ROC curve, and demonstrated to be indicative of pathological response (MP) (OR = 9.43, 95% CI = 1.54–57.75, p = 0.015) and comprehensive response (OR = 0.05, 95% CI = 0–0.58, p = 0.02) to NAC in all patients. The changes of other potential miRNAs in Table 3 were not independent predictors of response, maybe because of the compromised sample size and small number of events.
Relationship between miRNA and prognosis
By the date of the survival analysis, three patients died, ten patients relapsed, and two patients were excluded from the analysis because of the loss of follow-up. The median DFS was 43.4 months (9.47–60.9 months). Among clinicopathological characteristics in Table 1, univariate analysis showed only Neo-Bioscore was significantly related to DFS (HR = 2.04, 95% CI = 1.05–3.97, p = 0.035). With respect to the predictive role on prognosis of miRNAs, the patients were divided into high/low expression groups according to the median level of miRNAs at different time points, and increased/decreased level groups according to the changes of miRNA expression from point A to point B. Univariate analysis showed neither miRNA expression nor miRNA dynamics after NAC were found to be related to DFS. However, after adjustments for Neo-Bioscore, the change of miR-125b-5p from point A to point B was found to be significantly correlated with DFS (Table 4).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Neo-Bioscore | 2.04 | 1.05–3.97 | 0.035 | 2.85 | 1.31–6.18 | 0.008 |
| miR-125b-5p increased from point A to point B | 3.10 | 0.87–10.99 | 0.081 | 5.86 | 1.39–24.62 | 0.016 |
Bioinformatic analysis
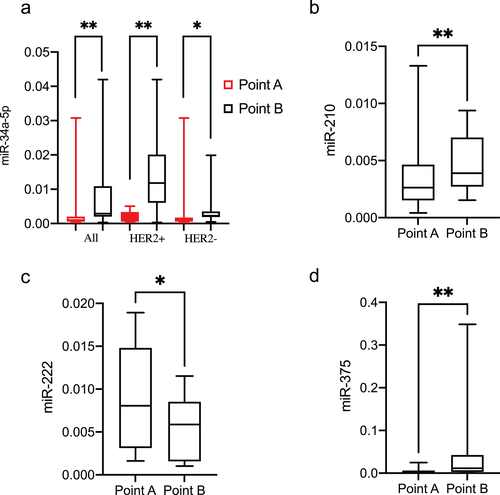
After the miRWalk procedure, 580 mRNAs of eight miRNAs were obtained (Figure S1). A total of 52 genes, targeted by at least two miRNAs, were selected for construction of a miRNA-mRNA network (Figure 3(a)). Transcription factor binding, chromatin binding, transcription regulator complex, negative regulation of cell population proliferation, and epithelial cell differentiation were the five most highly enriched items in GO analysis results (Figure 3(b)). Several known canonical cancer-associated or drug-resistance pathways were predicted by KEGG, including microRNA in cancer, PI3K-Akt signaling pathway, Hippo signaling pathway, MAPK signaling pathway, etc. (Figure 3(c)).
DISCUSSION
Several recent studies have reported that the circulating miRNAs were related to NAC effects; however, to date the conclusions have been controversial. The biological characteristics of BC to a great extent were affected by molecular subtypes, and a number of differentially expressed miRNAs have been identified among different subgroups.40 Hence, it is necessary to investigate the predictive effect of circulating miRNA expression on NAC response in specific subsets. In the present study, we used a dynamic method, in which the expression and change of miRNAs over time were correlated to clinical and pathological response and survival of luminal B BCs. Given the heterogeneity of this subtype, we also analyzed and did find some disparities between different HER2 statuses.
As for the relationship between miRNAs and NAC response, we used multiple response grading systems including clinical, pathological, and comprehensive evaluation. Consistent with published reports, our study showed the pCR rate was low in luminal B BC with only 18.9%, which may be the main reason of disagreement of miRNAs' predictive efficacy between different standards. Our analysis revealed miR-718, miR-4516, and miR-210 as predictors of chemo-sensitivity in luminal B cohort, especially in HER2− subgroup. Among those three miRNAs, the correlation between miR-718, miR-4516, and BC NAC effects has not been studied. In vitro study,41 miR-718 was reported might act as a tumor suppressor in BC progression and could rescue the effects of long non-coding RNAs (lncRNA) FBXL19-AS1 on BC tumorigenesis. By inference, it seems rational that high level of circulation miR-718 at baseline was associated with pCR in luminal B HER2− group. However, as for clinical assessment in whole patients, level of miR-718 in responders was the contrary, which is most likely resulted from including different HER2 status. Moreover, unlike miR-4516 and other miRNAs, changes of miR-718 during NAC were not found to be associated with response. miR-4516 was another relative new tumor suppressor miRNA.42 Kim et al.43 found that miR-4516 suppressed the proliferation of TNBC cells by targeting FOSL1, which suggests that miR-4516 can be used as an anticancer drug for TNBC. In our luminal B HER2− patients, lower level of miR-4516 at baseline and further increased during NAC predicted the response. Especially in dynamics analysis, upregulation of miR-4516 in the responders was consistent among different standards, except for pCR, supporting this miRNA as early markers of response to NAC. As for miR-210, several studies20, 21, 24, 44 have investigated the relationship between this miRNA and BC NAC treatment outcome mostly in HER2 positive patients, but with opposite results. Contrary to our results, which indicated miR-210 upregulation at baseline was related with pathological response; Jung et al.20 found miR-210 expression at baseline was significantly higher in HER2+ patients with residual disease than in those achieving pCR. In our study, NAC also led to further increase of miR-210 in insensitive patients, independent of assessment standards, implying that miR-210 might be involved in the mechanism underpinning resistance to anthracyclines/taxanes. The other studies21, 24, 44 showed no significant differences in miR-210 expression between responders and non-responders. The role of miR-210 in NAC remains controversial and subtype-related disparity needs further research. Apart from the above three miRNAs, miR-125b-5p was another luminal B HER2− specific miRNA in our study. Lower level of miR-125b-5p at point B and markedly decreased expression during NAC predicted poor clinical response. In TNBC or HER2+ BC patients, previous studies22, 24 showed miR-125b-5p was not associated with NAC clinical and pathological response. However, some other studies,12, 26, 45 in which BC subtype was not classified, found that miR-125b-5p was exhibiting higher expression level in non-responsive patients. Only Kassem et al.17 detected miR-125b-5p expression levels were higher in responsive patients like us, but the result was not significant.
In the luminal B HER2+ cohort of our study, the rate of responders was 76.9% and 38.5% based on pathological response (MP) and pathological response (pCR), respectively. Maybe this is the reason why we could not consistently identify chemo-induced decrease in miR-222 in the sensitive patients. In addition, previous studies focusing on the association between miR-222 and NAC response were mostly according to RECIST criteria. Among them, Rodriguez-Martinez et al.46 and Ritter et al.25 failed to detect the association, but Chen et al.44 found miR-222 expression levels were evidently higher in ineffective group comparing to effective group after NAC, and baseline miR-222 overexpression was considered as one of predictive markers of response to NAC in hormone receptor positive and HER2− BC.23 The present results showed that the changes of let-7g levels reflected a dynamic process in luminal B HER2+ breast cancer, and therefore, could be used to monitor pCR after NAC and surgery. There were no more luminal B HER2+ specific miRNAs determined.
Across luminal B and its two subgroups, we consistently identified chemo-induced increase in miR-34a-5p in the sensitive patients except who reached pCR. However, in a study14 which included 25 BC patients receiving NAC and achieving pathological partial response or pCR, results showed upregulation of both plasma and tumor miR-34a after chemotherapy. This disagreement might result from the low pCR rates of luminal B BC. Previous data47 indicated upregulated miR-34a-5p could inhibit the expression of Notch1 and increases sensitivity of acquired Adriamycin resistance MCF-7 cells to Adriamycin, which could be one of the underlying molecular mechanisms. Meanwhile, negative12, 17, 24, 44, 48 and opposite27 results also exist, so this preliminary speculation needs verification in future research. As for miR-375, consistent changes after NAC were not observed across the different subgroups in our results. However, low level of miR-375 at different time points and markedly increased expression during NAC might be associated with good response in luminal B HER2− patients. To date, few studies focused on this miRNA. In 68 luminal A BC patients who received neoadjuvant chemotherapy with epirubicin plus paclitaxel, miR-375 did not predict the chemosensitivity. Therefore, the expression and potential mechanism of miR-375 in BC are still controversial.49-51
Another major finding of our study was the association between the change of miR-125b-5p and DFS by adjustments for Neo-Bioscore. Neo-Bioscore systems incorporate aspects of tumor biology into staging and have been valuable for predicting the survival of patients after NAC.34 Our research did show that only Neo-Bioscore was significantly related to DFS. Even NAC responses based on different assessments were unable to discriminate the prognosis of the patients, which shows from a side view that pCR is not a good endpoint for luminal B BC. Liu et al.26 has demonstrated that HER2− patients with lower serum miR-125b-5p expression at baseline and during the NAC had favorable DFS, but they failed to observe this relationship in HER2+ patients.24 Another study48 reported that the OS and DFS of patients with residual invasive tumor were better for those demonstrating high miR-125b-5p. All these earlier studies did not find that the change of miR-125b-5p was related to prognosis. Our results showed patients with decreased levels of miR-125b-5p during NAC had a better DFS than those with increased levels, and the underlying mechanism needs further studies.
The major limitations of present study should also be highlighted. Because of the limited size and number of events, the power of analysis was compromised. Because of the limitation of candidate miRNA strategy, we cannot reveal the differentiation expressed miRNAs comprehensively. However, an important strength is we focused on luminal B BC, a specific and less studied subtype of BC, and separate exploration and analysis were carried out according to different HER2 status, minimizing the confounding effect of disease biology. Moreover, in consideration of poor reproducibility between previous studies, different categorization of chemotherapy response was used to assess the agreement of miRNAs' predictive efficacy. Further studies in larger series of patients with luminal B BC are necessary to determine whether those miRNAs are specific biomarkers of chemo-sensitivity and prognosis and in vitro or in vivo studies should be performed to elucidate the molecular function.
ACKNOWLEDGMENTS
This study was supported by grants from the National Key R and D Program of China (no. 2016YFC0904900), National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (no. 2018ZX09201014, no. 2017ZX09101001, and no. 2017ZX09304028), National Natural Science Foundation of China (no. 81872940, 81973395 and 82073935) and Beijing Natural Science Foundation (no. 7171012).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.