Effects of mediastinal lymph node dissection in colorectal cancer-related pulmonary metastasectomy
Funding information: National Research Foundation of Korea, Grant/Award Number: the Ministry of Education / 2019R1I1A1A01055513
Abstract
Background
The benefits of mediastinal lymph node dissection (MLND) in colorectal cancer-related pulmonary metastasectomy (PM) have been poorly reported. This study aimed to determine whether MLND affects survival in patients undergoing PM and to identify the prognostic factors for survival.
Methods
We retrospectively reviewed 275 patients who had undergone colorectal cancer-related PM from January 2010 to December 2016. MLND was defined as the resection of at least six mediastinal lymph node stations according to the International Association for the Study of Lung Cancer criteria (N1, ≥3 stations; N2, ≥3 stations). The propensity score matching method was used to reduce bias.
Results
Thirty-three (12%) patients underwent MLND, and 13 (4.7%) patients had mediastinal lymph node involvement. This study showed no difference in 5-year overall survival (no MLND, 52.7% vs. MLND, 53.5%; p = 0.81). On multivariable analysis, negative prognostic factors for overall survival were preoperative carcinoembryonic antigen (CEA) level (p < 0.001), a higher number of metastatic nodules (p < 0.001), metastatic nodule size ≥2 cm (p < 0.001), and lymph node involvement (p = 0.006).
Conclusions
Mediastinal lymph node involvement, preoperative CEA level, higher metastatic nodule number, and nodule size negatively affected survival whereas MLND in PM was not associated with survival.
INTRODUCTION
As the most common cancer of the gastrointestinal tract, colorectal cancer (CRC) is the third most common malignancy worldwide and the third most common cancer-related cause of death.1 Approximately 50% of all patients develop distant metastasis, either as synchronous metastasis diagnosed at the time of initial cancer detection or as metachronous metastasis diagnosed in the follow-up period.2The liver and lung have been reported to be the most common distant metastasis sites (35% and 10%–20%, respectively).2, 3 However, developments in chemotherapy and targeted therapy have prolonged overall survival after surgery. In selected patients, pulmonary metastasectomy (PM) is considered a treatment option providing a survival advantage. Pulmonary resection was first reported in 1882.4 Despite a paucity of randomized clinical trial data, untreated patients with metastatic disease have a 5-year survival rate of <5%,5 whereas resection of isolated pulmonary metastasis can increase the 5-year survival rate up to >50% in selected patients.6
According to previous studies, many adverse prognostic factors such as numbers of metastatic lesions or size of metastatic nodules have been suggested, and mediastinal lymph node (MLN) involvement of CRC cells has also been suggested as a negative prognostic factor of survival after PM.7 However, mediastinal lymph node dissection (MLND) has not been performed routinely at the time of CRC-related PM, and little is known about the survival benefits of MLND combined with PM. This study aimed to investigate whether MLND affects overall survival (OS) and disease-free survival (DFS) in patients undergoing PM due to CRC and to identify predicting factors for OS in these patients.
METHODS
Patients
We retrospectively analyzed data from a prospective database at a single institution concerning 275 patients who had undergone CRC-related PM between January 2010 and December 2016. This study was approved by the Severance Hospital Institutional Review Board (4-2020-1348). Sex, age, CRC location, CRC staging (tumor-node-metastasis, American Joint Committee on Cancer seventh edition), disease-free interval (DFI), lung metastasis (location, number, and size), type of pulmonary resection (wedge resection, segmentectomy, lobectomy) and operative approach (video-assisted thoracoscopic surgery [VATS] or thoracotomy), lymph node status, number of dissected mediastinal lymph nodes, postoperative recurrence, OS, and DFS were investigated on the basis of medical records. In patients with multiple metastases, size was defined as the diameter of the largest nodule.
Preoperative work-up
Preoperative evaluation included clinical examination findings, blood test results, and thoracic and abdominal computed tomography (CT) scans. Staging procedures included chest, abdominal, and pelvic CT scans in all patients. Magnetic resonance imaging of the liver was performed in patients with suspected liver metastases. Positron emission tomography-CT had not been routinely undertaken due to medical insurance issues. The chest, abdominal, and pelvic CT, tumor marker; CEA, and physical examination were performed on all patients to detect extrathoracic disease. Pulmonary function tests had been performed preoperatively for all patients. Serum carcinoembryonic antigen (CEA) levels had been obtained routinely prior to PM. The cutoff value for the CEA level was 5, according to the normal range. All patients had signed an informed consent for surgery and for the inclusion of their clinical data in the database.
Surgical indications and definition of mediastinal lymph node dissection
Indications for surgery were based on Thomford criteria,8 as follows: (i) controlled or complete resection of the primary tumor, (ii) absence of extra-thoracic disease or extra-thoracic localizations assessed as amenable to local therapies, (iii) completely resectable lung metastasis, and; (iv) operatively tolerable cardiopulmonary reserve. PM was performed for patients with combined liver metastasis that could be controlled using local treatment. All decisions concerning PM were determined at a multidisciplinary meeting.
MLND was defined as the resection of at least six stations mediastinal lymph nodes, according to the International Association for the Study of Lung Cancer (IASLC) criteria (≥3 stations at N1 and ≥3 stations at N2).9 MLND was performed according to surgeon preference.
Follow-up
Patients were reviewed at an outpatient clinic 8 weeks post-PM, then at 6 month intervals thereafter. Chest CT scans were obtained every 6 months to monitor the disease course. Follow-up data were obtained from clinical records and correspondence from attending physicians. Time to death was defined as the time from surgery until death from any cause. All patients alive at the last follow-up were censored.
Statistical analysis
Propensity scores which were calculated from logistic regression models included the following variables: age, sex, location of primary cancer, preoperative CEA level, DFI, location of lesion, maximum metastatic nodule size, the number of metastatic nodules, initial stage of CRC, operative approach, and type of pulmonary resection, to represent the probability of being assigned to either a no-MLND or an MLND group. Patients in the no-MLND and MLND groups were matched in a 1:1 ratio, according to their propensity score, using the Matchlt method.10 In total, 33 matched patients from the no-MLND group and 33 matched patients from the MLND group were included in the analysis. The characteristics of both groups were compared before and after propensity score matching (PSM). Chi-square or Fisher's exact tests were used to compare categoric variables between the two groups, and continuous variables were analyzed using a Student's t-test. Continuous variables are expressed as means ± standard deviation and categorical data are expressed as frequencies and percentages. Survival curves were estimated using the Kaplan–Meier method with 95% confidence intervals (CIs). A log-rank test was used to assess statistical significance. A stepwise multivariable Cox proportional hazards model was used among statistically significant variables (p < 0.05) in the univariate model. Multivariable analysis was performed using a Cox proportional hazards regression model to evaluate the effects of multiple variables on survival. A p-value <0.05 indicated statistical significance. Statistical analyses were performed using SPSS version 25.0 (SPSS Inc.) software.
RESULTS
Patient baseline characteristics
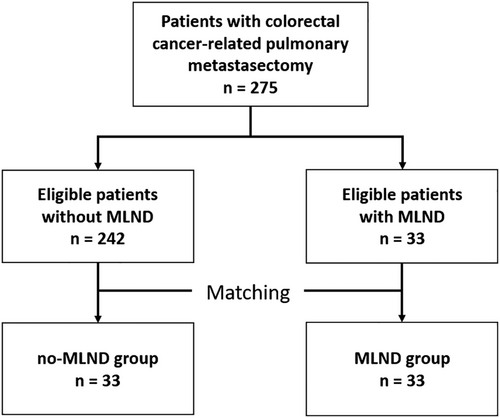
As shown in Figure 1, 275 patients were enrolled in this study. According to our criteria, 242 and 33 patients were classified into no-MLND and MLND groups, respectively. Median follow-up was 53.1 months (interquartile range [IQR] 38.2–80.0). Patient baseline clinical characteristics before PSM are shown in Table 1. The mean age was 60.9 ± 11.6 years (males, n = 165 [60%]); 220 (80%) patients had unilateral lung metastasis, and; the median number of metastatic nodules was 2 (range, 1–17). There were 176 (64.0%), 52 (18.9%), and 47 (17.1%) patients who had undergone CRC-related PM for lung metastasis for 1, 2, or ≥3 metastatic nodules, respectively. The mean size of the largest metastatic nodule was 1.4 ± 0.9 cm. There were 13 patients with a pathological diagnosis of MLN involvement. Nine patients were diagnosed with MLN involvement in preoperative imaging. Of 13 patients with a pathological diagnosis of MLN involvement after surgery, only five patients had been diagnosed with MLN involvement in preoperative imaging. MLN involvement had been confirmed in only 38.5% patients in preoperative imaging.
| Variables | Total n = 275 | MLND n = 33 | Before matching | After matching | ||
|---|---|---|---|---|---|---|
| No-MLND n = 242 | p-value | No-MLND n = 33 | p-value | |||
| Age | 60.9 ± 11.6 | 59.7 ± 12.1 | 61.0 ± 11.5 | 0.5 | 60.4 ± 12.6 | 0.8 |
| Sex | 0.9 | 0.6 | ||||
| Male | 165 (60%) | 19 (58%) | 146 (60%) | 17 (52%) | ||
| Female | 110 (40%) | 14 (42%) | 96 (40%) | 16 (48%) | ||
| Location of primary cancer | 0.2 | 0.8 | ||||
| Colon | 127 (46%) | 11 (33%) | 116 (48%) | 13 (39%) | ||
| Rectum | 148 (54%) | 22 (67%) | 126 (52%) | 20 (61%) | ||
| Preoperative CEA >5 (normal level) | 99 (36%) | 10 (30%) | 89 (37%) | 0.6 | 13 (39%) | 0.6 |
| Disease-free interval (months) | 22.6 ± 24.7 | 24.8 ± 17.0 | 22.4 ± 25.6 | 0.5 | 18.6 ± 17.3 | 0.2 |
| Direction | 1.0 | 0.8 | ||||
| Unilateral | 220 (80%) | 26 (79%) | 194 (80%) | 28 (85%) | ||
| Bilateral | 55 (20%) | 7 (21%) | 48 (20%) | 5 (15%) | ||
| Maximal nodule diameter (cm) | 1.4 ± 0.9 | 2.0 ± 1.1 | 1.3 ± 0.9 | 0.003 | 1.6 ± 0.9 | 0.1 |
| Maximal nodule diameter | <0.001 | 0.6 | ||||
| <2 cm | 228 (83%) | 19 (58%) | 209 (86%) | 22 (67%) | ||
| ≥2 cm | 47 (17%) | 14 (42%) | 33 (14%) | 11 (33%) | ||
| Number of metastatic nodule(s) | 0.9 | 0.4 | ||||
| 1 | 176 (64%) | 21 (64%) | 155 (64%) | 16 (49%) | ||
| 2 | 52 (19%) | 6 (18%) | 46 (19%) | 10 (30%) | ||
| ≥3 | 47 (17%) | 6 (18%) | 41 (17%) | 7 (21%) | ||
| Initial stage of colorectal cancer | 0.1 | 0.6 | ||||
| 1 | 17 (6%) | 2 (6%) | 15 (6%) | 3 (9%) | ||
| 2 | 52 (19%) | 9 (27%) | 43 (18%) | 6 (18%) | ||
| 3 | 102 (37%) | 16 (49%) | 86 (36%) | 14 (42%) | ||
| 4 | 102 (37%) | 6 (18%) | 96 (40%) | 10 (30%) | ||
| No information | 2 (1%) | - | 2 (0.8%) | |||
| Operative approach | 0.8 | 1.0 | ||||
| VATS | 249 (91%) | 29 (88%) | 220 (81%) | 30 (91%) | ||
| Open | 26 (9%) | 4 (12%) | 22 (9%) | 3 (9%) | ||
| Type of pulmonary resection | <0.001 | 0.3 | ||||
| Wedge resection | 216 (79%) | 5 (15%) | 211 (87%) | 8 (24%) | ||
| Segmentectomy | 19 (7%) | 5 (15%) | 14 (6%) | 8 (24%) | ||
| Lobectomy | 40 (14%) | 23 (70%) | 17 (7%) | 17 (52%) | ||
| Mediastinal lymph node involvement | 13 (5%) | 7 (21%) | 6 (3%) | <0.001 | 1 (3%) | 0.1 |
- Abbreviations: CEA, carcinoembryonic antigen; LNs, lymph nodes; VATS, video-assisted thoracoscopic surgery.
Propensity score matching
Prior to PSM, no differences were found in terms of primary cancer location, metastatic lesion location, DFI, metastatic nodule number, initial stage of CRC, and operative approach between the two groups. Patients in the MLND group were more likely to undergo lobectomy (p < 0.001) for a larger metastatic nodule (p < 0.001) than those in the no-MLND group. Differences between the two groups in terms of baseline characteristics could affect survival; therefore, the PSM method was used to reduce bias through minimizing the difference in covariates between the two groups.
After PSM, patients in both groups were matched in a 1:1 ratio according to their propensity score. Thirty-six matched patients from the no-MLND group and 36 patients from the MLND group were included in the final analysis. The distribution of clinical parameters included in the PSM is shown in Table 1. After minimizing differences in covariates between the two groups, factors concerning maximal pulmonary nodule size and type of pulmonary resection were well-balanced between the two groups after PSM.
Patient survival outcomes
In total, recurrence of metastasis post-PM occurred in 167 (60.7%) patients (Table 2). Pulmonary recurrence was observed in 91 (33.0%) patients, and 24 (8.7%) patients developed liver metastasis. Lymph node (LN) recurrence was observed in 11 (6.6%) patients; nine in the abdominal regional, one in the hilar region, and one in the supraclavicular fossa.
| Total n = 275 | After matching | ||
|---|---|---|---|
| MLND n = 33 | No-MLND n = 33 | ||
| Recurrence | 167 (60.7%) | 21 (63.6%) | 19 (57.6%) |
| Lung | 91 (54.5%) | 8 (24.2%) | 11 (33.3%) |
| Liver | 24 (14.4%) | 3 (9.1%) | 3 (9.1%) |
| Multiple | 19 (11.4%) | 5 (15.2%) | 2 (6.1%) |
| LN | 11 (6.6%) | 1 (3.0%) | |
| Local | 8 (4.8%) | ||
| Brain | 7 (4.2%) | 3 (9.1%) | 1 (3.0%) |
| Bone | 3 (1.8) | 2 (6.1%) | |
| Other | 4 (2.8%) | 1 (3.0%) | |
- Abbreviations: LN, lymph node; MLND, mediastinal lymph node metastasis.
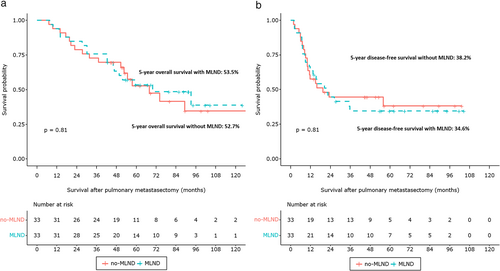
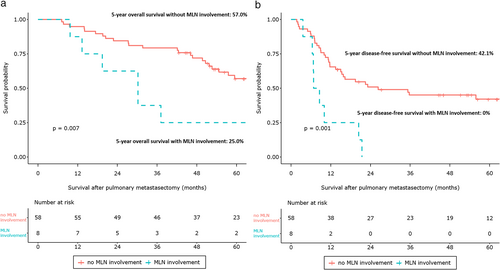
In total, 57.0% of patients had a 5-year OS and 36.8% of patients had a 5-year DFS. There was no significant difference in 5-year OS (no-MLND, 57.4% vs. MLND, 53.5%; p = 0.68) and 5-year DFS (no-MLND, 37.4% vs. MLND, 34.6%; p = 0.8) between the no-MLND and MLND groups (Figure S1). Moreover, MLN involvements were associated with inferior 5-year OS (no MLN involvement, 58.7% vs. MLN involvement, 23.1%; p < 0.001) and 5-year DFS (no MLN involvement, 38.6% vs. MLN involvement, 7.7%; p < 0.001, Figure S2). Similar results were obtained after PSM. Patient OS and DFS curves after matching the no-MLND and MLND groups are shown in Figure 2. There was no significant difference in 5-year OS between the two groups (no-MLND, 52.7% vs. MLND, 53.5%; p = 0.81). MLND had no significant association with 5-year DFS (no-MLND, 34.6% vs. MLND, 38.2%; p = 0.81). After PSM, MLN involvements were associated with poorer 5-year OS (no MLN involvement, 57.0% vs. MLN involvement, 25.0%; p = 0.007) and 5-year DFS (no MLN involvement, 42.1% vs. MLN involvement 0%; p = 0.001, Figure 3).
Risk factors for survival after pulmonary metastasectomy
In multivariable analysis, preoperative CEA level (hazard ratio [HR] 2.325, 95% CI: 1.642–3.294; p < 0.001), the number of metastatic nodules ≥3 (HR 3.003, 95% CI: 1.967–4.584; p < 0.001), the size of metastatic nodules ≥2 cm (HR 2.347, 95% CI: 1.530–3.599; p < 0.001), and mediastinal lymph node involvement (HR 2.432, 95% CI: 1.285–4.601; p = 0.006) remained independent prognostic factors (Table 3).
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | |
| Age | 1.010 | 0.995–1.026 | 0.2 | |||
| Male (vs. female) | 1.129 | 0.790–1.613 | 0.5 | |||
| Location of primary cancer | 0.890 | 0.630–1.257 | 0.5 | |||
| Preoperative CEA > 5 (normal range) | 2.241 | 1.586–3.167 | 0.000 | 2.325 | 1.642–3.294 | <0.001 |
| Disease-free interval (months) | 0.988 | 0.979–0.997 | 0.011 | 0.993 | 0.984–1.002 | 0.1 |
| Bilateral (vs. unilateral) | 2.044 | 1.388–3.009 | <0.001 | 1.083 | 0.637–1.840 | 0.8 |
| Maximal nodule size ≥2 cm (vs. <2 cm) | 2135 | 1.431–3.184 | <0.001 | 2.347 | 1.530–3.599 | <0.001 |
| Open (vs. VATS) | 3.254 | 2.052–5.162 | <0.001 | 1.435 | 0.828–2.488 | 0.2 |
| Type of pulmonary resection | ||||||
| Wedge resection | 1 | |||||
| Segmentectomy | 0.833 | 0.387–1.792 | 0.6 | |||
| Lobectomy | 1.190 | 0.744–1.904 | 0.5 | |||
| Number of metastatic nodules | ||||||
| 1 | 1 | |||||
| 2 | 1.367 | 0.872–2.144 | 0.2 | 1.373 | 0.874–2.158 | 0.2 |
| ≥3 | 2.650 | 1.749–4.015 | <0.001 | 3.003 | 1.967–4.584 | <0.001 |
| Lymph node dissection | 1.170 | 0.719–1.903 | 0.5 | |||
| Initial stage of colorectal cancer | 1 | |||||
| 1 | 1 | |||||
| 2 | 0.826 | 0.363–1.878 | 0.6 | |||
| 3 | 0.990 | 0.468–2.095 | 1.0 | |||
| 4 | 1.118 | 0.531–2.356 | 0.8 | |||
| Mediastinal lymph node involvement | 3.242 | 1.785–5.891 | 0.000 | 2.432 | 1.285–4.601 | 0.006 |
- Abbreviations: CI, confidence interval; HR, hazards ratio; CEA, carcinoembryonic antigen; VATS, video-assisted thoracoscopic surgery.
DISCUSSION
PM is a generally accepted treatment for selected patients with CRC-related pulmonary metastasis based on retrospective studies.6, 11, 12 In many institutions, PM has been regarded as routine practice; however, only one randomized controlled trial has been undertaken concerning the effects of PM, viz., the PulMiCC trial, which closed prematurely due to recruitment difficulties.13 The results of that study indicated that patients who had undergone surgical intervention had better survival outcomes than those who had been actively monitored.9 Criteria for PM were first proposed by Thomford et al. in 1965.8 Thoracic surgeons have accepted these criteria as general indications for surgery, with the basic principle of surgery being to remove all possible lesions while retaining the maximum lung tissue when operating for lung metastasis. Enucleation is the most ideal surgical method for lung reservation and in the case of multiple, metachronous pulmonary metastasis.14 However, with the development of VATS surgery, wedge resection is usually performed. Lobectomy has mainly been used for patients with central tumors, large tumors, or multiple nodules within the same lobe.15 Several retrospective studies have reported survival rates after surgical treatment of lung metastasis due to CRC, and reported risk factors have included the number and size of metastases, bilateral location, a short DFI, high preoperative CEA, and MLN involvement.11, 16, 17 In our study, preoperative CEA level, ≥3 metastatic nodules, metastatic nodules ≥2 cm, and MLN involvement were identified as risk factors concerning OS for CRC-related PM, as in previous studies.
Controversies remain concerning MLND during PM, which has not routinely been accepted as general practice because MLND can result in adhesion around the hilum, making repeated resection potentially challenging. Moreover, metastasis to the lung implies systemic disease. However, in surgery for CRC-related lung metastasis, MLND has occasionally been performed alongside PM. During anatomic resection, such as segmentectomy or lobectomy, regional lymph nodes could be unintentionally removed. However, when performing wedge resection, systematic MLND is intentionally performed. Therefore, we adopted the IASLC definition of MLND in this study, despite that definition relating specifically to lung cancer surgery, if systematic lymph node dissection had been performed. If MLN involvement was confirmed on MLND, obtaining an accurate prognostic prediction post-PM might be possible. According to previous studies, MLN involvement is an important negative prognostic factor in patients undergoing metastasectomy regardless of histology.6, 18 Some studies have reported MLN involvement in CRC as a relative contraindication to surgery.19 The sensitivity of detecting MLN involvement before PM in positron-emission tomography-CT and CT has been reported as only 35% and 25%, respectively.18 Therefore, the advantage of MLND is that an accurate prognosis post-PM appears to be obtainable through identifying MLN involvement, which is otherwise difficult to obtain using preoperative imaging studies. However, the therapeutic effect remains unclear. Therefore, we investigated whether performing MLND improved the survival rate. Survival rates were compared according to whether MLND had been undertaken. Based on our study results, performing further MLNDs to improve survival during PM appear unnecessary even though MLND can be helpful to determine prognosis in terms of MLN involvement.
Our study results are in agreement with those of previous studies that have investigated the effects of MLND on PM. Shiono et al.,15 Londero et al.20 not only analyzed CRC but included various types of primary cancer. Different types of primary cancer have differing clinical outcomes and mediastinal lymph node metastases, and such differences affect survival. Hamaji et al.18 only studied patients with CRC, but the two groups involved in their study differed in terms of surgical extent, such as wedge resection or lobectomy. Because differences in surgical extent also affect survival, we performed PSM including factors such as maximal metastatic nodule size, number of metastatic nodules, and type of pulmonary resection to minimize the difference between the two groups with and without MLND, to ensure a more accurate assessment of the effects of MLND.
This study had several limitations. First, this was a retrospective, single-center study with relatively small patient numbers, especially in terms of those who had received MLND. There was inevitably a small number of patients in the MLND group because mediastinal lymphadenectomy was not the standard treatment for pulmonary metastasectomy. In addition, the analyses of retrospective study were exploratory in nature. p-values may not be interpreted as confirmatory but rather descriptive. Second, MLND was performed mainly in accordance with surgeon preference. Finally, the criterion for MLND was adopted from criteria in relation to lung cancer. Despite these limitations, this study is likely to more clearly clarify the implications of MLND for survival through addressing potential between-group imbalances using PSM in patients with CRC-related pulmonary metastasis. Considering patient recruitment challenges in the PulMiCC trial, a randomized trial for MLND in PM might be difficult; therefore, a further and larger retrospective study may be helpful.
In conclusion, our retrospective study findings indicated that MLND during PM for CRC did not improve OS. Mediastinal lymph node involvement, preoperative CEA level, a higher metastatic nodule number, and a metastatic lesion ≥2 cm in size are poor prognostic factors of survival.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (2019R1I1A1A01055513). This study was conducted in accordance with the Declaration of Helsinki and was approved by the Severance Hospital Institutional Review Board (approval no: 4-2020-1348). The requirement for acquisition of informed consent from patients was waived because of the retrospective design of this study and anonymized data.
CONFLICT OF INTEREST
The authors declare no conflicts of interest for this article.




