Hexokinases II-mediated glycolysis governs susceptibility to crizotinib in ALK-positive non-small cell lung cancer
Funding information: Advanced Technology research project and the National Nature Science Foundation of China, Grant/Award Numbers: 81672284, 81802293; Chongqing Basic Science and Advanced Technology Research Program, Grant/Award Number: cstc2015jcyjBX0136
Abstract
Background
Activation of ALK leads to a high level of aerobic glycolysis related to crizotinib insensitivity in anaplastic lymphoma kinase-positive non-small cell lung cancer (ALK+ NSCLC). The strategy and mechanism of glycolysis inhibition in sensitizing ALK+ NSCLC cells to crizotinib requires further investigation.
Methods
The levels of glycolysis in H3122 and H2228 cells were evaluated through detection of glucose consumption and lactate production. MTT assay was used to explore the effects of glycolytic inhibitors on crizotinib sensitivity, and the potential mechanism of action were detected by colony formation, Ki67 incorporation assay, transwell assay, small interfering RNA technology and western blot analysis.
Results
ALK+ NSCLC cells exhibited significantly higher levels of glycolysis compared to ALK− NSCLC cells. Long-term exposure to crizotinib could decrease the sensitivity of ALK+ NSCLC cells to crizotinib via increasing the levels of glycolysis related to hexokinases II (HK2). Crizotinib in combination with glycolysis inhibitor 2-deoxy-D-glucose (2DG) synergistically inhibited proliferation, glycolysis, colony formation and invasion ability of ALK+ NSCLC cells. 2DG sensitization crizotinib might be associated with the inhibition of HK2-mediated glycolysis and P-ALK/AKT/mTOR signaling pathway in H3122 and H2228 cells.
Conclusions
These results indicate that HK2-mediated glycolysis plays a crucial role in the increased tolerance of ALK+ NSCLC cells to crizotinib. 2DG may sensitize ALK+ NSCLC to crizotinib via suppression of HK2-mediated glycolysis and the AKT/mTOR signaling pathway.
INTRODUCTION
Echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusion oncogene results in ALK activation and is a driving factor of lung tumorigenesis, which has been found in approximately 3%–7% of lung adenocarcinoma cases.1 ALK-positive non-small cell lung cancer is sensitive to multitarget tyrosine kinase inhibitor (TKI) crizotinib, approved by the Food and Drug Administration (FDA) in 2011.2, 3 To date, crizotinib remains a viable treatment option with good response and tolerable toxicity for patients with ALK+ NSCLC.4 However, most patients inevitably develop resistance to crizotinib after approximately one year, and drug resistance greatly limits the clinical application of crizotinib.5
ALK rearrangement and ALK activating mutations, which result in activation of ALK, are the most common resistance mechanisms to crizotinib. Several studies have reported that ALK-induced overexpression of HK2 contributed to activation of ALK inducing a metabolic shift toward aerobic glycolysis, especially in EML4-ALK positive lung cancer.6-8 Abnormal activated glycolysis in ALK+ NSCLC cells is characterized by an increased glucose uptake and lactate production, known as the Warburg effect. High rates of aerobic glycolysis in cancer are a metabolic adaptation and serve as a key process for cancer cells survival, growth, spread, and favoring drug resistance.9, 10 Taken together, aerobic glycolysis inhibition could be an effective strategy in cancer therapeutics.
The glycolysis inhibitor 2-deoxy-d-glucose (2DG), an analogue of glucose only allowing cells to undergo the first enzymatic reaction of glycolysis, affects the metabolism of glycolysis rate-limiting enzymes HK2 which plays a major role in the Warburg effect and cancer cell malignancy.11, 12 Safety of 2DG in humans has been established through both the clinical and preclinical studies, certain doses of 2DG have been proven to exert selective cytotoxic effects in a variety of cancer but not in normal tissues.13-15 2DG targets glucose metabolism and may represent a potential therapy of cancer.
This study demonstrated the relationship between high levels of glycolysis and crizotinb-tolerance of treatment in ALK+ NSCLC. Furthermore, we explored the effect and mechanism of 2DG on sensitizing ALK+ NSCLC cells to crizotinib.
METHODS
Cell culture and reagents
EML4-ALK-positive NSCLC cell lines were used in this study. NCI-H2228 was purchased from American Type Culture Collection (ATCC), and H3122 was obtained from Shanghai Bioleaf Biotech Co., Ltd. EGFR-TKI-sensitive PC-9 cells were gifted from Professor J. Xu and Dr M. Liu from Guangzhou Medical University (China). The EGFR-TKI primary resistant cell H1975 and other NSCLC cell lines, NCI-H1299 /NCI-H460/HCC827, were provided by the American Type Culture Collection. Cells were maintained in RPMI-1640 medium (HyClone) supplemented with 10% fetal bovine serum (FBS, GIBCO) and 100 U/ml penicillin (HyClone), 100 mg/ml streptomycin (HyClone). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2. Crizotinib (Cri) and 2-deoxy-d-glucose (2DG) were purchased from Selleck Chemicals, dissolved in dimethyl sulfoxide and stored at −20°C.
Growth inhibition assay
Cells were cultured in 96 well plates at a density of 3000 cells/well. Varying concentrations of 2DG (0, 0.125, 0.25, 0.5, 1, 2 mM) or crizotinib (0, 0.001, 0.01, 0.1,1 and 10 μM), were added to the media, and either 0 or 0.5 mM 2DG was added to the media containing a series of concentrations of crizotinib to test the efficacy of the combination. Following 48 h of culture, cells were subjected to 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazoliumbromide (MTT, Sigma-Aldrich) assay. All assays were performed in triplicate. Data were normalized as a percentage of controls. The combination index (CI) was calculated using the CompuSyn software program (ComboSyn, Inc.), assuming 1 as the cutoff. A CI < 1 represents a synergistic effect, a CI = 1 represents an addictive effect and a CI > 1 indicated an antagonistic effect.16
Ki67 incorporation assay
Cell proliferation activity was assessed by Ki67 incorporation assay using a Ki67 labeling and detection kit (Sigma-Aldrich Co.). Cells were treated with 0.5 mM 2DG, 0.4 μM Crizotinib, or both for 48 h, followed incubation for 12 h with Ki67 (1:200 dilution) and fixation. Cell nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) and viewed with a fluorescence microscope (Leica). At least 500 cells from three independent experiments were quantified. Data are expressed as the mean value of the percentage of positive cells ± standard error of the mean (SEM).
Colony-formation assay
A colony formation assay was used to detect cells multiplication capacity. Cells were seeded into 6-well plates at a density of 800 cells/well, and two days later, cells were treated with 0.5 mM 2DG, 0.4 μM crizotinib, or both and allowed to form colonies for two weeks and fixation. Cell clones were then stained with 0.1% crystal violet for 30 min at room temperature, followed by washing with flowing water and were then allowed to dry naturally. Colonies containing over 50 cells were counted.
Transwell assay
Cell invasion was measured using transwell chambers (8 μm pore size; Costar) with matrigel (BD Biosciences). Cell suspensions (2 × 105 cells) prepared in serum-free medium were added into the upper chamber of the transwell. The matched lower chamber contained 15% FBS medium. After incubation for 48 h, chambers with cells were fixed in formaldehyde and stained with 0.1% crystal violet for 30 min. Cells remaining on the upper chamber were removed with cotton tips, while those that had migrated onto the lower surface of the membrane were preserved, and counted under a microscope in five random fields at 200 × magnification.
Glucose consumption and lactate production analysis
Metabolites were quantified in freshly clarified cell supernatant and control medium. Glucose and lactate levels in culture supernatants were detected by a Glucose Assay Kit I and Lactate Assay Kit I (Eton Bioscience Inc.). Glycolytic rate is presented as relative glucose consumption and relative lactate production over 24 h per 106 cells.
Western blotting
Lysates from cells were prepared in RIPA buffer (Thermo Scientific) supplemented with protease/phosphatase inhibitors (Roche Diagnostics). Protein concentrations were determined by BCA kit (Millipore). Antibodies specific to ALK, phosphorylated ALK(Y1096), mTOR, phosphorylated mTOR (Ser2448), AKT, phosphorylated AKT (Thr308 and Ser473), p70S6K, phosphorylated p70S6K (Thr389), S6 phosphorylated S6 (Ser240/244) and HK2 were purchased from Cell Signaling Technology. GAPDH antibody was obtained from Bioss. Secondary antibodies (HRP-conjugated goat-anti-rabbit IgG) were from Thermo Fisher Scientific. Thirty micrograms of protein per lane were electrophoresed for the western blot analysis. The concentration of acrylamide / Bis in the gels varied and was dependent on the size of proteins being detected. A DAB HRP substrate kit (Vector) was used to visualize the signal.
Small interfering RNA assays
Small interfering RNA (siRNA) for human HK2 was obtained from GenePharma. SiRNA targeting the HK2coding region was as follows: sense, 5′-GGGCGGAUGUGUAUCAAUAdTdT-3′ and antisense,
5′-UAUUGAUACACAUCCGCCCdTdT-3′. HK2 siRNA and its negative control were each gently mixed with lipofectamine RNAi MAX (Invitrogen) in RPMI-1640 medium without FBS, penicillin or streptomycin, to a final concentration of 50 nM for 15 min at room temperature. Then, mixtures were added into each well of H2228 cells in 6-well plates. Six hours after transfection, cells were incubated with fresh complete medium for another 48 h before protein analysis and cell viability assay.
Plasmid transfection assays
HK2 expression (oe-HK2) plasmid was obtained from Shanghai Genechem Co., Ltd. H3122 cells were transiently transfected with this plasmid (0.015 μg/μl) and negative control (NC) plasmids (0.015 μg/μl) using lipofectamine 2000 transfection reagent (Invitrogen), according to the manufacturer's protocol.
RNA extraction and RT-qPCR
Total RNA from cells was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), RT-qPCR was performed using Quantinova SYBR Green PCR Kit (cat. no. 208054; Qiagen) on an CFX ConnectTM Real-Time System (Bio-Rad, Inc.), as previously described.17 Gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the relative expression level was calculated using the 2 − ΔΔCq method. The primer sequences used in the experiment were as follows: HK2 forward, 5′-CGCTCAACGACATTCGCACT-3′ and reverse, 5′-TTCACCAGGATAAGCCTCACC-3′.
Statistical analysis
Data are expressed as the mean ± SEM. SPSS 17.0 statistical software (SPSS) was applied for statistical analysis, and differences between groups were analyzed using one-way analysis of variance (ANOVA). A p-value <0.05 was considered statistically significant.
RESULTS
ALK+ NSCLC cells have higher glycolysis levels than ALK− NSCLC cells
According to previous studies,6, 7 ALK+ NSCLC cells exhibit high rates of glycolysis, and the glucose consumption and lactate production were detected in a series of NSCLC cells. The results showed higher levels of glycolysis (Figure 1(a), (b)) and higher expression of HK2 in ALK+ NSCLC cells than ALK− NSCLC cells (Figure 1(c), (d)).
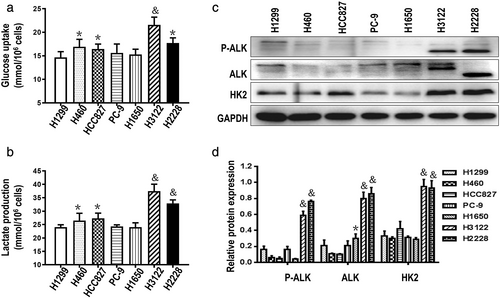
Sensitivity to crizotinib decreased and glycolysis level increased successively with increasing treatment duration of crizotinib in ALK+ NSCLC cells
To explore effects of glycolysis levels on crizotinib sensitivity in ALK+ NSCLC cells, H3122 and H2228 cells were treated respectively with crizotinib at the concentration of 0.2 μM and 0.4 μM18 for 1, 2, 3, and 4 months. After 4 months of crizotinib treatment, the sensitivity of H3122 and H2228 cells to crizotinib markedly decreased (Figure 2a,b), concurrently, relative glucose consumption and lactate production exhibited gradually increased in H3122 and H2228 cells (Figure 2c,d). As shown in Figure 2e,f, P-ALK expression was steadily downregulated, no obvious change in total ALK protein. While HK2 expression presented initial downregulation and followed recovery of high expression with prolongation of the time of crizotinib treatment in H2228 cells. Together all, increase of glycolysis levels and crizotinib tolerance could have a bearing on up-regulation of HK2 in ALK+ NSCLC cells with increasing treatment duration of crizotinib.
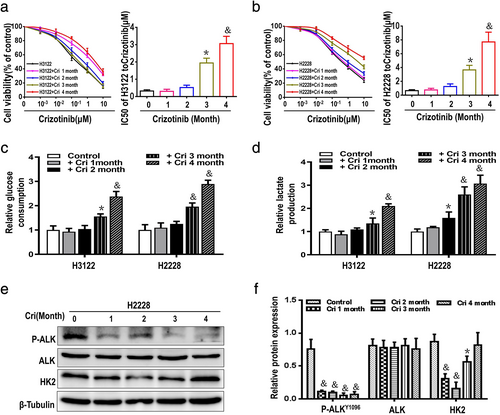
Elevated HK2 expression in ALK+ NSCLC cells plays a central role in reducing crizotinib-sensitivity
HK2 is a first rate-limiting enzyme in glycolysis, and we used small interfering RNA to HK2 in H3122 and H2228 cells. Compared to the negative control group, treatment with HK2-specific siRNA did not affect the expression of total ALK and phosphorylation of ALK (Figure 3(a), (b)), but enhanced crizotinib-induced cytotoxicity and markedly decreased the levels of glycolysis in H3122 and H2228 cells (Figure 3(c), (d)). In contrast, compared with the vector group, upregulation of HK2 in H3122 and H2228 cells transfected with HK2 overexpressed plasmids increased crizotinib-tolerance, but there was no evident changes in the expression of P-ALK/ALK protein (Figure 3(e)–(h)). Taken together, these results clearly indicated that HK2 protein plays an important role in regulating glycolysis and crizotinib-tolerance in ALK+ NSCLC cells.
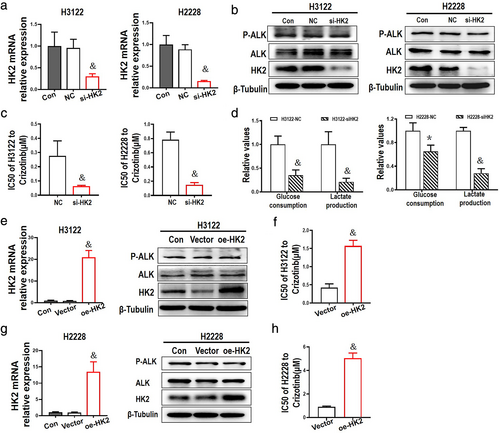
2DG synergistically enhanced crizotinib-induced cytotoxicity in ALK+ NSCLC cells
2DG, as an inhibitor of HK2, was chosen to inhibit the levels of glycolysis in ALK+ NSCLC cells. First, the cytotoxicity of 2DG was examined in ALK+ NSCLC cell lines H3122 and H2228, and a dose-dependent effect and sluggish cell growth inhibition of 2DG are shown in Figures 4(a) and (b). Following a dose–response treatment with 2DG for 48 h, the glucose consumption and lactate production clearly abated (Figure 4(c)), with a dramatic decrease in expression of HK2 induced by 2DG at concentrations of 0.5 mM or higher. There was also an influence on the expression of P-ALK, but no significant change in expression of ALK in H2228 cells (Figure 4(d), (e)).
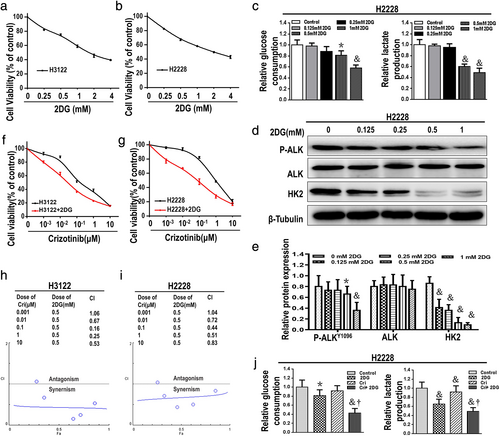
H3122 and H2228 cells were then treated with various doses of crizotinib and/or 2DG at a fixed dose concentration of 0.5 mM, followed by cell viability assessment with an MTT assay. The combination index (CI) values were less than 1 (CI < 1), with the exception of the combination of 2DG (0.5 mM) and crizoitinib (0.001 μM) in both H3122 and H2228 cells (Figure 4(f)–(i)). The levels of glycolysis showed a clear decrease in H2228 cells after 48 h of treatment with crizotinib and 2DG (Figure 4(j)). We were therefore able to confirm the synergistic interactions between 2DG and crizotinib.
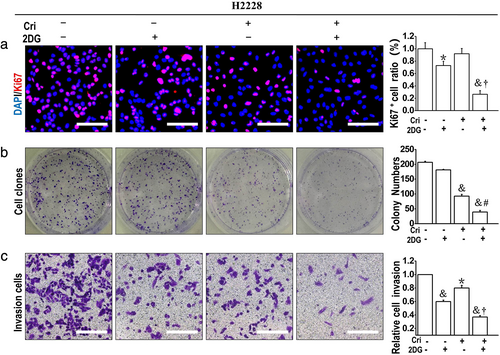
2DG augmented crizotinib-induced inhibitory effects on cell proliferation and invasion in ALK+ NSCLC cells
Then, we examined the effect of crizotinib and/or 2DG on proliferation activity by Ki67 incorporation assays and colony formation assays. Compared to the untreated and crizotinib treated groups respectively, the combination of crizotinib and 2DG significantly suppressed proliferation activity and colony formation, whereas the inhibitory effect of 2DG alone was not obvious (Figure 5(a), (b)). In addition, crizotinib or 2DG significantly inhibited the cell migration ability, and combined use of 2DG and crizotinib further augmented this effect (Figure 5(c)). These suggest that combination of crizotinib and 2DG synergistically suppresses the high proliferative activity and reverses aggressive phenotype of H2228 cells, compared to crizotinib alone.
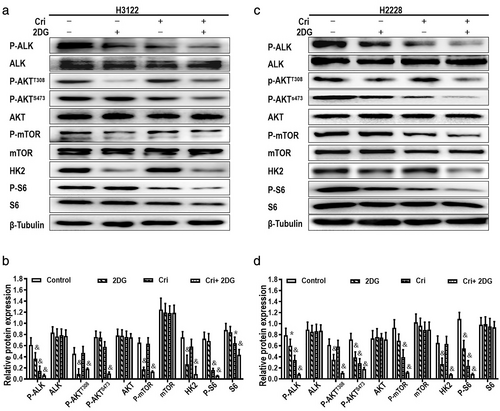
Combination of crizotinib and 2DG had a suppressive effect enhanced on AKT/mTOR signaling pathway in ALK+ NSCLC cells
Western blot analysis was used to further illustrate that the influence of a combination of 2DG and crizotinib on the signaling pathways are associated with glycolysis and downstream of ALK in H3122 cells (Figure 6(a), (b)) and H2228 cells (Figure 6(c), (d)). As shown in the results, 2DG or crizotinib alone partially decreased the phosphorylation of ALK; however, the levels of P-ALK, P-AKT, HK2, mTOR and S6 were obviously decreased in the combined treatment group, while total protein expression of ALK, AKT and S6 had no evident change. The results indicated that crizotinib combined with 2DG markedly inhibited the activation of AKT/mTOR pathway in H3122 and H2228 cells.
DISCUSSION
In the present study, it was determined that ALK+ NSCLC cells exhibit high levels of glycolysis, and reappear at high glycolysis levels after long-term treatment with crizotinib in which elevated HK2 expression play a crucial role. The combination of a glycolytic inhibitor (2DG) and ALK inhibitor (crizotinib) showed synergistic antitumor activity by abrogating hyperactivation of glycolysis to inhibit cell proliferation activity, colony-formation and the invasive ability of ALK+ NSCLC cells.
Aberrant activation of glycolysis has been recognized as a hallmark of cancer. It is generally known that high levels of glycolysis not only supplies rich resources of intermediate required for cell proliferation, but also accelerates the accumulation of lactate in tumor tissue resulting in acidification of the intracellular microenvironment favored for tumor metastasis and chemoresistance.19, 20 In this study, long-term exposure to crizotinib resulted in high levels of glycolysis and drug tolerance in H3122 and H2228 cell lines. Further research showed that the expression of P-ALK was continuously downregulated, accompanied by no change in total ALK protein, but high expression of HK2 was ultimately restored which contributed to the high levels of glycolysis in H2228 cells with duration of crizotinib. Thus, high levels of glycolysis contributed to cell survival and enhanced crizotinib-tolerance in ALK+ NSCLC cells.
Glycolytic inhibitor 2DG reportedly enhances the antitumor effects of TKI and some chemotherapeutic agents. A low concentration of 2DG in combination with specific agents exerts a synergistic therapeutic effect, although 2DG alone does not induce cell death for the majority of cancer cells.21, 22 In this study, glucose consumption and lactate production in H2228 cells were decreased in response to 2DG at a concentration of 0.5 mM which had little effect on cell viability. Moreover, cell proliferation was significantly suppressed in ALK+ NSCLC cells treated with crizotinib (IC25) combined with 0.5 mM 2DG. According to the results, we speculated the following possible mechanisms.
First, the trait of high glucolysis levels in ALK+ NSCLC cells may cause them to be more sensitive to glycolysis inhibitors. For inhibiting glycolysis in cancer cells, several small molecules were examined that target critical glycolytic enzymes, glucose transporters, and monocarboxylate transporters, such as HK2, LDHA, PFK, and PKM2.21 In Figure 1(c), compared to ALK− NSCLC cells, high expression of HK2 was detected in ALK+ NSCLC cells. The results of downregulation of HK2 used by small interfering RNA assays and overexpression of HK2 through lentivirus infection experiment indicated that HK2 mediated the levels of glycolysis and sensitivity to crizotinib in ALK+ NSCLC cells. HK2 expression was effectively suppressed by 2DG alone, or in combination with crizotinib, rendering HK2 susceptible to 2DG treatment. In conclusion, HK2 may play a central role in 2DG targeting glycolysis for inhibition of ALK+ NSCLC cell proliferation.
Second, ALK-rearrangement activates the AKT/mTOR signaling pathway, which is important for the proliferation of ALK+ NSCLC cells.23, 24 In Figures 3 and 6, there were obviously different effects on the expression of P-ALK between targeting intervention of HK2 and usage of 2DG. siRNA or overexpression methods directly modulate expression of HK2 and have no effects on the expression of P-ALK/ALK. However, with the exception of inhibiting HK2 expression, 2DG, as a glucose analogue, led to the reduction of lactate production and intracellular ATP level.25 The downregulation of P-ALK expression might be due to a decrease in the ATP level caused by 2DG treatment. AKT plays a critical role in cell growth by directly or indirectly activating mTOR.26 In this study, crizotinib or low concentration of 2DG alone were ineffective at downregulating the phosphorylation of AKT and mTOR in ALK+ NSCLC cells, while the combination of crizotinib and 2DG exerted a remarkable inhibitory effect on activation of AKT and mTOR. Previous studies have demonstrated that AKT is a regulator of glycolysis or indirectly activates the rate-limiting enzyme of glycolysis, rendering cancer cell survival dependent on glycolysis.7, 27 A glycolytic enzyme, such as HK2, is upregulated by activation of AKT/mTOR signaling.28 Thorough inhibition of AKT expression by combining 2DG and crizotinib further downregulated the expression of HK2, which resulted in reducing glycolysis levels to heighten crizotinib-induced cytotoxicity in H3122 and H2228 cells.
Furthermore, H2228 cell lines display a mesenchymal phenotype.29, 30 High levels of glycolysis are conducive to the malignant phenotype.31 2DG treatment apparently delays metastasis of osteosarcoma cells.32 The combination of 2DG and crizotinib suppressed H2228 cells invasion ability more effectively than crizotinib alone (Figure 5(c)). This suggests that treatment with glycolysis inhibitor and crizotinib may efficiently inhibit the invasiveness of ALK+ NSCLC cells.
In conclusion, ALK+ NSCLC cells present with enhanced glucolysis, targeting cancer cells glycolysis using glycolysis inhibitor, is a viable strategy that could sensitize the cells to first-line ALK-TKI crizotinib. Crizotinib combined with 2DG synergistically inhibit the proliferation of ALK+ NSCLC cells by depriving its abnormally hyperactivated levels of glycolysis, effectively downregulating the expression of HK2 and inhibiting the activation of ALK/AKT/mTOR signaling pathway in ALK+ NSCLC cells. However, a combination of glycolysis inhibitor and crizotinib may provide an effective strategy for improving the clinical outcomes of advanced ALK+ NSCLC patients, but the feasibility of this approach requires further confirmatory studies.
ACKNOWLEDGMENTS
This work was supported by Advanced Technology research project and the National Nature Science Foundation of China (81672284 and 81802293) and the Chongqing Basic Science and Advanced Technology Research Program (cstc2015jcyjBX0136).
CONFLICT OF INTEREST
No potential conflict of interest of this study was reported.




