Low-grade fibromyxoid sarcoma arising from the lung: A case report
Funding information: Kyushu University
Abstract
Low-grade fibromyxoid sarcoma (LGFMS) is a rare sarcoma subtype that most commonly arises in young adults. This tumor typically presents in the deep soft tissues of the proximal extremities or trunk as a painless mass. Although the most common site of LGFMS metastasis is the lung, it is rarely the primary site. Here, we report a case of primary pulmonary LGFMS. A 22-year-old asymptomatic man was referred to our hospital for investigation of a lung mass that had been discovered incidentally. Computed tomography (CT) showed a well-defined mass 4.0 cm in diameter in the upper lobe of the right lung. Malignancy was suggested by focal uptake of 18F-fluorodeoxyglucose positron-emission tomography (18-FDG-PET). Following surgery, postoperative histological analysis of the resected specimen demonstrated LGFMS based on histological and immunohistological findings. In particular, mucin 4 showed diffuse positivity in the spindle-shaped tumor cells. In conclusion, LGFMS can arise in the lungs, and physicians should consider this entity as a differential diagnosis for solitary lung mass in young adults.
INTRODUCTION
Low-grade fibromyxoid sarcoma (LGFMS) is a rare sarcoma first described by Evans in 1987.1 This tumor mainly arises in the proximal extremities and trunk as a painless mass in young adults. On histological examination, the tumor comprises slender, spindle-shaped cells with fibrous and myxoid areas. This tumor rarely presents in unusual locations such as the retroperitoneum, abdomen, head or neck and mediastinum.2 Primary pulmonary LGFMS is extremely rare. To the best of our knowledge after a search of the literature, only two cases of primary pulmonary LGFMS have been reported previously.3, 4 Due to its low nuclear grade and infrequent mitotic activity, systemic chemotherapy or radiation appears ineffective for LGFMS.5 Complete surgical resection is thus the standard treatment.3, 6 The heterogeneous histological appearance makes diagnosis challenging, but mucin 4 (MUC4) is a highly sensitive and specific immunohistological marker for LGFMS that could prove useful for a definitive diagnosis.
CASE REPORT
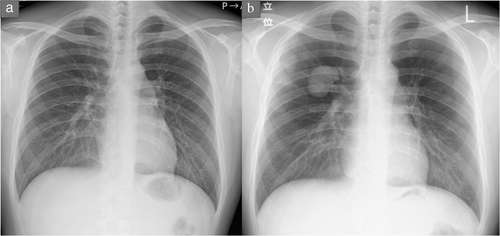
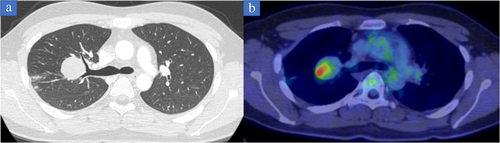
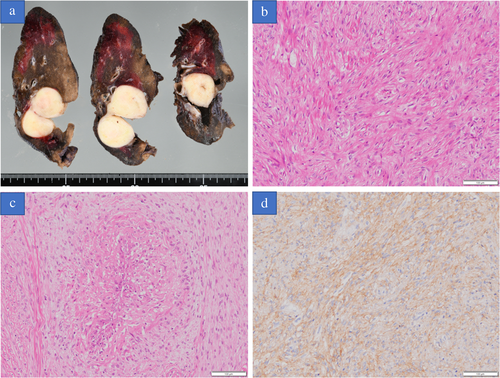
A 22-year-old asymptomatic man was referred to Iwate Medical University Hospital for detailed investigation of a lung mass discovered during a medical check. Chest X-ray showed a mass shadow in the right upper lung (Figure 1(b)). Computed tomography showed a 4.0 cm mass with peripheral atelectasis in the upper lobe of the right lung (Figure 2(a)). Preoperative 18F-fluorodeoxyglucose (18-FDG) positron emission tomography (PET) demonstrated focal uptake of FDG, with a maximum standardized uptake value of 5.59 in the lung mass (Figure 2(b)). These examinations excluded extrathoracic malignancies. As compared with the chest X-ray findings obtained 1.5 years previously, the mass demonstrated a rapid growth and a malignant lung tumor was strongly suspected (Figure 1(a)). Accordingly, to treat and make an exact diagnosis, thoracoscopic right upper lobectomy with mediastinal lymph node dissection was performed. Grossly, the tumor was well circumscribed and measured 4.5 × 4.0 × 3.0 cm. The cut surface was opalescent and solid (Figure 3(a)). Histological examination revealed bland-appearing spindle-shaped cells with a fibrous area (Figure 3(b)) and rosettes (Figure 3(c)). Immunohistologically, tumor cells were positive for bcl-2. No immunoreactivity was seen for antibodies to EMA, AE1/AE3, CD34 and negative staining was obtained for STAT-6, which ruled out a solitary fibrous tumor. These microscopic findings and diffuse positivity for MUC4 were compatible with LGFMS (Figure 3(d)), but no evidence of fused in sarcoma (FUS) translocation was seen from fluorescence in situ hybridization.
DISCUSSION
LGFMS was first described as a painless mass in soft tissue around the scapular and axillary-chest areas by Evans in 1987.1 This rare sarcoma mainly involves the deep soft tissues of the proximal extremities and trunk but occasionally arises in unusual areas. Chamberlain et al. reviewed previous cases regarding the location of LGFMS.2 Most tumors originated in the extremities (45.1%) or pelvis (18.6%), followed by the chest wall (13.7%), retroperitoneum (8.8%), abdomen (7.8%) and head or neck (5.9%). Despite the histologically benign appearance, LGFMS occasionally recurs and mainly metastasizes to the lung.1 Indeed, only two cases of primary pulmonary LGFMS have been reported previously.3, 4 The lung is thus extremely rare as a primary site for LGFMS, so metastasis from an extrathoracic site should to be ruled out. In the present case, preoperative imaging showed no evidence of any extrathoracic masses and the patient had no medical history of operations at other sites, so primary pulmonary LGFMS was diagnosed in the same way as previously reported cases.3, 4
Histologically, LGFMS comprises spindle-shaped cells with fibrous and myxoid areas. The heterogeneous histological findings make LGFMS difficult to distinguish from other sarcomas and benign tumors, and confirmation of the diagnosis by an expert, experienced soft-tissue pathologist is recommended.2 For this reason, preoperative diagnosis of primary pulmonary sarcoma such as LGFMS may be challenging. Porte et al. reported that preoperative tissue samples led to the correct pathological diagnosis in only 39% of primary pulmonary sarcoma patients.7 Therefore surgical resection in preference to preoperative pathological diagnosis may be reasonable in patients with primary pulmonary sarcomas. In LGFMS, MUC4 is positive in most cases and has been reported as a specific marker for this pathological entity. Doyle et al. reported that all 49 resected LGFMS cases showed positivity for MUC4 (100%).8 On the other hand, this tumor is genetically described by chromosomal translocations involving the FUS gene, most commonly as FUS-CREB3L2 (75%–95%), and FUS-CREB3L1 (5%–10%).2, 9, 10 In the present case, immunohistology showed diffuse positivity of spindle-shaped cells for MUC4 but no evidence of FUS translocation. These results suggest that MUC4 can help physicians establish the correct diagnosis in cases of LGFMS with FUS wild type.
Complete resection is the standard treatment strategy, similar to other primary pulmonary sarcomas.7, 11-13 On the other hand, conventional systematic therapy has limited efficacy in LGFMS. However, National Cooperative Sarcoma Groups are conducting a clinical trial on combination trabectedin and radiotherapy for some types of sarcomas including LGFMS (TRASTS study).14 These treatment modalities other than surgery warrant further investigation. To date, local and distance metastases have been reported after resection of LGFMS. The local recurrence rate has been reported as 1%–9%, and the metastasis rate as 6%–27% at a median follow-up of 24–60 months.2, 5, 6, 8 Interestingly, in Evans et al. report of 33 LGFMS cases with a median follow-up of 14 years, 50% of patients had metastasis and 42% died of the disease.15 These findings suggest a need for decades-long follow-up even when complete resection of LGFMS is performed, due to the potential for late recurrence and metastasis.
In conclusion, primary pulmonary sarcoma such as LGFMS should be listed as a differential diagnosis for solitary pulmonary nodules in young adults. To reach a definitive diagnosis, FUS gene translocation is useful; however, MUC4 is the most specific marker and useful in FUS wild type.
ACKNOWLEDGMENTS
We are grateful to Professor Yoshinao Oda (Kyushu University) for pathological advice on the diagnosis.
CONFLICT OF INTEREST
The authors report no conflicts of interest.




