Clinical impact of rebiopsy among patients with epidermal growth factor receptor-mutant lung adenocarcinoma in a real-world clinical setting
Abstract
Background
In this study, we investigated the risk factors of acquired T790M mutation among patients with lung adenocarcinoma with epidermal growth factor receptor (EGFR) tyrosine mutation who were treated with EGFR-tyrosine kinase inhibitors (TKIs). The aim was to identify the clinical impact of rebiopsy.
Methods
This multicenter, retrospective cohort study was conducted in South Korea from January 2007 to June 2017. Patients with adenocarcinoma with EGFR mutation who underwent rebiopsy and were treated with EGFR-TKIs were included.
Results
Of a total of 352 patients, T790M mutation was identified in 156 (41.9%) at the time of rebiopsy. The median duration from initial biopsy to rebiopsy was 17 months. Univariate logistic regression analysis revealed associations of exon 19 deletion (odds ratio [OR], 1.643; p = 0.026), absence of L858R (OR, 0.627; p = 0.042), and previous EGFR-TKI treatment duration (OR, 1.039; p < 0.001) with T790M mutation. Previous EGFR-TKI treatment duration (OR, 3.580; p < 0.001) was independently associated with T790M mutation. A multivariate Cox proportional hazard model revealed that brain metastasis at initial diagnosis (hazard ratio, 1.390; p = 0.050) tended to be associated with T790M mutation. Among the patients with T790M mutation at rebiopsy, the osimertinib user group (n = 90) had a better one-year survival (68.7 vs. 58.3%, p = 0.048) than the osimertinib nonuser group (n = 66).
Conclusions
Rebiopsy might affect the clinical course of patients with EGFR-mutant adenocarcinoma who receive EGFR-TKIs.
INTRODUCTION
Lung cancer is still the leading cause of cancer-related death worldwide.1 According to the histological classification, non-small cell lung cancer (NSCLC) accounts for over 80% of lung cancer cases,2 and adenocarcinoma is the most common subtype worldwide.3 Although immunotherapy, such as immune checkpoint inhibitors (ICIs), has recently been highlighted,4 epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are recommended as a first-line therapy for patients with advanced adenocarcinoma with EGFR mutation.5, 6
Unfortunately, acquired resistance occurs in the majority of patients treated with EGFR-TKIs, with various resistance mechanisms.7-9 A point mutation in exon 20 (T790M mutation) accounts for over half of the acquired resistance to EGFR-TKIs,10, 11 which has led to the development of osimertinib, a third-generation EGFR-TKI.12 Thus, it is important to identify T790M mutations in patients who are suspected to have acquired resistance to EGFR-TKIs in a clinical setting. There is growing evidence regarding repeat biopsy.13-15 However, in many previous studies, the number of patients was relatively small, or the main focus was on feasibility or diagnostic accuracy. In addition, there is a lack of data on the prevalence and clinical characteristics of acquired T790M mutation in a real-world setting.
Here, we investigated the risk factors of acquired T790M mutation among patients with lung adenocarcinoma with EGFR mutation who were treated with EGFR-TKIs. The intent was to identify the clinical impact of rebiopsy in a real-world clinical setting.
METHODS
Population cohort
This multicenter, retrospective cohort study involved “the lung cancer patients who were EGFR mutation positive in Korea” cohort. Data were gathered from The Korean Academy of Tuberculosis and Respiratory Disease. From January 2007 to June 2017, adenocarcinoma patients with EGFR mutation who underwent rebiopsy during the follow-up period and were treated with EGFR-TKIs were included in this study. Among the eligible patients, those who did not undergo an EGFR mutation test, had an interval of more than one month between biopsy and EGFR detection, or whose information about the EGFR mutation test was unknown, were excluded. Patients who initially had T790M mutation were also excluded (Figure 1).
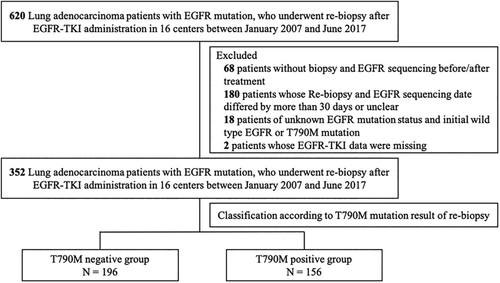
A standardized protocol was used to collect data regarding age, gender, smoking history, clinical stage at the time of initial diagnosis, brain metastasis, repeat biopsy performed during the follow-up period, and treatment information regarding conventional chemotherapy and EGFR-TKIs. The repeat biopsy information included the date and site of biopsy, histological diagnosis, timing correlation between biopsy and EGFR-TKI treatment, EGFR mutation status, and its detection method. The previous TKI treatment duration was set as the sum from the start date to the end date of each TKI administration. The study protocol was approved by the Institutional Review Board of Asan Medical Center (approval number: 2018-0092). Requirements for written informed consent were waived due to the retrospective nature of the present study and the use of anonymized patient data.
Evaluation of EGFR mutations
The peptide nucleic acid (PNA)-mediated PCR clamping method is the gold standard for the detection of EGFR mutations. The method uses formalin-fixed paraffin-embedded tumor tissue obtained via tissue biopsy. Previously, direct sequencing (DS) methods were commonly used to compare amplified sequences with GenBank EGFR through PCR. Recently, Mutyper methods have been used, in which circulating-free tumor DNA (ctDNA) is detected in plasma after collecting blood into an EDTA anticoagulation bottle. As this cohort was recruited from multiple institutions, various EGFR mutation detection techniques were used. They included direct sequencing and the aforementioned PNA clamping method and Mutyper as mentioned above. We used the PNAClamp EGFR, PANAqPCR, and PANAMutyper EGFR mutation detection kits (all from Panagene) to detect the various EGFR mutations.
Statistical analysis
All values are expressed as median and interquartile range (IQR) for continuous variables or percentages for categorical variables. Student's t-test or the Mann-Whitney U test was used to examine continuous data. A chi-square test or Fisher's exact test was used to examine the categorical data. Logistic regression and Cox regression analysis were performed to identify the factors affecting T790M mutation. Furthermore, variables with p < 0.2 in the univariate analysis were entered into the multivariate models. Survival analysis was performed using Kaplan-Meier analysis and log-rank test. IBM SPSS statistics v.20 (IBM, Armonk) was used for analyses. A statistically significant difference was defined as a p-value < 0.05.
RESULTS
Patient characteristics
A total of 352 patients with adenocarcinoma and EGFR mutations were included in this study. The median follow-up period was 22 months (interquartile range [IQR]: 15–36 months). The baseline characteristics of patients are summarized in Table 1. The median age was 58 years (IQR: 52–68 years); 34.9% patients were male and 29.0% were ever-smokers. Most patients (90.9%) initially had stage IV adenocarcinoma and the lungs were the most common initial biopsy site (67.5%). Regarding EGFR mutation, exon 19 deletion was the most common (60.8%), followed by L858R (34.7%), L861Q (5.4%), and G719X (2.3%). A total of 80.6% of patients were treated with an EGFR-TKI. They initially included gefitinib, erlotinib, or afatinib. Approximately 13.4% received cytotoxic chemotherapy.
| Characteristics | Total |
|---|---|
| Number (n) | 352 |
| Median age, years | 58 [52–68] |
| Male | 130 (34.9) |
| Ever-smoker | 108 (29.0) |
| Initial clinical stage | |
| I | 1 (0.3) |
| II | 2 (0.5) |
| III | 6 (1.6) |
| IV | 338 (90.9) |
| Initial biopsy site | |
| Lungs | 251 (67.5) |
| Lymph nodes | 69 (18.5) |
| Pleural effusion | 13 (3.5) |
| Othersa | 19 (5.1) |
| Initial EGFR mutation | |
| Exon 19 del | 214 (60.8) |
| L858R | 122 (34.7) |
| L861Q | 19 (5.4) |
| G719X | 8 (2.3) |
| Othersb | 11 (3.1) |
| Initial chemotherapy regimen | |
| Cytotoxic chemotherapy | 50 (13.4) |
| EGFR-TKI | 300 (80.6) |
| Gefitinib | 200 (53.8) |
| Erlotinib | 42 (11.3) |
| Afatinib | 57 (15.3) |
- Note: Data are presented as mean ± standard deviation, median [interquartile range], or number (%), unless otherwise indicated.
- Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
- a Others: Initial biopsy site was the brain in eight cases, the bronchus in two cases, pleura in two cases, liver in two cases, bone in two cases, the pericardial fluid in one case, pericardium in one case, and adrenal gland in one case.
- b Others: Four cases were E746_A750del, one case each presented p.D770_n771insG, I706T, exon 20 insertion, E746_T751del, E709G, codon 770, and 745aa.
Acquired T790M mutation
All eligible patients underwent repeat biopsy during the follow-up period. The median duration from initial biopsy to rebiopsy was 17 months (IQR 10–29 months). T790M mutation was identified in 156 (41.9%) patients at the time of rebiopsy. The comparison of characteristics according to the T790M mutation status at the time of rebiopsy is shown in Table 2. There were no significant differences in demographics, brain metastasis, rebiopsy site, and initial TKI treatment between the two groups. However, patients with T790M mutation had a longer duration between initial biopsy to rebiopsy (median, 19 vs. 16 months, p = 0.008) compared to the patients without T790M mutation. According to the initial EGFR profile, exon 19 deletion was more frequent in patients with T790M mutation (67.3 vs. 55.6%, p = 0.028), whereas L858R was less frequent in the T790M mutation group (28.8 vs. 39.3%, p = 0.043).
| Characteristics | Total | T790M positive | T790M negative | p-value |
|---|---|---|---|---|
| Patient numbers | 352 | 156 (41.9) | 196 (52.7) | |
| Median age, years | 58 [52–68] | 58 [50–68] | 59 [52–69] | 0.318 |
| Male | 130 (36.9) | 59 (37.8) | 71 (36.2) | 0.758 |
| Ever-smoker | 108 (30.7) | 49 (31.4) | 59 (30.1) | 0.384 |
| Duration from initial biopsy to rebiopsy [median] | 17 [10–29] | 19 [13–30] | 16 [9–28] | 0.008 |
| Brain metastasis | 126 (35.8) | 57 (36.5) | 69 (35.2) | 0.952 |
| Rebiopsy site | 0.369 | |||
| Lungs | 178 (50.6) | 75 (48.1) | 103 (52.6) | |
| Lymph nodes | 75 (21.3) | 32 (20.5) | 43 (21.9) | |
| BAL | 5 (1.4) | 5 (3.2) | 0 (0) | |
| Pleural fluid | 26 (7.4) | 12 (7.7) | 14 (7.1) | |
| Othersa | 68 (19.3) | 32 (20.5) | 36 (18.4) | |
| Initial EGFR profile | ||||
| Exon 19 del | 214 (60.8) | 105 (67.3) | 109 (55.6) | 0.028 |
| L858R | 122 (34.7) | 45 (28.8) | 77 (39.3) | 0.043 |
| L861Q | 19 (5.4) | 7 (4.5) | 12 (6.1) | 0.637 |
| G719X | 8 (2.3) | 2 (1.3) | 6 (3.1) | 0.309 |
| Othersb | 11 (3.1) | 5 (3.2) | 6 (3.1) | >0.999 |
| Initial TKI regimen | 0.460 | |||
| First-generation | 292 (83.0) | 131 (37.2) | 161 (45.7) | |
| Second-generation | 60 (17.0) | 25 (7.1) | 35 (9.9) |
- Note: Data are presented as mean ± standard deviation, median [interquartile range], or number (%) unless otherwise indicated.
- Abbreviations: BAL, bronchoalveolar lavage; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
- a Others:20 cases at liver, nine cases at pleura, 10 cases at bone, five cases each for ascitic fluid and cerebrospinal fluid, three cases at adrenal gland, two cases each at bronchus, brain, chest wall, pericardium, and pericardial fluid, one case each at breast, blood, kidney, large intestine, nasal cavity, and skin
- b Others: Four cases were E746_A750del, and one case each presented p.D770_n771insG, I706T, exon 20 insertion, E746_T751del, E709G, codon 770, and 745aa.
Treatment information according to T790M mutation status at the time of rebiopsy is summarized in Table S1. Although there were no significant differences in number, type, and the best response to EGFR-TKIs between the two groups, patients with T790M mutation had a longer EGFR-TKI treatment duration (median 15 vs. 11 months, p < 0.001) compared to patients without T790M mutation. Among the 156 patients with T790M mutation, 66 were treated with the third-generation TKI, osimertinib. In 90 patients who were not treated with osimertinib after repeat biopsy, 29 (32.2%) received conservative management, 24 (26.7%) participated in clinical trials, 18 (20%) received cytotoxic chemotherapy, and 19 (21.1%) received different types of EGFR-TKI.
Risk factors of acquired T790M mutation
To identify the risk factors of acquired T790M mutation, we performed logistic regression analysis (Table 3) and Cox regression analysis (Table 4). Univariate logistic analysis showed that the presence of exon 19 deletion (odds ratio [OR], 1.643; 95% CI: 1.061–2.545; p = 0.026) and the absence of L858R (OR, 0.627; 95% CI, 0.400–0.982; p = 0.042) were associated with T790M mutation. The previous EGFR-TKI treatment duration (OR, 1.039; 95% CI: 1.019–1.058; p < 0.001) was also associated with T790M mutation. Multivariate logistic analysis showed that previous EGFR-TKI treatment duration (OR, 3.580; 95% CI: 2.257–5.678; p < 0.001) was the only factor independently associated with T790M mutation. In addition, the presence of exon 19 deletion displayed a trend toward association with T790M mutation (OR, 1.509; 95% CI: 0.955–2.386; p = 0.078).
| Parameter | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 0.989 | 0.970–1.008 | 0.257 |
| Male | 0.934 | 0.604–1.443 | 0.758 |
| Ever-smoker | 0.241 | 0.027–2.131 | 0.201 |
| Brain metastasis | 1.039 | 0.801–1.347 | 0.772 |
| Same biopsy site as initial biopsy | 1.069 | 0.702–1.628 | 0.757 |
| Initial EGFR profile | |||
| Exon 19 del | 1.643 | 1.061–2.545 | 0.026 |
| L858R | 0.627 | 0.400–0.982 | 0.042 |
| L861Q | 0.720 | 0.277–1.875 | 0.502 |
| G719X | 0.411 | 0.082–2.066 | 0.281 |
| Other | 1.049 | 0.314–3.502 | 0.939 |
| Best response to TKI treatment (disease progression) | 0.272 | 0.076–0.971 | 0.045 |
| Previous EGFR-TKI days | 1.039 | 1.019–1.058 | < 0.001 |
| Multivariate analysis | |||
| Initial exon 19 del | 1.509 | 0.955–2.386 | 0.078 |
| Best response to TKI treatment (disease progression) | 3.089 | 0.817–11.688 | 0.097 |
| Previous EGFR-TKI days | 3.580 | 2.257–5.678 | <0.001 |
- Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
| Parameter | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.006 | 0.991–1.021 | 0.425 |
| Male | 0.933 | 0.793–1.098 | 0.401 |
| Ever-smoker | 0.550 | 0.148–2.042 | 0.372 |
| Brain metastasis | 1.439 | 1.035–2.001 | 0.030 |
| Same biopsy site as initial biopsy | 1.108 | 0.808–1.519 | 0.524 |
| Initial EGFR profile | |||
| Exon 19 del | 1.081 | 0.772–1.515 | 0.649 |
| L858R | 1.040 | 0.734–1.474 | 0.823 |
| L861Q | 1.043 | 0.488–2.230 | 0.914 |
| G719X | 0.548 | 0.135–2.214 | 0.398 |
| Other | 0.616 | 0.226–1.680 | 0.344 |
| Best response to TKI treatment (disease progression) | 0.444 | 0.141–1.394 | 0.164 |
| Previous EGFR-TKI days | 0.836 | 0.578–1.210 | 0.343 |
| Multivariable analysis | |||
| Brain metastasis | 1.390 | 0.999–1.933 | 0.050 |
- Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
The univariate Cox proportional hazard model showed that only brain metastasis at the initial biopsy was associated with an acquired T790M mutation (hazard ratio [HR], 1.439; 95% CI: 1.035–2.001; p = 0.030). In the multivariate Cox proportional hazard model, brain metastasis tended to be associated with T790M mutation. Patients with brain metastasis had a shorter duration between initial biopsy and rebiopsy (median, 15 vs. 19 months; p = 0.003) compared to patients without brain metastasis (Table S2). Patients with brain metastasis also had a shorter EGFR-TKI treatment duration (median, 12 vs. 14 months; p = 0.009) than patients without brain metastasis.
Survival analysis
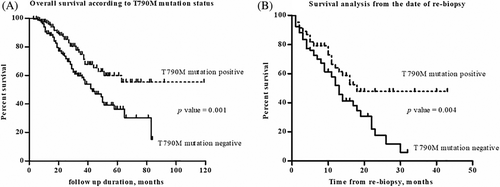
The T790M mutation positive group (n = 156) had an improved survival (median survival period: 43 months vs. not reached, p = 0.001) than the T790M mutation negative group (n = 196, Figure 2(a)). Even if the start date was the date of rebiopsy for the survival analysis, the T790M mutation positive group still had an improved survival (median survival period: 12 vs. 18 months, p = 0.004) than the T790M mutation negative group (Figure 2(b)).
Among the patients with T790M mutation at the time of rebiopsy, the osimertinib user group (n = 90) had a better one-year survival (68.7 vs. 58.3%, p = 0.048) than the osimertinib nonuser group (n = 66, Figure 3). The baseline and clinical characteristics in patients with T790M mutation treated with osimertinib are shown in Table S3. There were no significant differences in the clinical characteristics between the two groups.

DISCUSSION
In this study, acquired T790M mutation was identified at the time of rebiopsy in approximately 40% of patients with EGFR-mutant adenocarcinoma who were treated with EGFR-TKI. Exon 19 deletion, absence of L858R, previous EGFR-TKI treatment duration, and brain metastasis at initial diagnosis were associated with acquired T790M mutation. Previous EGFR-TKI treatment duration was also independently associated with acquired T790M mutation. However, the acquired T790M mutation group had improved survival compared to the T790M mutation negative group. In particular, the osimertinib user group had a better one-year survival than the osimertinib nonuser group among patients with acquired T790M mutation. These findings suggest that rebiopsy might affect the clinical course of patients with EGFR-mutant adenocarcinoma.
Acquired T790M mutation is regarded as one of the most important resistance mechanisms and clinical responses during EGFR-TKI treatment.16, 17 Among our cohort, acquired T790M mutation was identified in 41.9% of patients, which is comparable to previous studies.7, 18, 19 In a study of 37 patients with EGFR-TKI-resistant NSCLC, 18 patients (49%) showed acquired T790M mutation.7 In another study involving 57 EGFR mutation-positive NSCLC patients who had undergone repeat biopsy after failure of treatment with a first- or second-generation EGFR-TKI, acquired T790M mutation was identified in 27 patients (47%).18 A review identified T790M mutation in 51.9% of study patients suspected of having TKI resistance.19 However, in most previous studies, including this study, the frequency of acquired T790M mutation was investigated only in patients that received rebiopsy, which might induce selection bias. Further studies are needed to ascertain the exact frequency of T790M mutation among patients treated with EGFR-TKI.
Several factors are presently associated with the development of acquired T790M mutation. The presence of exon 19 deletion, absence of L858R, and previous EGFR-TKI treatment duration were associated with T790M mutation in logistic regression analysis. Brain metastasis at initial biopsy was associated with acquired T790M mutation in the Cox regression analysis. Previous studies support our results.20-23 An analysis of 25 studies involving 1,770 patients found that acquired T790M mutation (after treatment with EGFR-TKIs) was more common in patients with exon 19 deletion (53% vs. 36%, p < 0.001) than in patients with L858R mutation.20 Likewise, a study involving 111 patients with adenocarcinoma with EGFR mutation found that exon 19 deletion was independently associated with acquired T790M mutation (p = 0.003) in a multivariable analysis.21 In the present study, initial EGFR-TKI treatment (first- or second-generation TKIs) was not associated with acquired T790M mutation which is comparable with the results of a previous study.24 A study involving 233 NSCLC patients with EGFR mutation reported that the acquired resistance rate of T790M mutation was not different between EGFR-TKI types (42.9% of gefitinib users, 45.7% of erlotinib users, and 45.3% of afatinib users).24 However, other authors have previously described a higher incidence of T790M mutation in patients who received gefitinib or erlotinib (OR 7.1, p < 0.001) compared with patients who received afatinib.25 More large-scale studies are needed to reveal if there is an association between acquired T790M mutation and EGFR-TKI type. Using a logistic regression analysis, we found that previous EGFR-TKI treatment duration was independently associated with T790M mutation. Similarly, in a study involving 73 NSCLC patients treated with EGFR-TKIs, Matsuo et al.26 reported that a longer duration of treatment was independently associated with T790M mutation in a multivariate logistic analysis (≤10 vs. >10 months, OR 0.09, p < 0.001). These findings suggest that close monitoring of T790M mutation is needed, especially in long-term users of EGFR-TKIs. Brain metastasis is related to EGFR mutations in patients with lung adenocarcinoma and T790M mutation.22, 23 In a previous meta-analysis of 22 studies that included NSCLC patients with or without EGFR mutations, the presence of an EGFR mutation was associated with a markedly higher incidence of subsequent brain metastasis and an increasing trend in the incidence of initial brain metastasis.22 Another study with 212 NSCLC patients reported that T790M mutation was more common in patients with brain metastasis (n = 12/40, 30%, p < 0.01).23 Presently, the duration from initial biopsy to rebiopsy was significantly longer in patients without brain metastasis than in patients with brain metastasis (median 15 vs. 19 months, p = 0.003). Brain metastasis at initial diagnosis was associated with acquired T790M mutation. These findings reveal the importance of monitoring for acquired T790M mutation, especially in patients with brain metastasis.
Several previous studies have suggested that patients with acquired T790M mutation would have a favorable clinical course compared to patients with acquired resistance lacking the T790M mutation.16, 21, 27 In one of these studies, which involved 93 patients with EGFR-mutant lung adenocarcinoma, patients with T790M (n = 58, 62%) had better post-progression survival (median survival, 19 vs. 12 months; p = 0.036) than T790M-negative patients.16 In another study of 111 patients with EGFR-mutant lung adenocarcinoma, the acquired T790M-mutant group (n = 58, 52.3%) had a significantly longer overall survival (p = 0.010) compared to patients without T790M mutation.21 Although the exact mechanisms underlying these results are unclear, preclinical data has led to the conclusion that EGFR-mutant cell lines with acquired T790M mutation feature a more indolent growth than the parental cell lines.27 Osimertinib is an irreversible EGFR-TKI that selectively inhibits both EGFR-TKI sensitizing mutations and T790M-resistance mutations. The efficacy of osimertinib has been previously described.12, 28, 29 As a representative example, in the AURA study involving 419 patients with T790M-positive advanced NSCLC, the osimertinib group showed longer progression-free survival (10.1 vs. 4.4 months, p < 0.001) compared to patients treated with platinum therapy plus pemetrexed.29 Osimertinib treatment also showed clinical benefits in T790M-negative patients in a study involving 199 lung adenocarcinoma patients diagnosed with EGFR mutations.28 In the present study, while 42.3% of the T790M-positive group received osimertinib, it was only administered to 7.7% of the T790M negative group. This may have reflected insurance coverage in South Korea. The difference in osimertinib usage between the T790M positive and negative groups indicate that treatment might beneficially affect survival.
There were several limitations in this study. First, it was retrospective and only patients who underwent rebiopsy were included, and this may have resulted in selection bias. Second, the study included only Korean patients which might limit the generalizability of the results. However, the study was performed at several centers, and the baseline and clinical characteristics of the study patients were comparable with those in previous studies.15, 18, 19 Third, the initial biopsy and rebiopsy sites differed in 175/352 (49.7%) patients, which might influence the T790M mutation status. Fourth, data concerning other important resistance mechanisms to TKIs, such as human EGFR 2 amplification, mesenchymal-epithelial transition factor amplification, and epithelial-mesenchymal transition, were not collected during the study period.30 Finally, although we included a relatively large number of patients, the numbers were not adequate to ascertain meaningful comparisons within subgroups.
In conclusion, rebiopsy might influence the clinical course in adenocarcinoma patients with EGFR mutation who received EGFR-TKI. Further prospective studies involving larger populations with different ethnic populations are warranted.
ACKNOWLEDGMENTS
This study was supported by the Korea Academy of Tuberculosis and Respiratory diseases, Seoul, Korea, and by Panagene Korea.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.




