Autologous cytokine-induced killer (CIK) cells enhance the clinical response to PD-1 blocking antibodies in patients with advanced non-small cell lung cancer: A preliminary study
Abstract
Background
Programmed death-1 (PD-1) blocking antibodies have been shown to improve progression-free survival (PFS) and overall survival in a subset of patients with non–small cell lung cancer (NSCLC). However, the objective response rate with these agents remains low, and the vast majority of NSCLC patients require alternative combination treatment regimens to prolong their survival. The purpose of this study was to evaluate the clinical efficacy of autologous cytokine-induced killer (CIK) cell infusions combined with PD-1 blocking antibodies in patients with NSCLC.
Methods
In this preliminary study, we investigated the safety and immune function effectiveness of PD-1 blockade antibodies pembrolizumab or nivolumab administered in combination with or without autologous CIK cell infusions in 18 patients with advanced NSCLC. The peripheral blood mononuclear cells were isolated from these patients and the expression level of some cell surface molecules like PD-1 were detected using flow cytometry to reflect the effectiveness of this combination regimen.
Results
No treatment-related deaths occurred in either cohort. In comparison with the pretreatment level, CD3+CD56+CD16+ T cells were significantly increased with the combination therapy, while myeloid-derived suppressor cells were significantly increased with PD-1 blocking antibody therapy alone but not with combination therapy. Although the serum interleukin-4 level was downregulated following treatment with the combination regimen, interferon-γ levels were unchanged.
Conclusions
The purpose of this clinical study was to report the clinical efficacy and lack of exacerbated autoimmune adverse events with a combination of PD-1 blockade and CIK cell infusions in patients with advanced NSCLC, further supporting assessments of this combination in future clinical trials.
Introduction
In patients with advanced non–small cell lung cancer (NSCLC) who do not have targetable mutations but have programmed death-ligand 1 (PD-L1) expression on at least 50% of tumor cells, the PD-1 blocking antibody pembrolizumab has become a first-line treatment option as it has been found to achieve a significantly longer progression-free survival (PFS) and overall survival (OS) than platinum-based chemotherapy in a recent phase 3 trial in this group of patients.1 Nivolumab, another PD-1 blocking antibody, has also been reported to improve OS compared with docetaxel in patients with previously treated, advanced NSCLC.2, 3 In the CheckMate227 trial, the combination of nivolumab plus ipilimumab as first-line treatment for advanced NSCLC resulted in a longer duration of overall survival compared to chemotherapy but induced some significant adverse effects.4 In unselected patients, however, the proportion of NSCLC patients responding to the PD-1 blocking antibody alone has been reported to range from only 15% to 20%.3, 5
Cytokine-induced killer (CIK) cells, a nonspecific type of adoptive immunotherapy, have shown modest but encouraging results for solid tumors in clinical trials.6 Based on a previous single-institution study and a report from the International Registry on CIK cells,7 CIK cells improved the OS of patients with NSCLC.8 A previous in vitro study in lung cancer patients by our group9 showed that treatment with CIK cells before the administration of antibodies targeting PD-L1, lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1) improved the efficacy of CIK cell therapy. In a case report, we have also described a patient with NSCLC who exhibited clinical improvement following treatment with pembrolizumab plus CIK cells.10
Therefore, all previously published laboratory and clinical results suggest that the combination of CIK cells plus a PD-1 blocking antibody may be a promising treatment for NSCLC. Some clinical trials also reported this combination improved the clinical efficiency of NSCLC and renal cell carcinoma.11 We therefore conducted a retrospective study of PD-1 blocking antibodies (pembrolizumab or nivolumab) plus autologous CIK cells to assess the safety, effectiveness, and influence on immune function of this treatment in patients with advanced NSCLC.
Methods
Study design and participants
This was a retrospective study conducted at Tianjin Medical University Cancer Institute and Hospital, Tianjin, China. Eligible patients were aged 18 years or older with histologically or cytologically confirmed advanced (mainly stage IV) NSCLC and radiographic evidence of measurable disease according to the RECIST, version 1.1.12 The study protocol was approved by the Institutional Review Board of the hospital. The studies involving human participants were reviewed and approved by the Ethical Committee of Cancer Hospital of Tianjin Medical University, according to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Procedures
Patients were enrolled in this study to investigate the safety and effectiveness of PD-1 blocking antibody therapy plus CIK cell infusions versus PD-1 blocking antibody therapy alone. Treatment was continued until the occurrence of either disease progression or unacceptable toxicity. Administration of either regimen could be delayed for adverse events.
Adverse events were recorded and categorized using the Medical Dictionary for Regulatory Activities, version 18.1, and their severity was graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.0. Tumor responses were monitored using computed tomography (CT) after every two cycles of treatment until disease progression. Patients were followed for survival after discontinuing the study treatment. The median duration of follow-up was determined for all patients, and the median PFS and OS were assessed. OS was defined as the time of diagnosis until death.
Preparation of CIK cells
CIK cells were prepared as described previously.8, 13 Briefly, peripheral blood mononuclear cells (PBMCs) were collected from patients by using a Cobe Spectra Apheresis System (Caridian BCT). They were then cultured in AIM-V medium (Invitrogen) containing 50 ng/mL anti-CD3 antibody (eBioscience, San Diego, CA, USA), 100 U/mL recombinant human interleukin (IL)-1α (eBioscience), and 1000 U/mL interferon (IFN)-γ (PeproTech), at 37°C with 5% CO2 for 24 hours. Thereafter, 300 U/mL of recombinant human IL-2 (Proleukin) was added to the medium. The medium was then replaced with fresh IL-2, and the IFN-γ-containing medium was replaced every five days.
On days 13, 14, and 15, a total of 13.07 ± 1.37 × 109 CIK cells were harvested and transfused into the NSCLC patients separately. The percentage of vital CIK cells ranged from 97% to 99%. The CD3+ CD56+ subset ranged from 25% to 30% of the vital CIK cells. Phenotypic analyses of the CIK cells were performed each day before harvesting and transfusion into the patients, using the following mAbs: CD3-FITC, CD8-PE, CD314-APC (also known as anti-NKG2D), CD56-Percp-Cy5.5, and anti-DNAM-1.
All cell preparations were tested to ensure that they were free of bacterial, mycoplasma, or fungal contamination and contained <5 EU/mL endotoxin.
Flow cytometry and antibodies
PBMCs were isolated from heparinized venous blood by centrifugation on a Ficoll-Paque gradient (GE Healthcare, Uppsala, Sweden) within four hours of venipuncture. The cells were then surface stained in phosphate-buffered saline, 1% ferrous-benzoic-xylenol, and 0.01% sodium azide; fixed in 2% (wt/vol) paraformaldehyde; and analyzed by flow cytometry on a FACSCalibur (BD Biosciences). Forkhead box P3 protein (FOXP3) was stained intracellularly according to the kit's protocol.
Data analysis was conducted using FlowJo version 7.6.2 software (Tree Star, Ashland, OR, USA). The antibodies used for surface staining included the following: CD3/CD4/CD8/PD-1/CD16+C56+/CD25/CD45/Lin1/HLADR/CD33/CD11b/CD14/CD15. The antibodies used for intracellular staining included anti-human FOXP3. Myeloid-derived suppressor cells (MDSCs) were defined as Lin1–HLADR–CD33+ 11b+.
Enzyme-linked immunosorbent assays (ELISAs)
Plasma from the patients' peripheral blood samples was prepared on ice and stored at −80°C until use. ELISA kits for analyses of IFN-γ, IL-2, IL-4, IL-6, and IL-17 were all purchased from eBioscience (San Diego, California, USA).
Statistical analysis
All patients who received at least one dose of the study drugs were eligible for safety and efficacy assessments. Patient demographics and frequency of adverse events were summarized with descriptive statistics. Two-sided 95% exact confidence intervals (CIs) were calculated for the proportion of patients achieving an objective response using the Clopper-Pearson method. Estimated time-to-event endpoints were calculated via the Kaplan-Meier method, with two-sided 95% CIs for medians and rates calculated using standard methods. Prism software (GraphPad) was used to analyze all data and to determine the statistical significance of differences among treatment groups by applying an unpaired Student's t-test. P-values less than 0.05 were considered statistically significant.
Results
Patients
Between 15 May 2015, and 30 November 2016, 18 patients with advanced NSCLC were assigned to treatment with either a PD-1 blocking antibody plus CIK cells (n = 7) or a PD-1 blocking antibody alone (n = 11; Table 1) based on patient preferences. All 18 patients were treated and assessed for safety and clinical responses. The PD-L1 test was carried out with the standard 22C3 antibody (Roche) on tissues from eight patients, three patients were in the combination cohort and five patients were in the PD-1 antibody alone cohort. All other tissues for other patients were not able to be reached or not good enough to be tested. All three patients tested were PD-L1 negative in the combination cohort. Among the five patients in the PD-1 antibody alone cohort, three patients were PD-L1 negative and two patients were PD-L1 positive. One patient was PD-L1 ≥ 50%. The baseline characteristics of the patients are summarized in Table 1.
| PD-1 blocking antibody + CIK cells (n = 7) | PD-1 blocking antibody alone (n = 11) | P-value (Fisher's exact test) | |
|---|---|---|---|
| Age, years (mean ± SD) | 60.00 ± 13.66 | 57.36 ± 7.74 | 0.6060* |
| Sex | |||
| Male | 4 (57.14) | 7 (63.64) | 1.0000 |
| Female | 3 (42.86) | 4 (36.36) | |
| ECOG performance status | |||
| 0–1 | 0 (0.00) | 3 (27.27) | 0.2451 |
| >1 | 7 (100.00) | 8 (72.73) | |
| Disease stage | |||
| IIIB | 0 (0.00) | 2 (18.18) | 0.4967 |
| IV | 7 (100.00) | 9 (81.82) | |
| CNS metastases | |||
| No | 5 (71.43) | 8 (72.73) | 1.0000 |
| Yes | 2 (28.57) | 3 (27.27) | |
| Histology | |||
| Squamous | 4 (57.14) | 2 (18.18) | 0.1414 |
| Nonsquamous | 3 (42.86) | 9 (81.82) | |
| EGFR mutations | |||
| No | 6 (85.71) | 9 (81.8%) | 1.0000 |
| Yes | 1 (14.29) | 2 (18.2%) | |
| Previous surgery | |||
| No | 3 (42.86) | 7 (63.64) | 0.6305 |
| Yes | 4 (57.14) | 4 (36.36) | |
| Previous radiotherapy | |||
| No | 3 (42.86) | 8 (72.73) | 0.3322 |
| Yes | 4 (57.14) | 3 (27.27) | |
| Previous systemic therapy | |||
| Chemotherapy | 4 (50.00) | 9 (81.82) | 1.0000 |
| TKIs | 1 (12.50) | 2 (18.18) | |
| Number of previous systemic therapies | |||
| 0 | 3 (42.86) | 2 (18.18) | 0.0586 |
| 1 | 4 (57.14) | 3 (27.27) | |
| >1 | 0 (0.00) | 6 (54.55) | |
| PD-1 status | |||
| unknown | 4 (57.14) | 6 (54.55) | 0.6512 |
| <1% | 3 (42.86) | 3 (27.27) | |
| >=1% | 0 (0.00) | 2 (18.18) | |
| PD-1 blocking antibody | |||
| Pembrolizumab | 5 (71.43) | 8 (72.73) | 1.0000 |
| Nivolumab | 2 (28.57) | 3 (27.27) | |
- * t-test.
- CIK, cytokine-induced killer; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Safety
After four years follow-up, at the end of the study (August 2019), four patients (57.1%) in the combination therapy cohort (PD-1 blocking antibody + CIK cells) and all patients (100%) in the PD-1 blocking antibody therapy alone cohort had died from disease progression. The remaining three patients (42.9%) in the combination therapy cohort and no patients (0%) in the PD-1 blocking antibody therapy alone cohort remained on treatment at the time of database lock.
One patient in the combination cohort suffered from grade 3 pneumonia and grade 1 hypothyroidism, which led to a delay in the continuation of treatment. One patient with EGFR exon20 mutation in the combination cohort suffered from grade 2 exfoliative dermatitis. One patient suffered from grade 2 adrenal insufficiency and one suffered from hypothyroidism in the PD-1 blocking antibody alone cohort. No grade 4 or serious treatment-related adverse events occurred in either cohort. No treatment-related deaths had occurred at the time of analysis, and no increase in the occurrence of adverse events was evident in the combination therapy cohort in comparison with the PD-1 blocking antibody therapy alone cohort.
Clinical response
At the time of analysis, the median follow-up duration was 28.4 months in the combination therapy cohort and 18.6 months in the PD-1 blocking antibody therapy alone cohort. At data cutoff on 13 August 2019, all patients had discontinued treatment because of disease progression or death.
The objective response rate (ORR) was 42.9% (3/7) in the combination therapy cohort. 28.6% (2/7) patients in the combination cohort had a complete response (CR), and 14.3% (1/7) patient had a partial response (PR). No patient in the PD-1 blocking antibody therapy alone cohort had a CR, and only one had PR. The ORR was 9.1% (1/11) in the PD-1 blocking antibody alone cohort (Table 2; Fig 1a,b). The disease control rate (DCR; ie, CR + PR + SD) was 57.1% (4/7) in the combination therapy cohort versus 45.5% (5/11) in the PD-1 blocking antibody therapy alone cohort (P = 0.6499; Table 2).
| PD-1 blocking antibody + CIK cells (n = 7) | PD-1 blocking antibody alone (n = 11) | |
|---|---|---|
| Best overall response: | ||
| Complete response | 2 (28.57) | 0 |
| Partial response | 1 (14.29) | 1 (9.09) |
| Stable disease | 1 (14.29) | 4 (36.36) |
| Progressive disease | 3 (42.86) | 6 (54.55) |
| Disease control rate (%) | 4 (57.14) | 5 (45.45) |
| Objective response rate (%) | 3 (42.86) | 1 (9.09) |
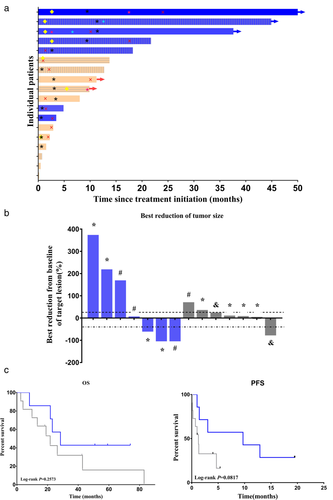
 , Complete response;
, Complete response;  , Partial response;
, Partial response;  , Stable disease;
, Stable disease;  , Progressive disease;
, Progressive disease;  , lost to follow-up;
, lost to follow-up;  , Alive;
, Alive;  , End of PD-1 treatment;
, End of PD-1 treatment;  , PDL-1 status unknow;
, PDL-1 status unknow;  , PDL-1 status < 1%;
, PDL-1 status < 1%;  , PDL-1 status > = 1;
, PDL-1 status > = 1;  , Anti-PD-1 Ab + CIK cells;
, Anti-PD-1 Ab + CIK cells;  , Anti-PD-1 Ab alone. (b) Plots of tumor regression from baseline as measured by RECIST, version 1.1. The upper dotted line represents 20% progression and the lower dotted line represents the RECIST boundary for a complete response or partial response at 30%. There were four patients in the PD-1 alone group who died quickly before the tumor response assessment
, Anti-PD-1 Ab alone. (b) Plots of tumor regression from baseline as measured by RECIST, version 1.1. The upper dotted line represents 20% progression and the lower dotted line represents the RECIST boundary for a complete response or partial response at 30%. There were four patients in the PD-1 alone group who died quickly before the tumor response assessment  , Anti-PD-1 Ab + CIK cells;
, Anti-PD-1 Ab + CIK cells;  , Anti-PD-1 Ab alone;
, Anti-PD-1 Ab alone;  , PD-1 unknown;
, PD-1 unknown;  , PD-1 <1%;
, PD-1 <1%;  , PD-1 >1%. (c) Kaplan-Meier estimate of overall survival (OS) in all patients
, PD-1 >1%. (c) Kaplan-Meier estimate of overall survival (OS) in all patients  , Anti-PD-1 Ab alone;
, Anti-PD-1 Ab alone;  , Anti-PD-1 Ab + CIK cells. (d) Kaplan-Meier estimate of progression-free survival (PFS) in all patients
, Anti-PD-1 Ab + CIK cells. (d) Kaplan-Meier estimate of progression-free survival (PFS) in all patients  , Anti-PD-1 Ab alone;
, Anti-PD-1 Ab alone;  , Anti-PD-1 Ab + CIK cells.
, Anti-PD-1 Ab + CIK cells.The median OS was 28.4 months in the PD-1 blocking antibody therapy alone cohort, whereas the median OS for the combination group was 21.7 months (Fig 1c). The median PFS was 9.7 months in the combination cohort and 1.3 months in the PD-1 blocking antibody therapy alone cohort (Fig 1d). Although survival was not significantly prolonged in the combination therapy cohort, fewer deaths occurred than in the PD-1 blocking antibody therapy alone cohort.
Two patients in the PD-1 blocking antibody therapy alone cohort were confirmed to be epidermal growth factor receptor (EGFR)-mutant and no treatment response was achieved. One of the seven patients in the combination therapy cohort was EGFR-mutant. This patient suffered from grade II exfoliative dermatitis with no clinical response.
Effects on immune function
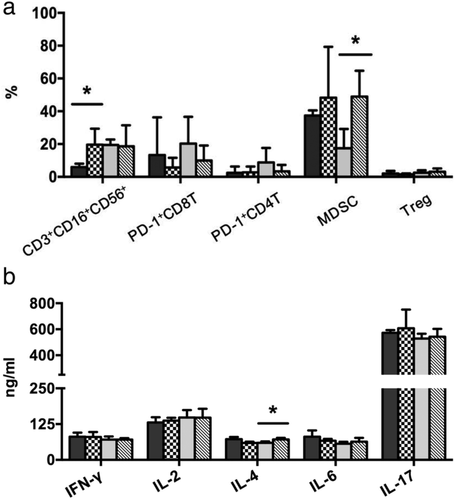
Immune profiling was conducted in patients who agreed to this testing. CD3+ CD16+ CD56+ T cells were significantly increased in the combination therapy cohort after treatment (P = 0.0339). The PD-1+CD8+ T cell level was decreased in both cohorts, while the PD-1+CD4+ T cell level was decreased in the PD-1 blocking antibody therapy alone cohort, but not in the combination therapy cohort. Interestingly, the MDSC count was significantly increased after treatment (P = 0.0184) in the PD-1 blocking antibody therapy alone cohort but not in the combination therapy cohort. The regulatory T cell count did not change significantly in either cohort (Fig 2a).
 α-PD-1 Ab + CIK (pre);
α-PD-1 Ab + CIK (pre);  α-PD-1 Ab + CIK (post);
α-PD-1 Ab + CIK (post);  CIK alone (pre);
CIK alone (pre);  CIK alone (post). (b) ELISA analyses of IFN-γ, IL-2, IL-4, IL-6, and IL-17 expression in the patient groups. Results are expressed as means ± SEM or as medians and interquartile range
CIK alone (post). (b) ELISA analyses of IFN-γ, IL-2, IL-4, IL-6, and IL-17 expression in the patient groups. Results are expressed as means ± SEM or as medians and interquartile range  α-PD-1 Ab + CIK (pre);
α-PD-1 Ab + CIK (pre);  α-PD-1 Ab + CIK (post);
α-PD-1 Ab + CIK (post);  CIK alone (pre);
CIK alone (pre);  CIK alone (post). *Indicates statistically significant differences between pre- and post-treatment levels in the patient cohorts (determined by independent samples t-tests).
CIK alone (post). *Indicates statistically significant differences between pre- and post-treatment levels in the patient cohorts (determined by independent samples t-tests).We also collected serum samples to analyze differences in the production of IFN-γ, IL-2, IL-4, IL-6, and IL-17 before and after treatment. Although the serum IL-4 level was significantly increased after treatment (P = 0.0212) in the PD-1 blocking antibody therapy alone cohort, it was slightly decreased in the combination therapy cohort. The IL-6 level was slightly increased in the PD-1 blocking antibody therapy alone cohort but slightly decreased in the combination therapy cohort, while the IL-17 level was slightly increased in both cohorts. No significant increase or decrease of IFN-γ and IL-2 level was observed in either cohort (Fig 2b).
Discussion
The antitumor activity of CIK cells is mainly associated with cluster of differentiation CD3+ CD16+CD56+ T cells.14 The PD-1 pathway serves as a checkpoint to limit T cell–mediated immune responses.15 By expressing PD-1 ligands on the cell surface and engaging PD-1 receptor-positive immune effector cells, tumors can co-opt the PD-1 pathway to evade an immune response.16 At present, however, the clinical efficacy of PD-1 blocking antibodies is still limited, and combination regimens are needed to improve patient outcomes.
The findings of the present retrospective study indicate that treatment with PD-1 blocking antibodies plus CIK cell infusions is tolerable, with no increase in the occurrence of adverse events in comparison with PD-1 blocking antibody therapy alone. The combination regimen also showed encouraging clinical activity in terms of disease control and ORRs.
Over median follow-up periods of 28.4 and 18.6 months in the two cohorts, no treatment-related deaths had occurred at the time of analysis, and no significant increases in pulmonary, gastrointestinal, or renal toxicities or discontinuations due to treatment-related adverse events were noted when CIK cell infusions were given together with PD-1 blocking antibody therapy. The proportion of patients with advanced NSCLC achieving an objective response (CR + PR) was higher in the combination therapy cohort than in the PD-1 blocking antibody alone cohort (42.9% vs. 9.1%, respectively), with no evidence of an increased occurrence of adverse events. In addition, the DCR was also higher in the combination therapy cohort than in the PD-1 blocking antibody alone cohort (57.1% vs. 45.5%, respectively), although the difference was not statistically significant (P = 0.2451). Furthermore, the combination regimen significantly increased the CD3+ CD16+ CD56+ T cell population after treatment; these cells play a central role in innate antitumor immune regulation.
A limitation of this study was the small sample size and the retrospective study but not the prospective one. Also, there were only moderate differences in the clinical response between the combination therapy and PD-1 blocking antibody therapy alone cohorts, which might be related to the short duration of follow-up. Moreover, there were two patients with EGFR mutations in the PD-1 blocking antibody alone cohort but one in the combination therapy cohort, and it is now believed that EGFR mutations are associated with low response rates to the PD-1 pathway blockade in NSCLC.17
Another limitation of this study was that the tumor PD-L1 expression level was not determined in all patients. In the recently reported phase 3 KEYNOTE-024 study,1 in which pembrolizumab was compared with platinum-based chemotherapy in patients with tumor PD-L1 expressed on at least 50% of tumor cells, a better objective response was achieved with pembrolizumab than with platinum-based chemotherapy. Randomized studies are needed to examine whether the addition of CIK cells to PD-1 blocking antibody therapy will indeed enhance the clinical benefit compared with anti-PD-1 blocking antibody therapy alone across a range of PD-L1 expression levels.
Also, in our retrospective study, we found that more patients in the PD-1 antibody alone cohort were nonsquamous cell lung cancer patients (81.8%, n = 11) with fewer in the combination cohort (42.86%, n = 7). This may also influence the clinical result.
In the patients we studied, we found no evidence of increased toxicity in the combination therapy cohort. Based on the tolerability of this regimen and its promising clinical activity, we hypothesized that the addition of CIK cells to PD-1 blockade might be important for maintaining long-term responses. After integrating observations from our previous case report in which the combination of CIK cells plus a PD-1 blocking antibody was associated with improved activity in a NSCLC patient with thrombocytopenia,10 CIK cells plus PD-1 blockade was selected for further investigation in patients with advanced NSCLC.
In patients with lung cancer, our previous in vitro study showed that transfusion of CIK cells before administration of antibodies targeting PD-L1, LAG-3, TIM-3, and CEACAM-1 may improve the efficacy of CIK cell therapy in patients with NSCLC.9 In the present study, we found that the CD3+ CD16+ CD56+ T cell count was sharply increased after treatment in the combination therapy cohort. Therefore CD3+ CD16+ CD56+ T cell-targeted therapy can be applied to any type of tumor and also to any one individual, regardless of the human leukocyte antigen type.18 In the present study, the significant increase of CD3+ CD16+ CD56+ T cells after CIK cell infusions may have played an important role in the enhanced clinical response in the combination therapy cohort. Interestingly, we found that the MDSC count was significantly increased after treatment in the PD-1 blocking antibody therapy alone cohort, but not in the combination therapy cohort. Also, the IL-4 level was significantly increased in the PD-1 blocking antibody therapy alone cohort but not in the combination therapy cohort. The main tumor regulation mechanism of MDSCs is via immune suppression, and activated MDSCs are known to secrete chemokines, cytokines, and enzymes that contribute to tumor cell invasion, proliferation, survival, adhesion, and chemoattraction, resulting in a crosstalk that can impact tumor progression, invasion, and metastasis. IL-4 has been reported to be a major cytokine produced by MDSCs in tumor microenvironments on immune evasion.19
Previous studies have reported that MDSCs may be one of the factors that contribute to resistance to anti-PD-1 treatment.20-22 Recently, Kim et al.23 reported that eradication of MDSCs in a mouse melanoma model was able to reverse resistance to anti-CTLA-4 treatment. On the basis of our results, combination therapy with CIK cells plus a PD-1 blocking antibody may increase CD3+ CD16+ CD56+ T cells and thereby reverse the resistance to the anti-PD-1 antibody treatment and enhance the clinical response in patients with NSCLC.
In conclusion, a pressing clinical challenge in NSCLC is the identification of optimal combination strategies that will increase the proportion of patients who benefit from treatment. The combination of CIK cells plus a PD-1 blocking antibody appears to be well tolerated and have promising clinical activity, including high response rates and the potential for deep and durable responses. The findings of this study may provide some evidence of the clinical benefit of CIK cells administered in combination with a PD-1 blocking antibody for patients with advanced NSCLC.
Acknowledgments
This work was supported by the National Key R&D Program (2018YFC1313400) and three grants from Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. 81702268, No. 81602926 and No. 81601492) and the National Key Technologies R&D Program (No. 2015BAI12B12). It was also supported by grants from Natural Science Foundation of Tianjin (No. 18JCYBJC93400) and CSCO-MSD research fund (Y-MSD2020-0161).
Disclosure
The authors report no conflicts of interest in this work.




