Cloning short DNA into plasmids by one-step PCR
Abstract
Background
Plasmid construction of small fragments of interest (such as insertion of small fragment marker genes, expression of shRNA, siRNA, etc) is the basis of many biomolecular experiments. Here, we describe a method to clone short DNA into vectors by polymerase chain reaction (PCR), named one-step PCR cloning. Our method uses PCR to amplify the entire circular plasmid. The PCR was performed by the primers containing the gene of short DNA with overlapping sequences between 10–15 bp. The PCR products were then transformed into E. coli and cyclized by homologous recombination in vivo.
Methods
The pEGFP-N1-HA plasmid was constructed by one-step PCR and transformation. Cells were transfected with pEGFP-N1-HA and pEGFP-N1 plasmid using TurboFect transfection reagent. Protein expression was detected by western blotting and the HA-GFP fusion protein was detected by confocal microscopy.
Results
The pEGFP-N1-HA plasmid was successfully constructed and HA expression in cells.
Conclusions
Free from the limitations of restriction enzyme sites and omitting the ligation process, our method offers a flexible and economical option of plasmid construction.
Key points
Significant findings of the study
A method to clone short DNA into plasmids was found.
What this study adds
Our study provides a flexible and economical option to clone short DNA into plasmids.
Introduction
Plasmid vectors with small or multiple-tags, and small RNAs are commonly used in biomedical studies. Current methods of plasmid construction require obtaining the gene of interest from the amplification of the DNA template or chemically synthesized oligonucleotides.1-3 Obtaining a gene fragment of interest from the DNA library is the most commonly used method.1 It relies on digestion of DNA by type II restriction enzymes, and cutting the plasmid DNA circles with the same restriction nuclease to create linear DNA molecules. Finally, the gene of interest and the linearized vector are separately purified, mixed together at a certain molar ratio, and ligated with T4 DNA ligase to form recombinant DNA circles.4-7 This process involves cumbersome subcloning procedures including designing primers, digestion, ligation and verification of recombinant plasmids.8, 9 Therefore, its efficiency is dependent on both the restriction enzyme and the DNA ligase. In addition, unwanted nucleotide sequences may be added to the insert, and result in undesirable changes in the final products.10 The chemical synthesis method is also called oligo overlap cloning, which can be used to add a short stretch of DNA to a plasmid, such as adding short tags (HA-tag or Flag) and cloning shRNAs.3, 11 The DNA fragment of interest with restriction site is obtained through chemical synthesis, and the linearized vector with sticky ends is obtained by digesting the vector with restriction endonuclease.11, 12 Both methods share similar limitations.
To overcome these limitations, several PCR-based cloning protocols have been proposed to skip the use of DNA ligases and restriction endonucleases. Similar to our method, these methods are based on homologous recombination. The PCR products are flanked by 15 to 60 bp sequences, which exactly match the ends of the linear vector.13 These include in vitro Gateway recombination cloning technology,14 seamless ligation cloning extract (SLiCE)15 and RecET,16 etc. The SLiCE method from a commonly used laboratory E. coli strain, JM109 and DH5α, can efficiently function as an effective alternative to commercially available seamless DNA cloning kits.15 It also includes in vivo recombination methods, such as relying on RecA recombination enzymes, Red/ET recombination systems,17, 18 and DH5α competent cells.19 These methods require preparing the target gene and linearized vector, respectively. The target gene can also be inserted by the QuikChange site-directed mutagenesis method.20 Similar to our one-step PCR method, the target fragment can be inserted at any amplifiable site of the vector. The difference is that when we designed the primers, we divided the target sequence into three parts, the target sequence at the 5′ end of the forward primer, the target sequence at the 5′ end of the reverse primer, and a 15 bp homology arm with overlapped region for target gene. In the QuikChange site-directed mutagenesis method, the sequence of the target fragment was designed at the 5′ end of the forward primer, while the 5′ end of the reverse primer contained only a 15 bp homologous arm. Compared with the QuikChange site-directed mutagenesis method, our one-step PCR method reduces both the cost for primer synthesis and the formation of primer dimers.
Although DNA inserts below 90 bp can be inserted using the SLiCE method,21 it is still difficult in most plasmid construction methods to insert short DNA fragments less than 150 bp.22 DNA inserts below 150 bp can be directly synthesized, but it is cost prohibitive with limited productivity. Also, the effects of synthesizing two short oligonucleotides below 150 bp still depend on the efficiency of annealing, endonuclease, and ligase, etc. The fidelity also significantly decreases when synthesizing oligonucleotides between 100 bp and 150 bp. Therefore, with these traditional methods it is especially inappropriate to construct plasmids with DNA fragments below 150 bp. In this study, we amplified a linear vector including the short target gene below 150 bp by one-step PCR.
A DNA repair system in E. coli, homologous recombination, which can cyclize linearized vectors with homology arms, has been previously reported.10, 22 Employing the characteristics of E. coli, we developed a method for constructing plasmids based on one-step PCR and homologous recombination. Previously reported methods of constructing plasmid using homologous recombination of E. coli are all for genes longer than 150 bp.22
Here, we describe an efficient and flexible method based on one-step PCR and homologous recombination for constructing a plasmid containing the fragment of interest below 150 bp. We constructed the pEGFP-N1-HA plasmid by inserting the target fragment HA-tag into the vector pEGFP-N1 for proof-of-principle validation.
Methods
Cell culture
Human 293T cells and HeLa cells were purchased from American Tissue Culture Collection (ATCC) and maintained in our laboratory. Cells were maintained in Dulbecco's modified Eagle's medium (HyClone, Logan City, Utah, USA) containing 10% fetal bovine serum (Biological Industries, Cromwell, USA) and 1% penicillin-streptomycin in a humidified incubator with 5% CO2 and 37°C.
Construction of the pEGFP-N1-HA plasmid
The pEGFP-N1 vector (4733 bp) used in this study was provided by TaKaRa. The sequence of HA-tag was 5′-TACCCATACGACGTCCCAGACTACGCT-3′ (27 bp). The linearized vector pEGFP-N1 carrying the HA-tag was obtained by PCR. The PCR products were verified by gel electrophoresis. The original template plasmids were digested by DpnI enzyme (purified PCR products). Linearized vectors were transformed into E. coli and self-recombinant plasmids based on homologous recombination.
Sequences of primers of HA-tag were as following: forward, 5′- TAC GAC GTC CCA GAC TAC GCT AAG CTT CGA ATT CTG CAG TCG AC-3′; reverse, 5′-CTA CCG GAC TCA GAT CTC GAG CTC ATG TAC CCA TAC GAC GTC CCA-3′. As for PCR reactions, each 20 μL sample in the PCR thermocycler (Gene-Explorer Touch, Zhengzhou, China) contained 1 μL DNA template (30 ng/μL), 1 μL forward primer (10 nM), 1 μL reverse primer (10 nM), 7 μL double distilled H2O, and 10 μL I-5 2× high fidelity master mix (MCLAB, San Francisco, USA). A negative control replacing master mix with double distilled H2O was employed. The PCR cycling profile for all reactions was as follows: initial denaturation at 98°C for 2 minutes, denaturation of template at 98°C for 10 seconds, annealing at 64°C for 15 seconds, and extension at 72°C for 75 seconds, amplification for 18 cycles with the final extension at 72°C for 1 minute.
The PCR products (4774 bp, containing homologous sequences) were verified by electrophoresis on 0.9% agarose gel. The remaining 17 μL PCR products were mixed with 0.5 μL DpnI enzyme (BioLabs, Ipswich, USA), centrifuged, and incubated at 37°C for 3 hours.
Transformation of the pEGFP-N1-HA plasmid into E. coli
E. coli competent DH5α (Transgene Biotech, Beijing, China) (50 μL) was thawed on ice and mixed with 5 μL PCR products. The mixture was then incubated on ice for 30 minutes and transferred into a water bath at 42°C for 45 seconds followed by incubation on ice for 2 minutes. The mixture was added to 1 mL prewarmed Luria Broth (LB) and gently shaken at 37°C and 150 rpm for 70 minutes. Finally, cells were plated on fresh LB-agar plates supplemented with 50 μg/mL Kanamycin (BBI, Shanghai China), and incubated overnight at 37°C. The monoclonal colonies were picked up and sequenced. The length of the pEGFP-N1-HA plasmid was 4.76 kb.
A colony of transformed E. coli cells was picked and grown in 1 mL LB at 37°C for 4 hours. Then, 200 μL were taken for DNA sequencing. The remaining cells were added into 250 mL LB and incubated overnight at 37°C. Plasmid DNA was extracted with the GoldHi Endofree Plasmid Miniprep Kit (Cwbio, Beijing, China) according to the manufacturer's instructions.
Western blotting
Cells were transfected using TurboFect Transfection reagent (Thermo, Waltham American) according to the manufacturer's instructions. Cells were lysed in ice-cold lysis buffer 48 hours later, and lysates were centrifuged at 12000 rpm for 20 minutes at 4°C. After measuring the protein content using a bicin-choninic acid (BCA) assay, equal amounts of protein (1 g/mL) were electrophoresed by SDS-PAGE on 12% acrylamide gels and transferred onto a PVDF membrane. The membranes were blocked by 5% skim milk in Tris-buffered saline with 0.1% Tween (TBST) for 1 hour, and then incubated with anti-HA antibody (Santa Cruz Biotechnology, Dallas, USA) and anti-GFP antibody (Santa Cruz Biotechnology, Dallas, USA) at room temperature for 2 hours. After three washes with TBST, membranes were incubated with a secondary polyclonal antibody to rabbit IgG conjugated with HRP at room temperature for 1 hour. Labeled proteins were detected using an ECL substrate (Mei5bio, Beijing, China).
Fluorescence imaging
Cells were fixed with 4% paraformaldehyde (Sangon, Shanghai, China) for 20 minutes, permeabilized with 0.5% PBS-Triton X-100 (BBI, Shanghai, China) for 18 minutes, and blocked with 4% bovine serum albumin (BioFroxx, Einhausen, Germany) in PBS at room temperature for 90 minutes. The slides were then incubated with anti-HA antibody (Santa Cruz, Dallas, USA) for 2 hours at room temperature, and subsequently incubated with secondary antibody at room temperature for 2 hours. Nuclei were stained with Hoechst (Sigma, Saint Louis, USA). Images were captured using a confocal laser scanning microscopy (Zeiss, Oberkochen, Germany).
Statistical analysis
All the data were normalized to the control. Data are shown as mean ± SD and analyzed with t-test using the GraphPad Prism (GraphPad, USA). P < 0.05 was considered statistically significant.
Results
pEGFP-N1-HA plasmid successfully constructed using our method
Compared with the traditional construction method in Fig 1a, the schematic map of our method is illustrated in Fig 1b. The linearized vector was obtained by one-step PCR. After annealing, the fragment of HA-tag was directly cloned into the pEGFP-N1 vector. The new constructed plasmid, pEGFP-N1-HA, was subjected to agarose-gel electrophoresis. As shown in Fig 1c, PCR products were absent in the negative control, while the pEGFP-N1-HA reaction system showed a 4.7 kb PCR product. The sequencing result of the pEGFP-N1-HA plasmid construction was identical to the GenBank sequence (see Fig 1d), which confirmed the electrophoresis results.
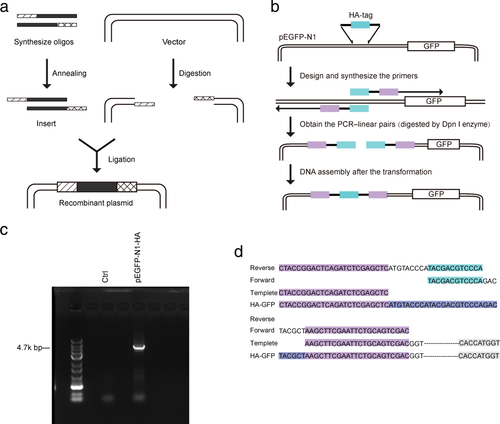
 ) Overlapping sequences of the templates (
) Overlapping sequences of the templates ( ) Homogenous arms. First, primers were designed and synthesized. The PCR-linear pairs were then obtained. Finally, the PCR products were transformed into chemically competent E. coli. (c) Electrophoresis on SDS-SAGE gel of the PCR products. The size of the pEGFP-N1-HA plasmid was about 4.7 kb. (d) Diagram of the PCR-linear pairs cyclization. Both the forward and reverse primer have a homologous region, which can be circularized in competent E. coli (
) Homogenous arms. First, primers were designed and synthesized. The PCR-linear pairs were then obtained. Finally, the PCR products were transformed into chemically competent E. coli. (c) Electrophoresis on SDS-SAGE gel of the PCR products. The size of the pEGFP-N1-HA plasmid was about 4.7 kb. (d) Diagram of the PCR-linear pairs cyclization. Both the forward and reverse primer have a homologous region, which can be circularized in competent E. coli ( ) Overlapping sequences of the templates (
) Overlapping sequences of the templates ( ) Homogenous arms (
) Homogenous arms ( ) HA-tag (
) HA-tag ( ) GFP.
) GFP.HA-tag was expressed in 293T cells
The HA-tag expression in 293T cells was detected by western blotting. As shown in Fig 2a, HA-tag was successfully expressed in cells. The HA-GFP fusion protein was clearly detected by confocal microscopy (Fig 2b).
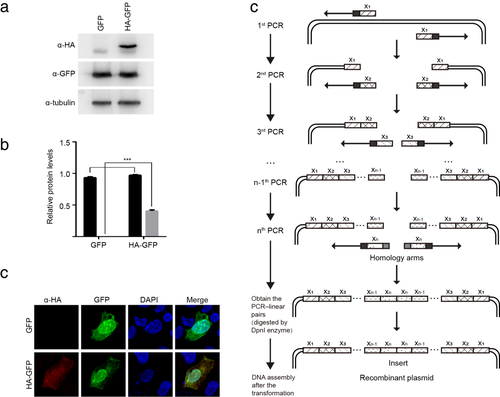
 ) α-GFP (
) α-GFP ( ) α-HA. The values were normalized by the intensities of the corresponding α-tubulin band. (c) The expression of GFP and HA proteins in 293A cells transformed with pEGFP-N1 or pEGFP-N1-HA was detected by confocal microscopy. (d) Schematic diagram of our one-step PCR method for inserting large fragments. The large fragment insertion is segmented into a plurality of fragments with a length of less than 150 bp according to a one-step PCR method. PCR-linear pairs with large fragment insertion are obtained by multiple PCR amplification, digested by DpnI to eliminate the parent DNA, and directly transformed into competent E. coli. * indicates P < 0.05, ** indicates P < 0.01, and *** indicates P < 0.001.
) α-HA. The values were normalized by the intensities of the corresponding α-tubulin band. (c) The expression of GFP and HA proteins in 293A cells transformed with pEGFP-N1 or pEGFP-N1-HA was detected by confocal microscopy. (d) Schematic diagram of our one-step PCR method for inserting large fragments. The large fragment insertion is segmented into a plurality of fragments with a length of less than 150 bp according to a one-step PCR method. PCR-linear pairs with large fragment insertion are obtained by multiple PCR amplification, digested by DpnI to eliminate the parent DNA, and directly transformed into competent E. coli. * indicates P < 0.05, ** indicates P < 0.01, and *** indicates P < 0.001.Discussion
To efficiently and rapidly construct plasmids with inserts below 150 bp has prominent practical applications, such as small peptide fusion proteins, small interfering RNAs (siRNAs) or short hairpin RNAs (shRNAs). Traditional plasmid construction depends on restriction enzymes and ligases which are time-consuming and labor-intensive. There have been many developments in constructing recombinant vectors in vitro using recombinases and the endogenous recombinases of E. coli. Seamless ligation cloning extract (SLiCE) method is a commonly used method, which utilizes bacterial cell extracts to assemble multiple DNA fragments into recombinant DNA molecules in a single in vitro recombination reaction.23 Compared with our method, the advantage of SLiCE is that SLiCE can simultaneously assemble multiple DNA fragments without using recombinase, restriction enzyme and DNA ligase. However, SLiCE involves the extraction of bacterial solution and the preparation of linearized vectors and target fragments. The recombination efficiency of different bacterial cell extracts is also different. There have been studies using the recombination system of E. coli competent DH5α. This method required neither engineering bacteria, nor recombinase and bacterial cell extracts. However, the linearized vector and the target fragment have to be prepared, respectively. Therefore, these have to be digested by DpnI enzyme and transformed.19, 24 The biggest difference between this method and our method is that our method only requires one step of PCR to obtain the linearized vector with the target fragment. In addition, our method is also more flexible in position selection than the existing one-step PCR method for constructing shRNA plasmid.20 Our method is based on homologous recombination, which is different from the method for constructing site-directed mutagenesis25 and generation of shRNA expression vectors20 in previous reports. Compared to these previously reported methods, our method is more flexible in selecting insertion sites. The DNA fragment of interest can be inserted at any position other than the promoter. Compared with direct synthesis of short oligonucleotides, our method is more economic. We also compared the cloning efficiency and accuracy of constructing the pEGFP-N1-HA plasmid by our one-step PCR method and the In-Fusion cloning method. Our one-step PCR method synthesized 89 bp DNA, about 47% less than 131 bp of the In-Fusion cloning. There were more procedures in the In-Fusion cloning, such as DNA fragment purification from PCR enzymatic reactions, in vitro recombination, etc. According to the sequencing results, there was the same accuracy between our one-step PCR method and the In-Fusion cloning method (data not shown).
Although our method was specifically designed for plasmids with small inserts below 150 bp, it can also be applied to construct plasmids with large fragments (Fig 2c). Small fragments can be obtained by PCR one by one, and ligated to form a large DNA fragment. Our method has several limitations. The efficiency may not be constant because the plasmid template used in PCR amplification has some transformation potential.25 In order to eliminate the influence of the plasmid template, it is necessary to digest the template by DpnI endonuclease. The DpnI digestion time of the PCR products plays key roles in the success of our method. DNA polymerase may also induce errors. The error rate may be high when PCR is used to amplify genomic sequences. The amplified genomic sequences are longer, and the DNA polymerase mismatch rate is higher.26, 27 The use of high-fidelity Taq enzyme, such as Pfu DNA polymerase,28 can reduce the mismatch rate in PCR, which will also increase the cost.
Overall, our method combines the advantages of both PCR-based strategies and homologous recombination (Figure S1). Due to its simplicity and versatility, our method makes it possible to rapidly and cost-effectively construct plasmids with inserts below 150 bp, which will greatly contribute to the functional study of small peptides and RNA interference.
Acknowledgments
Funding was provided by the National Natural Science Foundation of China (Grant No. 81770580) and the Sichuan Science and Technology Program (Grant No. 2019YJ0050).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.




