Glutaredoxin 1 regulates macrophage polarization through mediating glutathionylation of STAT1
Abstract
Background
Macrophage polarization affects tumor growth, metabolism, and many other tumor processes. M1 macrophages can promote antitumor immunity response. Signal transducer and activator of transcription 1 (STAT1) is one of the critical transcription factors in this process, which promotes the expression of a series of inflammatory molecules. STAT1 has been reported as a potential target of reactive oxygen species (ROS)-induced glutathionylation, while the glutathionylation of STAT1 in macrophages and its underlying regulatory mechanism remains unclear. Glutaredoxin 1 (Grx1) functions as a deglutathionylation enzyme, which regulates the activities of many proteins through reversing glutathionylation.
Methods
GeneChip and RT-qPCR was first applied to test the mRNA level of Grx1 in M1 macrophages. Western blot was then used to evaluate the variations of the Grx1 protein expression in M1 macrophages. Next, immunoprecipitation was used to investigate the glutathionylated STAT1 in both wild-type and Grx1−/− mouse macrophages. Finally, the induced alterations of STAT1 activity and function by Grx1 in M1 macrophage were examined by western blot and RT-qPCR.
Results
In M1-type macrophages, the levels of Grx1 were elevated. Glutathionylation of STAT1 was negatively regulated by Grx1. Furthermore, depletion of Grx1 increased the activity of STAT1, and thereby promoted the mRNA level of C-X-C motif chemokine ligand 9 (CXCL9) during M1-type polarization of macrophages.
Conclusions
Grx1 controlled deglutathionylation of STAT1, which in turn might regulate M1-type polarization of macrophages.
Introduction
Macrophages are essential cellular components of the innate immune system, and not only participate in various physiological processes such as inflammatory response, wound healing and antigen presentation, but also play vital roles in inflammation-related diseases such as tumor, diabetes, and atherosclerosis. Macrophages are characterized by high functional plasticity. In response to various stimuli or under different pathological conditions, macrophages can be differentiated into two main metabolic and functional phenotypes, referred to as classical M1 type “polarization” and alternative M2 type “polarization.”1
Macrophages are one of the major immune cells in the tumor microenvironment, which are called tumor-associated macrophages (TAMs). TAMs play an important role in the process of tumorigenesis and development. Tumor cells are usually in a high metabolic state, which might result in insufficient nutrients and hypoxia. They adapt to the environment by changing their metabolism, which leads to the accumulation of lactic acid, nitric oxide (NO), reactive oxygen species (ROS), prostaglandins, and arachidonic acid. After gathering metabolic byproducts, the tumor microenvironment exhibits an oxidative and inflammatory status, which affects the functions of TAMs.2, 3 Previous studies have shown that in the tumor microenvironment, the M1-like TAMs induced by γ-interferon (IFN-γ) produce a series of proinflammatory cytokines, nitric oxide, ROS, etc., thereby functioning as antitumor immunity,4 whereas, M2-like TAMs may play an opposite role by promoting tumor development.5 Thus, targeting TAMs and changing their polarized phenotypes is one of the significant directions of current antitumor strategies.
ROS can regulate the M1-type polarization of macrophages through redox modifications, such as glutathionylation. ROS promote the binding of glutathione (GSH) to the free thiol (-SH) on the protein cysteine to form a protein-glutathione mixed disulfide adduct (Pr-SSG).6 Deglutathionylation is mainly regulated by glutaredoxin (Grx), which transfers the modified thiol group of the target protein to its own thiol group.7 Recently, multiple studies have shown that in certain cells such as alveolar epithelium, Grx1 positively regulates the NF-κB signal through deglutathionylation activity, and promotes the production of cytokines such as TNF-α during inflammatory response.8
However, in a model of LPS-induced acute pneumonia, neutrophil infiltration could still be observed in the lungs of Grx1−/− mice. In IL-17A stimulated alveolar epithelial cells, although siGrx1 decreased the expressions of some NF-κB-regulated genes (KC, CCL20), the level of IL-6 was still increased compared to the control.9 These results suggest that in addition to the NF-κB signaling pathway, Grx1 might negatively regulate M1-type polarization of macrophages through other molecules. STAT1 is one of the most important transcription factors in the M1-type polarization of macrophages. Phosphorylated STAT1 translocates into the nuclear to promote the expression of many genes related to M1-type polarization, such as CXCL9.10 Recently, one group reported that STAT1 was the target of glutathionylation in microglia BV2 cells, and that glutathionylated STAT1 (STAT1-SSG) contributed to its activation.11 However, the role of Grx1/STAT1-SSG in M1 macrophage polarization is still unknown.
Based on these studies, we first detected the Grx1 levels in M1 polarized macrophages and the glutathionylated STAT1. Grx1−/− mouse peritoneal macrophages were then used to investigate the effect of Grx1 on glutathionylation and activity of STAT1. Finally, we examined the changes of M1 macrophage biomarker levels. This study suggests that Grx1/Pr-SSG might be an important regulatory mechanism in controlling STAT1 functions in M1-type macrophage polarization.
Methods
Mouse macrophage cell line culture
A mouse macrophage cell line RAW264.7 was purchased from the Cell Bank of the Chinese Academic of Sciences (Shanghai, China). RAW 264.7 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco) which contained 10% (v/v) heat-inactivated fetal bovine serum (Biological Industries, BI) and antibiotics (100 μg/mL streptomycin and 10 U/mL penicillin) at 37°C in a humidified atmosphere with 5% CO2.
Mice
Grx1−/− mice were obtained from Cyagen (KOCMP-23992-Glrx) and fed in the animal facilities of West China School of Basic Medical Sciences and Forensic Medicine at Sichuan University. Age-matched C57BL/6 mice were obtained from Dashuo as WT controls. All the mouse-related procedures were approved by the ethics committee of the West China School of Basic Medical Sciences and Forensic Medicine at Sichuan University.
Primary mouse alveolar macrophage isolation and culture
First, the mice were sacrificed and the lungs of each mouse were perfused with 1 mL phosphate buffered saline (PBS). The PBS fluid was then aspirated from the lungs to isolate the alveolar macrophages. We repeated this step several times. Next, cells were centrifuged for 15 minutes at 1600 rpm and washed twice with DMEM. The cells were cultured as the mouse macrophage cell line. After 24 hours, the alveolar macrophage cells were washed twice with PBS to remove the nonadherent cells, and the remaining adherent cells were kept for subsequent experiments.
Primary mouse peritoneal macrophage isolation and culture
First, 3 mL broth (29 g thioglycolate/100 mL H2O, kept in the dark at 4°C for more than two weeks) was injected into the peritoneal cavity of the mice. Three days later, they were sacrificed and disinfected with 75% alcohol. Each mouse was then injected with 5 mL of DMEM medium into the abdominal cavity. Next, the abdomen was massaged for 1 minute and the medium was then extracted. This step was repeated three times. The peritoneal eluate of each mouse was centrifuged for 15 minutes at 1600 rpm, and the supernatant discarded. PBS buffer was subsequently used to wash the isolated macrophages three times. Finally, the primary peritoneal macrophages were cultured as described above. After 24 hours, the cells were washed twice with PBS to scour off the nonadherent cells, and the remainder of the cells were kept for subsequent experiments.
Induction of M1 macrophage polarization
Lipopolysaccharides (LPS: 1 μg/mL, Sigma-Aldrich, USA) or/and IFN-γ (20 ng/mL or 50 ng/mL, Immune tools, Germany) were added to the macrophage culture medium at certain time points to obtain M1 phenotype macrophages.
Real-time fluorescence quantitative polymerase chain reaction (RT-qPCR)
Glrx and CXCL9 were tested via RT-qPCR (β-actin as a control). Total RNA was extracted by UNIQ-10 Column Total RNA Purification Kit (Sangon Biotech, China). mRNA (2 μg) was reverse transcribed by HiScript II Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme, China). The synthesized cDNA was amplified with ChamQ Universal SYBR qPCR Master Mix (Vazyme, China). The RT-qPCR was accomplished by CFX96 Real-Time PCR. The primers were used to amplify cDNAs as follow:
5′-CAGTGGTGGAAGTCGGCTC-3′, 5′- GCCATGACATCGTTCATCTGT-3′ for Grx1 (Glrx).
5′-GGAGTTCGAGGAACCCTAGTG-3′, 5′-GGGATTTGTAGTGGATCGTGC -3′ for CXCL9.
5′-ATGACCCAAGCCGAGAAGG-3′, 5′-CGGCCAAGTCTTAGAGTTGTTG −3′ for β-actin.
Coimmunoprecipitation
2 mL H2O2 or PBS was added into the cell culture medium and incubated for 10 minutes. Cells were lysed, and anti-GSH antibody (Abcam, USA) was then added to the cell lysate, which was incubated at 4°C for 12 hours. Next, agarose beads were added, and the reactions were incubated at 4°C for a further 2 hours. The supernatant was centrifuged, and the agarose beads were washed three times with PBS. Then, SDS loading buffer was added into a certain volume of PBS containing agarose beads. The mixture was denatured at 100°C for 10 minutes and then subjected to SDS-PAGE as described below.
Western blot
RIPA buffer was added into M1-type polarized macrophages, which were incubated on ice for 30 minutes. During incubation, samples were shaken every 10 minutes, and then centrifuged at 12 000 rpm for 15 minutes at 4°C. SDS loading buffer was added into the supernatant before being denatured at 100°C for 10 minutes, which was then followed by SDS-PAGE. After transferring the proteins to the PVDF membranes, the membranes were blocked with 5% skim milk for two hours at room temperature. The corresponding primary antibodies (anti-Grx1, anti-STAT1, antiphosphorylated STAT1, antiactin or anti-HMGN2 [Cell Signaling Technology, USA]) were then diluted, and the membranes incubated with primary antibodies at 4°C overnight. After washing the membranes three times with PBST, the secondary antibodies corresponding to each antibody were diluted 1:1000 and incubated at room temperature for two hours. The membranes were then washed three times with PBST before imaging.
Statistical analysis
Each result was repeated at least three times and analyzed by GraphPad prism 6.0 statistical software.
Results
mRNA level of Glrx was upregulated in M1-type macrophages
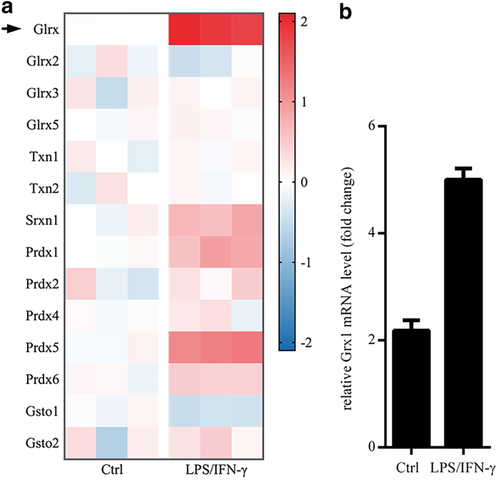
In mammalian cells, several groups of molecules are involved in regulating redox modification, such as Grx, Thioredoxin (Trx, gene name: Txn), peroxiredoxin (Prx, gene name: Prdx), and glutathione S-transferase Ω (Gsto). The Grx family in mammalian cells mainly includes Grx1 (gene name: Glrx), Grx2, Grx3 and Grx5. Grx1 is widely distributed in cytoplasm, nuclei and extracellularly. Grx2 and Grx5 are mainly located in mitochondria, and Grx3 is located in the nucleus in most cases.12, 13 We first tested the levels of Grx and the redox-related molecules in M1-type polarization of macrophages by GeneChip. We found that the expression of Grx1 (Glrx), in LPS/IFN-γ induced M1-type macrophages from mice, was increased at the highest level compared to others (Fig 1a). Later, we verified the mRNA level of Glrx in macrophages under the stimulus of LPS/IFN-γ via RT-qPCR. Undoubtedly, we achieved a similar result with GeneChip (Fig 1b). The results indicated that Grx1, rather than other members of the Grx family or other redox-related factors, might participate in regulating M1-type polarization of macrophages.
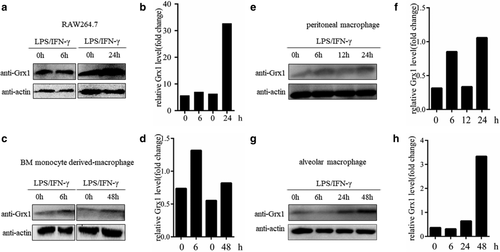
Grx1 protein expression was elevated in M1-type macrophages
As is already known, macrophages are resident cells in various tissues of the body.14 Therefore, we examined the Grx1 protein levels during M1-type polarization of macrophages from multiple sources. The mouse macrophage cell line RAW264.7 was first tested. After the induction of polarization to M1 type for 24 hours, the expression level of Grx1 protein increased significantly (Fig 2a,b). In addition, we tested some primary macrophages, including bone marrow monocyte derived-macrophages (BMDMs), peritoneal macrophages (PMs), and alveolar macrophages (AMs) under induction. Similar results were observed (Fig 2c–h). In addition, we also tested the effects of induction time on Grx1 protein levels in PMs and AMs. The results showed that as the time of LPS/IFN-γ treatment increased, the expression level of Grx1 also gradually increased (Fig 2e–h). Together, our results demonstrated that Grx1 might play an indispensable role in the process of M1-type macrophage polarization.
Grx1 regulated the glutathionylation of STAT1 in macrophages
Combined with previous reports, we speculated that STAT1 might be the target of Grx1 and ROS-mediated glutathionylation in M1 macrophages. We first tested the level of glutathionylated STAT1 in RAW264.7 cells. RAW264.7 cells, pretreated with H2O2 for 10 minutes, were lysed. Glutathionylated proteins in the cell lysates were coimmunoprecipitated with anti-GSH agarose beads, and incubated with anti-STAT1 antibody. As shown in Fig 3a,b, a band corresponding to STAT1-SSG was detected in the macrophages. Meanwhile, we used macrophages isolated from the peritoneal cavities of mice to check whether a STAT1-SSG signal could be detected. Consistently, we achieved similar outputs as RAW264.7 cells (Fig 3c,d), which confirmed that STAT1 was modified by glutathionylation in macrophages.
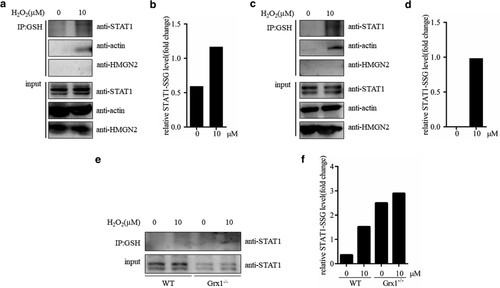
Next, we used peritoneal macrophages separated from wild-type (WT) and Grx1−/− mice to investigate the effect of Grx1 on the STAT1 glutathionylation. The lysates of WT and Grx1−/− peritoneal macrophages were performed to anti-GSH coimmunoprecipitation. As shown in Fig 3e,f, STAT1-SSG signal in Grx1−/− cells was much stronger than that in WT. Because of different exposure time, no STAT1-SSG was observed in H2O2 treated WT macrophages. These results indicated that Grx1 might inhibit glutathionylation of STAT1 in macrophages.
Grx1 depressed activity and function of STAT1 in macrophage M1 polarization
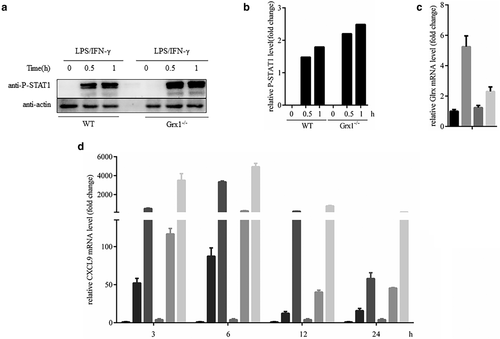
To determine the roles of Grx1/Pr-SSG in regulating STAT1 function in M1 macrophages, we examined the effect of depletion of Grx1 on STAT1 activity during M1 type polarization. First, we checked whether Grx1 would affect the phosphorylation of STAT1 (P-STAT1). As shown in Fig 4a,b, Grx1−/− peritoneal macrophages treated with LPS/IFN-γ showed higher levels of P-STAT1 than wild-type cells at each time point checked, and suggested that Grx1 might inhibit STAT1 activity during the process of M1-type macrophage polarization.
 Ctrl,
Ctrl,  LPS/IFN-γ,
LPS/IFN-γ,  IFN-γ 20 ng/mL,
IFN-γ 20 ng/mL,  IFN-γ 50 ng/mL (d) PMs from WT and Grx1−/− mice were treated by LPS/IFN-γ, IFN-γ 20 ng/mL and IFN-γ 50 ng/mL at 3, 6, 12 or 24h before using RT-qPCR to detect the mRNA levels of CXCL9.
IFN-γ 50 ng/mL (d) PMs from WT and Grx1−/− mice were treated by LPS/IFN-γ, IFN-γ 20 ng/mL and IFN-γ 50 ng/mL at 3, 6, 12 or 24h before using RT-qPCR to detect the mRNA levels of CXCL9.  WT Ctrl,
WT Ctrl,  WT IFN-γ 20 ng/mL,
WT IFN-γ 20 ng/mL,  WT IFN-γ 50 ng/mL,
WT IFN-γ 50 ng/mL,  Grx1−/− Ctrl,
Grx1−/− Ctrl,  Grx1−/− IFN-γ 20 ng/mL,
Grx1−/− IFN-γ 20 ng/mL,  Grx1−/− IFN-γ 50 ng/mL
Grx1−/− IFN-γ 50 ng/mLIn addition to the STAT1 cascade, LPS also activated NF-κB signaling, which is also the target of Grx1/Pr-SSG. Many studies have shown that STAT1 is the key transcriptional mediator of macrophage responses to IFN-γ.15-17 Thus, we mainly focused on the downstream molecules of the STAT1 signaling pathway after IFN-γ stimulation in the following experiments, such as CXCL9 and some proinflammatory cytokines.17-19 We speculated that Grx1/STAT1-SSG might affect the M1 polarization of macrophages. Herein, we first tested the mRNA level of Glrx in peritoneal macrophages stimulated by IFN-γ for 24 hours. The results showed that IFN-γ could also upregulate the levels of Glrx in peritoneal macrophages (Fig 4c). Next, we measured the mRNA levels of CXCL9 in WT and Grx1−/− mouse peritoneal macrophages with IFN-γ treatment for certain hours. Grx1−/− peritoneal macrophages showed higher mRNA levels of CXCL9 than WT macrophages at each time point (Fig 4d). These results suggested that Grx1 might reduce the activity of STAT1, thereby restraining the M1-type polarization of macrophages.
Discussion
In our study, we revealed that the expressions of Grx1 in M1-type macrophages were increased in both mRNA and protein levels. Furthermore, by studying Grx1−/− mouse macrophages, we demonstrated that Grx1 might reverse the glutathionylation of STAT1, and regulate M1-type polarization of macrophages through suppression of STAT1 activity. These results might provide some clues to understanding the regulatory mechanism of M1-type polarization of macrophages.
Under oxidative stress, the free sulfhydryl groups on protein cysteine can be oxidized to sulfenic acid (RSOH), sulfonation acid (RSO2H), and sulfonic acid (RSO3H).20 As a reversible oxidative modification, glutathionylation protects target proteins from inactivation caused by irreversible sulfination and sulfonation.21 More importantly, the protein glutathionylation also directly participates in regulating cellular signals, and thereby is involved in a variety of cellular physiological or pathological processes.22 Our group has reported glutathionylation plays an important role in actin dynamics23 and regulates the affinity of integrin α4 with its ligands in neutrophils.6
Catalyzed by activated Janus tyrosine kinase (JAK), STAT is phosphorylated and forms activated dimers, which then enter the nucleus combining with targeted DNA motifs to regulate gene transcription and participate in a variety of physiological or pathological processes.24 Considering that the phosphorylation of STAT1 occurs at the early stage after macrophages are appropriately stimulated,25, 26 we chose to test the levels of P-STAT1 at 0.5 hours/1 hour after stimulation. Many studies have clarified the relationship between oxidative stress and STAT1 in different aspects.27 Hypoxia-induced oxidative stress causes glutathionylation of STAT1 in the brain, which abnormally activates the STAT1 signaling pathway and promotes BV2 cells to transform into proinflammatory phenotype.28 PACAP, which induces ROS, upregulates the phosphorylation level of STAT1.29 In MCF-7 breast cancer cell line, H2O2 has been reported to enhance the glutathionylation of STAT1, and promote IFN-induced apoptosis of tumor cells.27 In line with these findings, we showed that in macrophages, Grx1 inhibited STAT1 activity through its thioltransferase activity during the process of M1-type polarization and suppressed the expression of the canonical inflammatory genes such as CXCL9.
Simultaneously, many studies have shown that Grx1 participates in some inflammatory responses related to M1 macrophages by regulating NF-κB activity.30 However, Grx1/Pr-SSG seems to play an opposite role in contrast to STAT1 activity. Disruption of Grx1 promotes the glutathionylation of IκB kinase β (IKKβ) in the NF-κB cascade, which reduces the nuclear translocation of RelA, and subsequently inhibits the DNA binding activity of NF-κB, thus downregulating the levels of TNF-α and some other proinflammatory factors in response to LPS.9, 31 In addition, NF-κB components, p50 and RelA, are also regulated by glutathionylation, and thereby their ability to bind to DNA is reduced.30 Therefore, in the M1-type polarization of macrophages with high ROS levels, Grx1/Pr-SSG may control the inflammatory function of macrophages through balancing STAT1 and NF-κB pathways.
However, it has been previously reported that some M1 marker molecules, such as iNOS, are reduced in Grx1−/− alveolar macrophages after administration of LPS.32 The conflict might be due to the fact that IFN-γ rather than LPS was used in our experiments. Thus, in our model STAT1 might be the superior transcriptional mediator. In IFN-α/β/γ treated MCF-7 breast cancer cells, the phosphorylation of STAT1 was upregulated and reached a peak at 48 hours, thereby inducing apoptosis.29 Many studies have revealed that STAT1 signaling is primarily activated by IFN-γ in macrophages.33, 34 Interestingly, some groups have reported that IFN-γ even negatively regulated components of the LPS response.33, 35 These support our results that disruption of Grx1 promoted the activity of STAT1 in IFN-γ stimulated macrophages and consequently upregulated the transcription of downstream target genes such as CXCL9. However, the expressions of other M1-related molecules during the process of IFN-γ stimulation need to be investigated in the future.
The environment in which tumor cells are grown is referred as the tumor microenvironment (TME). This includes the structure, function, and metabolism of the tissue in which the tumor is located, and is closely related to occurrence and metastasis of the tumor. The high metabolic rate of tumor cells makes the TME in a state of high oxidative stress.36, 37 Increasingly, more studies have revealed that the recruitment, differentiation of macrophages, the secretion of various cytokines as well as growth factors in the TME are involved in tumor growth and metastasis. These macrophages, called tumor-associated macrophages (TAMs), are abundant in most malignant solid tumors and play a key role in the mechanism of tumor metastasis and antitumor immunity. Numerous groups have illustrated that in TAMs, M1-like macrophages enhance antitumor immunity, while M2-like macrophages inhibit antitumor immunity and promote tumor development.38 Our experiments showed that Grx1 mediated M1 polarization of macrophages by regulating STAT1 activity, indicating that Grx1/Pr-SSG might play an important role in the tumorigenesis and development of tumors through regulating macrophage polarization, which remains to be further explored.
Acknowledgments
This study was supported by Sichuan Association for Science and Technology (No. 2018RCTJ04), Innovation and Entrepreneurship Training Program for Sichuan University Students (No. C2019106935, no. C2020113908), National Natural Science Foundation of China (No. 31401188, no. 81470931), Science Foundation for Excellent Youth Scholars of Sichuan University Grant (No. 2016SCU04A08).
Disclosure
No authors report any conflict of interest.




