Diabetes and stroke: An important complication
糖尿病和卒中:一个重要的并发症
1 POTENTIAL MECHANISMS OF DIABETES-RELATED ASSOCIATION WITH STROKE
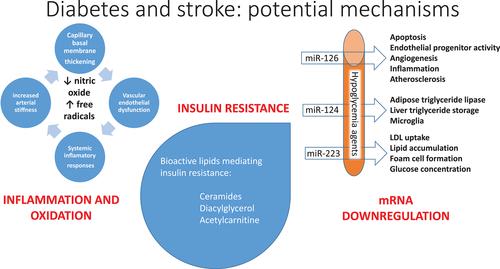
Diabetes is associated with a plethora of metabolic changes which may underlie the propensity to development of stroke (Figure 1).1 Insulin resistance has multiple drivers, is inextricably linked to type 2 diabetes, and is associated with coronary artery2 and cerebrovascular3 atherosclerosis. Insulin resistance is mediated by increased levels of bioactive lipids, including ceramides4 and diacylglycerol,5 playing a role in cell death in numerous tissues.6 Hyperglycemia leads to formation of advanced glycation end products and to activation of the polyol pathway, protein kinase C pathway, and the poly-adenosine diphosphate-ribose polymerase (PARP) and hexosamine pathways, all increasing oxidative stress, with formation of such reactive oxygen species as the hydroxyl radical, superoxide anion, peroxides, reducing endogenous antioxidants including superoxide dismutase, glutathione, and ascorbic acid and eventuating in oxidative damage of DNA, proteins, and lipids, causing cellular necrosis or apoptosis.1 Hyperglycemia directly and indirectly increases circulating levels of T cells and macrophages as well as inflammatory and prothrombotic factors, reduces endothelial function, and leads to glycation and oxidation of lipoproteins, increasing their atherogenicity.1 A specific mediator of oxidative stress involves activation of the transient receptor potential melastatin (TRMP) 2 calcium channel by ischemia/reperfusion injury, a pathway upregulated in diabetes and insulin-resistant states and mediating neuronal cell death in association with oxidative stress.7 Diabetes leads to reductions in levels of key microRNAs, miRNA-126, miRNA-124, and miRNA-223, which are also downregulated in stroke and which have anti-inflammatory and neuroprotective effects.8
2 THE ASSOCIATION OF DIABETES WITH STROKE
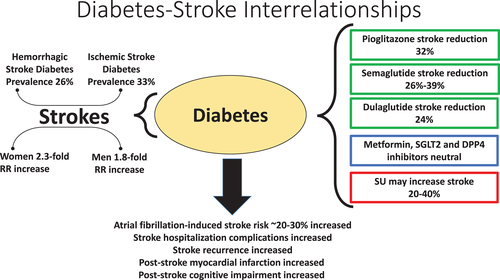
There can be no doubt of the strong evidence of an association of diabetes with stroke (Figure 2). A recent summary of epidemiologic studies showed that diagnosed diabetes is present on average in 26% of persons with hemorrhagic stroke and 33% of those with ischemic stroke, for an overall average of 28%, and that if glycosylated hemoglobin (HbA1c) is used to include undiagnosed diabetes, the prevalence of diabetes among persons with stroke is 37%, although these estimates vary by a great deal in different studies.9 This is not as clearly the case with prediabetes. A UK study showed that there was a similar relative increase in stroke with HbA1c above vs below median among persons both with systolic blood pressure above and below the median in those of European ethnicity, but that this was true to a lesser extent in persons of South Asian ethnicity in the higher systolic blood pressure subset, and that there was no relative increase in stroke with higher HbA1c among those with lower systolic blood pressure.10 A Mendelian randomization study showed that genetic predisposition to type 2 diabetes was more clearly associated with stroke than were genetic factors associated with hyperglycemia and hyperinsulinemia. Looked at in terms of stroke risk, men with diabetes have a 1.8-fold increase in relative risk of stroke and women a 2.3-fold increase,11 although absolute stroke risk is greatest among men.12, 13 The Emerging Risk Factors Collaboration meta-analysis of 102 prospective studies with ~8.5 million person-years of follow-up showed that diabetes increased ischemic and hemorrhagic stroke risk 2.27-fold and 1.56-fold, respectively.14 There may be a particularly marked increase in stroke among persons with type 1 diabetes.15 Similar effects of diabetes in increasing stroke risk are reported in studies in the United States,16 in the Middle East,17 in China,18 and in Taiwan,19 with the latter studies important as there is evidence of greater likelihood of stroke in much of Asia than in Western Europe and North America.20
Diabetes is not only associated with stroke but also with risk of adverse stroke outcome.9, 10 In a study of hospital complications with stroke in Thailand, diabetes was associated with increased risk of sepsis and acute kidney injury, and to a lesser extent with cardiovascular events, with pneumonia and urinary infection, and with in-hospital mortality.21 Diabetes increases the likelihood of stroke recurrence,22 and diabetes, but not prediabetes, has been reported to be associated with increased risk of developing cognitive impairment and dementia, particularly that occurring after stroke.23
An important cause of stroke is atrial fibrillation, and in a study of this relationship, persons with diabetes, although more likely to be anticoagulated with warfarin than nondiabetic persons with atrial fibrillation, had greater risk of stroke, although with improvement during the period from 1992 to 2019.24 A meta-analysis of studies of atrial fibrillation showed that those with diabetes had greater risk of stroke as well as of other vascular events and of mortality, with similar risk reduction comparing warfarin with direct oral anticoagulants.25
3 THE ASSOCIATION OF LONG-TERM GLYCEMIA WITH STROKE
Two large epidemiologic studies give evidence of an association of hyperglycemia with atherosclerotic cardiovascular disease. In the Swedish National Diabetes Register of 271 174 persons, stroke correlated with HbA1c levels to a greater extent than did myocardial infarction, with increase in risk beginning at a HbA1c level around 7%.26 Similarly, in a recently published 7.3-year follow-up of 406 271 persons with type 2 diabetes and 2 086 440 nondiabetic persons at mean age 64, those with diabetes had 10.1 vs 7.3 stroke events per 1000 person years, and the risk of ischemic stroke as well as the mortality from such events increased linearly as HbA1c levels increased.27 In a 5.4 year follow-up of 7209 persons with type 2 diabetes in Hong Kong, 372 developed stroke, with HbA1c a significant risk factor.28 The Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) observational 29-year follow-up of 1441 persons with type 1 diabetes similarly showed significant correlation of HbA1c with stroke.29
In the United Kingdom Prospective Diabetes Study (UKPDS), however, stroke occurred in 99 of 3776 persons with newly diagnosed type 2 diabetes during 7.9 years of follow-up, without relationship to HbA1c or fasting glucose.12 Furthermore, patients with type 2 diabetes at high cardiovascular risk randomized to intensive glycemic control for a mean of 3.7 years in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial failed to have reduction in stroke rate over a median 8.8 years of observation,30 and those similarly randomized in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial31 and in the Veterans Affairs Diabetes Trial (VADT)32 also failed to have reduction in stroke with intensive glycemic control over 6- and 15-year follow-up, respectively.
4 ACUTE ASSOCIATION OF GLYCEMIA WITH STROKE
There is striking evidence that acute hyperglycemia at presentation with stroke is a marker of risk, both of poor functional outcome and of mortality.33 Several studies have addressed the question of whether intervention to control glycemia might be of benefit in such patients. The Stroke Hyperglycemia Insulin Network Effort (SHINE) randomized clinical trial included adult patients with known diabetes whose blood glucose exceeded 110 mg/dL and those not known to have diabetes with glucose exceeding 150 mg/dL; there was no demonstrable difference in stroke outcome, but severe hypoglycemia (glucose <40 mg/dL) occurred in 2.6% of the intervention group but in none of those randomized to standard treatment.34 Three meta-analyses similarly showed lack of outcome benefit and increase in hypoglycemia with intensive glucose lowering.35-37
5 RELATIONSHIPS OF SPECIFIC GLUCOSE-LOWERING AGENTS TO STROKE
In contrast to the disappointing evidence of intervention specifically aimed at glucose lowering, there is growing evidence favoring certain glucose-lowering treatments, while suggesting that other glucose-lowering medicines might best be avoided in terms of stroke outcome (FIGURE 2).38, 39 There is no evidence of increase or reduction in stroke occurrence with metformin, but stroke outcome may benefit in persons treated with this agent.40 In a study of 132 737 metformin-treated type 2 diabetes persons requiring a second agent, sulfonylurea (SU) and insulin treatment were associated with 28% and 77% increases in likelihood of stroke, respectively, while glucagon-like peptide 1 receptor agonists (GLP-1RA) were associated with significant 35% reductions in stroke, and sodium glucose cotransporter 2 inhibitors (SGLT2i) and thiazolidinediones (TZD) were associated with nonsignificant 44% and 27% reductions in stroke, using dipeptidyl peptidase IV inhibitors (DPP-IVi) as the comparator group.41
5.1 Sulfonylureas
There are theoretical grounds for thinking that activation of neural SU receptors might have benefit in stroke, but a study with parenteral glyburide failed to show improvement in survival.42 Furthermore, in animal models, SU, which block an adenosine triphosphate–sensitive potassium (KATP) channel, increase brain injury, while diazoxide, a KATP channel opener, reduces brain injury, and a meta-analysis of 17 studies of SU treatment of >27 000 persons showed 35% to 41% greater stroke morbidity with SU than among persons receiving comparators.43, 44 Another meta-analysis of 82 randomized controlled trials showed higher stroke risk with SU than with DPP-IVi, GLP-1RA, and insulin.45 A propensity score-matched study of nearly 30 000 persons treated either with metformin or SU showed the latter to be associated with a 25% increase in stroke,46 and in a study of >200 000 persons in the Korean National Health Insurance dataset, metformin was associated with a significant 16% lower likelihood of stroke than the SU glimepiride.47 Of particular note, unlike DPP-IVi, GLP-1RA, and SGLT2i, SU are recognized to cause both severe and nonsevere hypoglycemia, either of which may be associated with stroke.48
5.2 Thiazolidinediones
The Insulin Resistance Intervention After Stroke (IRIS) study randomized 3851 nondiabetic persons with objective evidence of insulin resistance after stroke to either the TZD pioglitazone (PGZ) or placebo, demonstrating a significant 24% reduction in the primary outcome of stroke or myocardial infarction, with a 19% reduction in stroke.49 PGZ was associated with a 22% reduction in stroke in a meta-analysis of five studies of 6840 persons,50 and a more recent meta-analysis including IRIS reported a 32% reduction in stroke with PGZ.51 A 25% to 48% reduction in stroke was reported in approximately 8000 PGZ-treated persons in two UK cohorts52 In a population-based study of 7574 persons using PGZ and 32 337 comparators from the Taiwan National Health Insurance Research Database, those receiving low-, intermediate-, or high-dose PGZ showed likelihood of ischemic stroke 0.79-fold, 0.74-fold, and 0.66-fold, respectively, that of comparators.53
5.3 Glucagon-like peptide 1 receptor agonists
Other than with the short-acting agent lixisenatide, the GLP-1RA are associated with numeric reduction in stroke, significantly so with semaglutide, the oral and parenteral preparations associated with 26% and 39% reductions, and with dulaglutide, associated with 24% reduction in stroke, with a meta-analysis of GLP-1RA trials involving 56 004 participants showing a significant 16% reduction in stroke rates.54 Furthermore, there is a suggestion that dulaglutide is associated with a reduction in the likelihood of substantial cognitive impairment independent of its effect on recognized cerebrovascular events.55
5.4 Sodium-glucose co-transporter-2 inhibitors
In a meta-analysis of randomized controlled trials of 75 540 participants, the SGLT2i were neither associated with increase nor with decrease in stroke risk.56 However, the Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT2 Inhibitors (CVD-REAL) propensity score–matched follow-up of 205 160 persons treated with SGLT2i vs other agents showed stroke event rates of 0.42 vs 0.58 per 100 person-years, yielding a significant 20% event reduction.57 In the Canadian Network for Observational Drug Effect Studies (CNODES), administrative health care databases from seven Canadian provinces and the United Kingdom during the period of 2013 to 2018 showed a significant 22% lower ischemic stroke rate with SGLT2i than with DPP-IVi, although adjustment for age, sex, diabetes duration, and propensity score led to a hazard rate of 0.85, failing to achieve statistical significance.58
6 SUMMARY AND FUTURE DIRECTIONS
The strong association of diabetes with stroke has long been appreciated. The long-term degree of hyperglycemia correlates with stroke likelihood, although there is no evidence that intensive glycemic treatment reduces stroke. Similarly, it appears that acute hyperglycemia at the time of stroke is associated with worse outcome, although there is no evidence that intensive glycemic treatment at the time of stroke improves outcome. There is, however, evidence of reduction in likelihood of stroke with two classes of glucose-lowering agents, the TZD and the GLP-1RA, as well as the evidence of harm with SU.
Some of the insights into mechanisms of the association of diabetes with stroke may allow development of novel treatment approaches to reduce diabetes-related neuronal damage. Diabetes treatment approaches with evidence of pre-stroke prevention might play roles in post-stroke protection. Certainly, the effects of GLP-1RA are highly encouraging, and one wonders whether there might be a role of such treatment in post-stroke management. The SGLT2i have anti-inflammatory, antioxidant, and potentially neuroprotective effects.59 PGZ may mediate changes in miRNA in diabetes with stroke, suggesting a potential mechanism of benefit,60 in addition to its role in reducing insulin resistance. We know that diabetes is associated with vitamin D deficiency, which has a variety of effects both on insulin secretion and on insulin action,61 and that vitamin D deficiency is associated with decreased endothelial function, with prothrombotic and proinflammatory effects, and with reduction in neuronal survival.62 Treatment with vitamin D or its analogs could be envisioned as another post-stroke approach. Might specific delivery systems for neuroprotective miRNA be utilized after stroke? Would inhibitors of transient receptor potential cation channel, subfamily M, member 2 (also known as TRPM2) allow reduction in neuronal calcium, in turn decreasing mitochondrial dysfunction, leading to lower levels of oxidative stress and preventing cell death?63 Inhibition of phosphodiesterase 5 might potentiate protein kinase B/AKT activity, improving insulin action and preventing the cycle of vascular injury of diabetes-related stroke.64
Given the estimated global lifetime risk of stroke from the age of 25 years onward of nearly one quarter of persons, with lifetime risk somewhat greater than one third in Southeast and East Asia,18 and given the doubling of risk among persons with diabetes,12 further understanding of approaches to cerebrovascular disease prevention and to post-stroke protection in diabetes appears an urgent priority.
1 糖尿病与卒中相关的潜在机制
糖尿病与多种代谢变化有关,这可能是卒中发生的基础。胰岛素抵抗有多个驱动因素,与2型糖尿病有着千丝万缕的联系,并与冠状动脉和脑血管动脉粥样硬化有关。胰岛素抵抗是由生物活性脂质(包括神经酰胺和二酰甘油)水平升高所介导的,在许多组织中参与促细胞死亡的作用。高血糖导致晚期糖基化终末产物的形成和多元醇途径、蛋白激酶C途径、聚腺苷二磷酸核糖聚合酶(PARP)和己糖胺途径的激活,所有这些途径都增加了氧化应激,形成了诸如羟自由基、超氧阴离子、过氧化物等活性氧化物,减少了包括超氧化物歧化酶、谷胱甘肽和抗坏血酸在内的内源性抗氧化剂,最终导致DNA、蛋白质和脂质的氧化损伤,造成细胞坏死。高血糖直接或间接增加T细胞和巨噬细胞以及炎症和高凝因子的水平,降低内皮功能,并导致脂蛋白糖基化和氧化,增加其致动脉粥样硬化作用。氧化应激的一种特异性介质通过缺血/再灌注损伤激活瞬时受体电位 (TRMP)2型钙通道,这是糖尿病和胰岛素抵抗状态下上调的一条途径,并介导与氧化应激相关的神经细胞死亡。糖尿病导致关键microRNAs包括miRNA-126、miRNA-124和miRNA-223等水平降低,这些具有抗炎和神经保护作用的microRNA在卒中时也下调。
2 糖尿病与卒中的关系
毫无疑问,强有力的证据表明糖尿病与卒中有关。最近的流行病学研究总结显示,在出血性卒中和缺血性卒中中,确诊糖尿病的平均发病率分别为26%和33%,总体平均值为28%,如果使用糖化血红蛋白(HbA1c)囊括未确诊的糖尿病,卒中患者中糖尿病的患病率为37%,尽管这些估计在不同的研究中存在很大差异。糖尿病前期的情况则并不明显。英国的一项研究表明,在欧洲民族中,HbA1c高于中位数和低于中位数的卒中人数与收缩压高于和低于中位数的人群中有类似的相对增加,但在收缩压较高的南亚种族人群中,这一点的程度较小,而在收缩压较低的人群中,HbA1c较高的卒中患者并没有相对增加。孟德尔随机化研究表明,2型糖尿病的遗传易感性与卒中的关系比与高血糖和高胰岛素血症相关的遗传因素更明显。从卒中风险的角度来看,男性糖尿病患者的相对风险增加1.8倍,女性增加2.3倍,尽管男性的绝对卒中风险最高。对102项前瞻性研究进行新风险因素协作meta分析,包括约850万人-年的随访,结果显示糖尿病使缺血性和出血性卒中的风险分别增加2.27倍和1.56倍。 1型糖尿病患者的卒中发病率可能特别显著增加。在美国、中东和中国的研究中也报道了糖尿病增加卒中风险的类似影响,其中后者的研究很重要,因为有证据表明亚洲大部分地区比西欧和北美更有可能卒中。
糖尿病不仅与卒中有关,还与不良卒中结局的风险有关。在泰国一项关于卒中并发症研究中,糖尿病与败血症和急性肾损伤的风险增加有关,与心血管事件的相关风险较小,与肺炎和泌尿系感染有关,还与住院死亡率有关。糖尿病增加卒中复发的可能性。 糖尿病而不是糖尿病前期,与认知障碍和痴呆症的风险增加有关,特别在卒中后。
卒中的一个重要原因是房颤,在一项相关研究中,糖尿病患者虽然比非糖尿病房颤患者更可能接受华法林抗凝治疗,但卒中风险更高,尽管在1992至2019年期间有所改善。对房颤的一项meta分析表明,糖尿病患者发生卒中以及其他血管事件和死亡率的风险更高,华法林和直接口服抗凝剂相比,风险降低相似
3 长期血糖与卒中的关系
两项大型流行病学研究提供了高血糖与动脉粥样硬化性心血管疾病相关的证据。在瑞典国家糖尿病登记271174人中,卒中与HbA1c水平的相关性比心肌梗死更大,HbA1c水平从约7%开始风险增加。同样,在最近发表的一项对406271名2型糖尿病患者和2086440名非糖尿病患者(平均年龄64岁)的7.3年随访中,糖尿病患者每1000人发生10.1次卒中事件,非糖尿病患者每1000人发生7.3次卒中事件,而缺血性卒中的风险和此类事件的死亡率随着HbA1c水平的上升而线性增加。在对香港7209名2型糖尿病患者的5.4年随访中,372人发生了卒中,表明HbA1c是一个显著的危险因素。糖尿病控制和并发症试验(DCCT)以及糖尿病干预和并发症流行病学(EDIC)对1441名1型糖尿病患者进行了长达29年的观察性随访,结果同样显示HbA1c与卒中显著相关。
然而,英国前瞻性糖尿病研究(UKPDS)在7.9年的随访中,3776名新诊断的2型糖尿病患者中有99人发生卒中,与HbA1c或空腹血糖无关。此外,在控制糖尿病心血管风险的行动(ACCORD)试验中,具有高心血管风险的2型糖尿病患者随机接受强化血糖控制平均3.7年,未能在平均8.8年的观察期间降低卒中发生率。那些类似ADVANCE和VADT试验进行随机化的研究分别在6年和15年的随访中进行强化血糖控制,也未能减少卒中发生。
4 血糖与卒中的急性关系
有明显的证据表明,卒中时出现的急性高血糖是风险的标志,患者功能不良和死亡率都很高。有几项研究提出了这样一个问题,即控制血糖的干预措施是否对这类患者有好处。卒中高血糖胰岛素网络效力(SHINE)随机临床试验包括血糖超过110 mg/dL的已知糖尿病成人患者和血糖超过150 mg/dL的未知糖尿病患者;在卒中结局方面没有明显差异,但在干预组中有2.6%发生严重低血糖(血糖<40 mg/dL),而随机接受标准治疗的患者中没有一人发生严重低血糖。三项meta分析相似地显示,高强度降糖缺乏益处,并伴随着增加低血糖的风险。
5 特异性降糖药与卒中的关系
与专门针对降糖的干预措施令人失望的证据相反,越来越多的证据支持某些降糖治疗的好处,就卒中结果而言,最好避免使用其他降糖药物。没有证据表明二甲双胍增加或减少卒中的发生,但服用这种药物的人可能使卒中结局受益。在一项对132 737名需要第二种药物的2型糖尿病患者进行的研究中,磺脲类药物(SU)和胰岛素治疗使卒中的可能性分别增加28%和77%,而胰高血糖素样肽1受体激动剂(GLP-1RA)与显著降低35%的卒中有关。以二肽基肽酶IV抑制剂(DPP-IVi)为对照组,葡萄糖共转运蛋白2抑制剂(SGLT2i)和噻唑烷二酮(TZD)与卒中发病率降低44%和27%相关,但差异没有显著性。
5.1 磺胺类
有理论依据认为激活神经SU受体可能对卒中有好处,但一项关于注射用格列本脲的研究未能显示提高存活率。此外,在动物模型中,SU阻断了三磷酸腺苷敏感钾(KATP)通道,增加了脑损伤,而二氮嗪是一种KATP通道开放剂,可以减少脑损伤,对17项关于SU治疗的研究进行的meta分析显示,SU治疗超过2.7万人的卒中发病率比接受对照治疗的人高35%到41%。另一项对82个随机对照试验的meta分析显示,SU比DPP-IVi、GLP-1RA和胰岛素有更高的卒中风险。一项对接受二甲双胍或SU治疗的近3万人进行的倾向得分匹配研究显示,后者与卒中增加25%有关。在韩国国民健康保险数据集中的一项超过20万人的研究中,二甲双胍与卒中的可能性比SU格列美脲显著降低16%。特别值得注意的是,与DPP-IVi、GLP-1RA和SGLT2i不同,SU被认为可以引起严重和非严重的低血糖,其中任何一种都可能与卒中有关。
5.2 噻唑烷二酮
卒中后胰岛素抵抗干预(IRIS)研究随机选择了3851名卒中后胰岛素抵抗的非糖尿病患者,服用TZD吡格列酮(PGZ)或安慰剂,显示卒中或心肌梗死的主要预后显著降低24%,卒中发生率降低19%。在一项对6840人的5项研究的meta分析中,PGZ与减少22%的卒中有关。一项包括IRIS在内的更新的meta分析报告称,服用PGZ可以减少32%的卒中。据报道,在两个英国队列中,大约8000名接受PGZ治疗的人卒中减少了25%到48%。在一项以人群为基础的研究中,使用PGZ的7574名患者与32337名对照者相比,服用低、中、高剂量PGZ的人患缺血性卒中的可能性分别是对照组的0.79倍、0.74倍和0.66倍。
5.3 胰高血糖素样肽1受体激动剂
除了使用短效药物利西拉肽(lixisenatide)外,GLP-1RA还与卒中的减少有关,特别是塞马鲁肽,口服和肠外制剂分别减少26%和39%,以及与度拉鲁肽相关卒中减少24%,对涉及56004名参与者的GLP-1RA试验的meta分析显示,卒中发生率显著降低16%。此外,还有一种观点认为,度拉鲁肽与广泛性认知损害的可能性降低有关,而与其对公认的脑血管事件的影响无关。
5.4 钠-葡萄糖共转运蛋白-2抑制剂
在对75540名参与者进行的随机对照试验的meta分析中,SGLT2i与卒中风险的增加或降低无关。然而,新使用SGLT2i(CVD-REAL)倾向评分匹配的205160人与其他药物治疗的患者心血管结局的有效性比较显示,卒中事件发生率为0.42比0.58/100人年,显著减少了20%的心血管事件发生率。在加拿大药物效应观察研究网络(CNODES)中,2013年至2018年期间,来自加拿大7个省和英国的行政卫生保健数据库显示,使用SGLT2i的缺血性卒中发病率比使用DPP-IVi显著降低22%,尽管对年龄、性别、糖尿病病程和倾向评分进行了调整,风险比率为0.85%,但未能达到统计学意义。
6 总结与未来发展方向
长期以来,糖尿病与卒中之间的密切联系一直受到人们的重视。长期高血糖与卒中的可能性相关,尽管没有证据表明强化血糖治疗可以减少卒中。同样,似乎卒中时的急性高血糖与较差的预后相关,尽管没有证据表明卒中时的强化血糖治疗能改善预后。然而,有证据表明,使用TZD和GLP-1RA这两类降糖剂可以降低卒中的可能性,也有证据表明使用SU会造成损害。
对糖尿病与卒中相关机制的一些见解可能有助于开发新的治疗方法,以减少糖尿病相关的神经元损伤。具有卒中前预防证据的糖尿病治疗方法可能在卒中后的保护方面发挥作用。当然,GLP-1RA的效果非常鼓舞人心,人们想知道这种治疗是否在卒中后的治疗中起到了作用。SGLT2i具有抗炎、抗氧化和潜在的神经保护作用。PGZ可能介导糖尿病合并卒中时miRNA变化,这表明除了在降低胰岛素抵抗方面的作用外,还有一种潜在的有益机制。我们知道糖尿病与维生素D缺乏有关,维生素D缺乏对胰岛素分泌和胰岛素作用有多种影响。维生素D缺乏与内皮功能降低、血栓形成和促炎作用以及神经元存活率降低有关。维生素D或其类似物的治疗可以被设想为卒中后的另一种方法。卒中后是否可以利用特定的神经保护性miRNA递送系统?TRPM2抑制剂是否可以减少神经元钙离子,进而减少线粒体功能障碍,从而降低氧化应激水平,防止细胞死亡?抑制磷酸二酯酶5可能增强蛋白激酶B/AKT活性,改善胰岛素作用,预防糖尿病相关卒中的血管损伤。
考虑到全球有近四分之一的人从25岁以后有卒中的终生风险,东南亚和东亚的终生卒中风险略高于三分之一,且糖尿病患者的风险翻了一番,进一步了解预防脑血管疾病和糖尿病卒中后保护的方法似乎是当务之急。




