Thiotepa-busulfan-fludarabine Compared to Treosulfan-based Conditioning for Haploidentical Transplant With Posttransplant Cyclophosphamide in Patients With Acute Myeloid Leukemia in Remission: A Study From the Acute Leukemia Working Party of the EBMT
Supplemental digital content is available for this article.
Graphical Abstract
Abstract
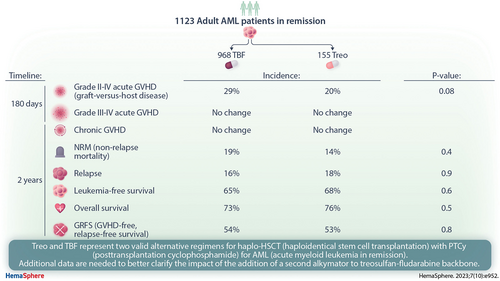
We conducted a registry analysis including adult acute myeloid leukemia (AML) patients in remission who had received thiotepa, busulfan, and fludarabine (TBF) or treosulfan-based (Treo) conditioning for haplo-hematopoietic stem cell transplant (HSCT) with posttransplant cyclophosphamide (PTCy) between 2010 and 2020. A total of 1123 patients met the inclusion criteria (968 received TBF and 155 received Treo). A 1:1 matched-pair analysis was performed on 142 TBF and 142 Treo patients. In the Treo group, 68% of patients received treosulfan at a dose ≥36 g/m2 and 54% of patients received a second alkylator (thiotepa or melphalan). We observed a trend toward increased incidence of grade II–IV acute (a) graft-versus-host disease (GVHD) at 180 days in the TBF group compared with Treo (29% versus 20%; P = 0.08), while incidence of grade III–IV aGVHD was not statistically different. Similarly, the incidence of chronic (c) GVHD was not statistically different in the 2 groups. Incidence of nonrelapse mortality at 2 years was 19% in TBF and 14% in Treo (P = 0.4). Relapse incidence at 2 years was not statistically different in the 2 groups (16% and 18% in TBF and Treo, respectively; P = 0.9). Leukemia-free survival, overall survival, and GVHD-free, relapse-free survival was 65% versus 68% (P = 0.6), 73% versus 76% (P = 0.5), and 54% versus 53% (P = 0.8) in TBF versus Treo, respectively. In conclusion, we did not find a significant difference between the 2 conditioning in the present study; Treo and TBF represent 2 valid alternative regimens for haplo-HSCT with PTCy for AML in remission.
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation using a haploidentical donor (haplo-HSCT) represents an alternative option as consolidation of remission for patients with acute myeloid leukemia (AML) lacking a HLA-matched sibling donor (MSD) or unrelated donor (UD). The development of posttransplant cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis has led to a dramatic increase in the use of haplo-HSCT worldwide, leading to survival rates that approach those observed after HLA-matched transplants.1-3 Nevertheless, the conditioning regimen that best suits this unique transplant platform remains to be elucidated. The original nonmyeloablative conditioning pioneered by the Baltimore and Seattle groups including fludarabine, cyclophosphamide, and low-dose total body irradiation demonstrated low rates of GVHD and nonrelapse mortality (NRM), with the downside of significant relapse.4 The combination of thiotepa, busulfan, and fludarabine (TBF), initially designed to favor engraftment in cord blood transplant,5 was then administered as conditioning regimen for haplo-HSCT in Europe, resulting in excellent antileukemic activity with a limit of toxicity particularly with alkylators at higher doses.6-8 In fact, in the context of haplo-HSCT with PTCy, the administration of cyclophosphamide shortly after the 2 alkylators-based conditioning may increase organ toxicity,9 and a regimen associated with reduced tissue injury could better fit this platform. Treosulfan is an alkylating drug with strong myelotoxic and immunosuppressive properties,10,11 which has demonstrated a strong antileukemic activity combined with reduced extra-hematological toxicity,12 making it an ideal candidate to be incorporated in conditioning regimens for HSCT in AML.13-15 Treosulfan has been recently approved in Europe on the basis of the results of a randomized trial conducted on patients with AML and myelodysplastic syndrome (MDS) receiving MSD or UD HSCT.16,17 Nevertheless, evidence from treosulfan-based (Treo) preparatory regimens in haplo-HSCT for AML is limited to data from single-center experience.18,19 Hence, we designed the present study to compare the outcomes of Treo with TBF conditioning in a cohort of patients receiving haplo-HSCT with PTCy for AML in complete remission (CR).
METHODS
Study design and data collection
This is a registry-based retrospective study from the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT), which is a working group of >600 transplant centers that are required to report all consecutive HSCT and follow-up data annually. Audits are routinely performed to determine the accuracy of the data. Each patient provides consent for HSCT and authorization for the use of HSCT data for research purposes according to the guidelines of the Declaration of Helsinki. The Institutional Review Board of the ALWP of the EBMT granted ethical approval for this study. Eligibility criteria included age >18 years, diagnosis of AML, first haplo-HSCT prepared with either Treo or TBF conditioning between 2010 and 2020. Only patients undergoing transplant in first or second CR (CR1/CR2) were included. NRM was defined as death from any cause in the absence of prior disease recurrence. Disease relapse was defined according to the standard hematologic criteria. Leukemia-free survival (LFS) was defined as survival without relapse. Overall survival (OS) was calculated from the day of transplant until death from any cause or last follow-up. GVHD-free, relapse-free survival (GRFS) was defined by the first of the following events: acute (a) GVHD grades III to IV, severe chronic (c) GVHD, relapse, or death.20 CRFS was defined by the first of the following events: cGVHD (any grade), relapse, or death. Patients with no event were censored at last contact. The cause of death of patients who experienced relapse at any time before death was considered relapse related. Acute and cGVHD were graded according to the standard criteria.21 All outcomes were measured from the time of stem cell infusion.
Conditioning regimens and GVHD prophylaxis
The conditioning regimen was selected according to the participating center discretion. Dose intensity for the 2 compared regimens was defined according to the EBMT criteria22,23 Myeloablative conditioning (MAC) was defined as a busulfan dose of 9.6 mg/Kg for TBF and a treosulfan dose of 42 g/m2 for Treo. Reduced intensity conditioning (RIC) was defined as a busulfan dose of 6.4 mg/Kg for TBF and a treosulfan dose ≤36 g/m2 for Treo. In the Treo group, a second alkylating agent was allowed at standard doses (thiotepa: 5 or 10 mg/Kg; melphalan: ≤140 mg/m2). Only patients who received unmanipulated haplo-HSCT with PTCy as part of GVHD prophylaxis were included in the analysis. Additional antithymocyte globulin (ATG) was allowed.
Statistical methods
Patient-, disease-, and transplant-related characteristics were compared using the χ2 or Fisher exact test for categorical variables, and the Mann-Whitney U test was used for continuous variables. Probabilities of LFS, OS, and GRFS were estimated using the Kaplan-Meier method,24 whereas NRM, relapse, and GVHD were estimated using cumulative incidence analysis considering competing risks.25 To minimize the effect of confounding factors, a propensity score matching analysis was done. For each patient receiving Treo, a separate matched control was identified using exact and propensity score-matched criteria with a caliper of 0·10. Exact matching was used for disease status at HSCT, cytogenetic risk, conditioning intensity, and stem cell source. The propensity score was based on the following variables: age, de novo versus secondary AML, year of HSCT, donor/recipient gender, Karnofsky performance status, ATG administration, and donor/recipient cytomegalovirus serostatus, The patients were well matched, with standardized mean difference estimates of <10% for all matched parameters. Comparisons were performed using Cox model and cluster-robust standard errors was used to account for dependence between observations within matched pairs. Results were expressed as the hazard ratio (HR) with the 95% confidence interval (95% CI). We also run univariate and multivariate analyses on the entire population, including variables differing significantly between the groups, factors known to be associated with outcomes, and a center frailty effect to take into account the heterogeneity across centers in a Cox proportional-hazards model. All tests were 2-sided with a type 1 error rate fixed at 0.05. Statistical analyses were performed with SPSS 25.0 (IBM Corp., Armonk, NY) and R 4.0.2 (R Core Team 2020). R: A language and environment for statistical computing. R: Foundation for Statistical Computing, Vienna, Austria (URL: https://www.R-project.org).
RESULTS
Patient, disease, and transplant characteristics
A total of 1123 patients fulfilled the inclusion criteria for the present analysis. Among them, 968 received TBF and 155 Treo conditioning. After 1:1 pair matching, we were able to compare 142 patients receiving Treo with 142 patients receiving TBF. The main characteristics of the global population and of the pair-matched groups are summarized in Tables 1 and 2, respectively. Median age at transplant was 58 years (range, 18–76); cytogenetic risk was adverse in 16% of the patients. Most (82%) patients underwent transplant in CR1. Median year of transplant was 2019, female donors/male recipients were observed in 22% of patients, and intensity of the conditioning was MAC or RIC in 53% and 47% of patients, respectively. Graft source was peripheral blood stem cells (PBSCs) in 87% of patients in both groups. Within the Treo group, all patients received treosulfan and fludarabine as a conditioning backbone. A second alkylator was administered to 54% of patients (thiotepa: 45%; melphalan: 9%). Dose of treosulfan was 30 mg/m2, 36 mg/m2, or 42 mg/m2 in 32%, 14%, and 54% of patients, respectively. ATG was administered in addition to PTCy as GVHD prophylaxis in 13% of TBF and 11% of Treo patients, respectively. Median follow-up was 15 (range, 12–24) and 18 (range, 14–22) months in TBF and Treo, respectively.
Engraftment, NRM, and GVHD
The engraftment rate was 96% in TBF and 94% in Treo (P = 0.7). Incidence of NRM at 2 years was 19% (95% CI, 13-27) in TBF and 14% (95% CI, 8-21) in Treo (HR, 1.4 [95% CI, 0.74-2.65]; P = 0.3). The leading transplant-related causes of death were GVHD in TBF and infection in Treo group, respectively. Sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) was the cause of death in 2 patients (6%) receiving TBF, while no SOS/VOD-related death was observed in patients receiving Treo. The complete list of causes of death and their relative incidence is reported in Suppl. Table S1.
| Characteristic | TBF (n = 968) | Treo (n = 155) | P-value |
|---|---|---|---|
| Median age at transplant, y (range) | 53 (18–74) | 59 (18–76) | <0.001 |
| Patient gender | |||
| Male | 526 (54) | 92 (59) | 0.24 |
| Female | 442 (46) | 63 (41) | |
| Secondary AML | 112 (12) | 24 (16) | 0.17 |
| Cytogenetic risk (MRC) | 0.16 | ||
| Good | 54 (6) | 10 (7) | |
| Intermediate | 529 (54) | 98 (63) | |
| Adverse | 190 (20) | 25 (16) | |
| Missing | 195 (20) | 22 (14) | |
| FLT3-ITD mutation | 0.9 | ||
| FLT3-ITD | 178 (35) | 30 (34) | |
| FLT3 wt | 335 (65) | 58 (66) | |
| Missing | 455 | 67 | |
| NPM1 mutation | 0.7 | ||
| NPM1 mutated | 183 (38) | 29 (36) | |
| NPM1 wt | 293 (62) | 51 (64) | |
| Missing | 492 | 75 | |
| Disease status at HSCT | |||
| CR1 | 732 (76) | 128 (83) | 0.06 |
| CR2 | 236 (24) | 27 (17) | |
| Disease risk index | 0.34 | ||
| Low | 54 (6%) | 10 (7%) | |
| Intermediate | 716 (74%) | 121 (78%) | |
| High | 198 (20%) | 24 (15%) | |
| Karnofsky performance score | 0.95 | ||
| ≥90 | 795 (82) | 127 (82) | |
| <90 | 173 (18) | 28 (18) | |
| Median year of HSCT (range) | 2018 (2011–2020) | 2019 (2012–2020) | 0.001 |
| Conditioning intensity | 0.12 | ||
| MAC | 582 (60) | 83 (54) | |
| RIC | 386 (40) | 72 (46) | |
| GVHD prophylaxis (other than PTCy) | |||
| MMF + cyclosporine | 744 (77) | 28 (18) | |
| MMF + tacrolimus | 117 (12) | 55 (36) | |
| MMF + sirolimus | 13 (1) | 64 (41) | |
| Other | 94 (10) | 8 (5) | |
| ATG | 0.48 | ||
| Yes | 119 (12) | 16 (10) | |
| No | 849 (88) | 139 (90) | |
| Stem cell source | < 0.001 | ||
| PBSC | 500 (52) | 136 (88) | |
| BM | 468 (48) | 19 (12) | |
| Female donor/male recipient | 184 (19) | 34 (22) | 0.4 |
| Median follow-up, mo (95% CI) | 27 (25-30) | 18 (15-23) | < 0.001 |
- AML = acute myeloid leukemia; ATG = antithymocyte globulin; BM = bone marrow; CI = confidence interval; CR = complete remission; HSCT = hematopoietic stem cell transplant; MAC = myeloablative conditioning; MMF = mycophenolate mofetil; MRC = Medical Research Council Cytogenetic Classification; PBSC = peripheral blood stem cells; RIC = reduced intensity conditioning; TBF = thiotepa, busulfan, fludarabine; Treo = treosulfan-based regimen; wt = wild type.
- Unless otherwise noted, data are expressed as n (%).
| Characteristic | TBF (n = 142) | Treo (n = 142) | P-value |
|---|---|---|---|
| Median age at transplant, y (range) | 58 (21–72) | 58 (18–76) | 0.47 |
| Patient gender | |||
| Male | 81 (57) | 83 (59) | 0.81 |
| Female | 61 (43) | 59 (41) | |
| Secondary AML | 13 (9) | 22 (16) | 0.1 |
| Cytogenetic risk (MRC) | 0.52 | ||
| Good | 7 (5) | 9 (6) | |
| Intermediate | 84 (59) | 91 (64) | |
| Adverse | 23 (17) | 23 (17) | |
| Missing | 28 (19) | 19 (13) | |
| FLT3-ITD mutation | 0.79 | ||
| FLT3-ITD | 28 (34) | 30 (36) | |
| FLT3 wt | 54 (66) | 53 (64) | |
| Missing | 60 | 59 | |
| NPM1 mutation | 0.65 | ||
| NPM1 mutated | 33 (42) | 29 (39) | |
| NPM1 wt | 45 (58) | 46 (61) | |
| Missing | 64 | 67 | |
| Disease status at HSCT | 1 | ||
| CR1 | 116 (82) | 116 (82) | |
| CR2 | 26 (18) | 26 (18) | |
| Karnofsky performance score | 0.38 | ||
| ≥90 | 116 (82) | 119 (84) | |
| <90 | 26 (18) | 23 (16) | |
| Median year of HSCT (range) | 2019 (2014–2020) | 2019 (2012–2020) | 0.39 |
| Conditioning intensity | 1 | ||
| MAC | 75 (53) | 75 (53) | |
| RIC | 67 (47) | 67 (47) | |
| GVHD prophylaxis (other than PTCy) | |||
| MMF + ciclosporine | 95 (67) | 25 (18) | |
| MMF + tacrolimus | 27 (19) | 50 (35) | |
| MMF + sirolimus | 4 (3) | 60 (42) | |
| Other | 16 (11) | 7 (5) | |
| ATG | 0.59 | ||
| Yes | 19 (13) | 16 (11) | |
| No | 123 (87) | 126 (89) | |
| Stem cell source | 1 | ||
| PBSC | 124 (87) | 124 (87) | |
| BM | 18 (13) | 18 (13) | |
| Female donor/male recipient | 31 (22) | 31 (22) | 1 |
| Median follow-up, mo (95% CI) | 15 (12-24) | 18 (14-22) | 0.5 |
- AML = acute myeloid leukemia; ATG = antithymocyte globulin; BM = bone marrow; CI = confidence interval; CR = complete remission; HSCT = hematopoietic stem cell transplant; MAC = myeloablative conditioning; MMF = mycophenolate mofetil; MRC = Medical Research Council Cytogenetic Classification; PBSC = peripheral blood stem cells; RIC = reduced intensity conditioning; TBF = thiotepa, busulfan, fludarabine; Treo = treosulfan-based regimen; wt = wild type.
- Unless otherwise noted, data are expressed as n (%).
We observed a trend toward higher incidence of grade II–IV aGVHD at 180 days in the TBF (29% [95% CI, 21-37]) compared with Treo (20% [95% CI, 13-27]) (HR, 1.48 [95% CI, 0.96-2.3]; P = 0.08) (Table 3). However, the incidence of grade III–IV aGVHD at 180 days was not statistically different in the 2 cohorts, being 12% (95% CI, 7-18) and 10% (95% CI, 6-16) in TBF and Treo, respectively (HR, 1.21 [95% CI, 0.67-2.17]; P = 0.53). The 2-year cumulative incidence of cGVHD and severe cGVHD did not significantly differ in the 2 groups, being 32% (95% CI, 23-42) versus 41% (95% CI, 30-51) (HR, 0.71 [95% CI, 0.44-1.12]; P = 0.14) and 13% (95% CI, 7-21) versus 12% (95% CI, 7-20) (HR, 1.07 [95% CI, 0.48-2.37]; P = 0.87) in TBF and Treo, respectively.
Relapse and survival outcomes
Relapse incidence at 2 years was not statistically different between the 2 conditioning regimens, being 16% (95% CI, 9-24) in TBF and 18% (95% CI, 11-27) in Treo (HR, 0.89 [95% CI, 0.44-1.8]; P = 0.75). Similarly, no significant difference was observed in 2-year LFS (65% [95% CI, 55-74] versus 68% [95% CI, 58-77]; HR, 1.14 [95% CI, 0.71-1.83]; P = 0.59) and OS (73% [95% CI, 63-80] versus 76% [95% CI, 67-83]; HR, 1.19 [95% CI, 0.7-2.03]; P = 0.53) in TBF and Treo groups, respectively (Table 4; Figure 1). GRFS at 2 years did not differ as well, 54% (95% CI, 46-63) in TBF and 53% (95% CI, 43-63) in Treo group, respectively (HR, 1.04 [95% CI, 0.73-1.49]; P = 0.83). CRFS at 2 years was 28% (95% CI, 19-38) and 39% (95% CI, 29-49) in TBF and Treo, respectively (HR, 0.81 [95% CI, 0.58-1.14]; P = 0.23). Results of univariate and multivariate analysis conducted on the global population are presented in the Suppl. Tables S2 and S3.
180-d Grade I–IV aGVHD (95% CI) |
180-d Grade III–IV aGVHD (95% CI) |
2-y cGVHD, Any Grade (95% CI) |
2-y cGVHD, Severe (95% CI) |
|
|---|---|---|---|---|
| TBF | 29% (21-37) | 12% (7-18) | 32% (23-42) | 13% (7-21) |
| Treo | 20% (13-27) | 10% (6-16) | 41% (30-51) | 12% (7-20) |
| HR (95% CI) | 1.48 (0.96-2.3) | 1.21 (0.67-2.17) | 0.71 (0.44-1.12) | 1.07 (0.48-2.37) |
| P-value | 0.08 | 0.53 | 0.14 | 0.87 |
- CI = confidence interval; d = days; GVHD = graft-vs-host disease; HR = hazard ratio; TBF = thiotepa, busulfan, fludarabine; Treo = treosulfan-based regimen; y = years.
DISCUSSION
Haplo-HSCT with PTCy-based GVHD prophylaxis is increasingly used worldwide and currently represents a valid alternative to MSD and UD transplant for consolidation of remission in patients with AML.26 Nevertheless, the conditioning regimen that best suits this peculiar transplant platform remains to be determined. The TBF protocol, which provides for the combination of 2 alkylators, is widely used in haplo-HSCT in Europe for different hematological malignancies. On the contrary, recent efforts to balance conditioning intensity with reduced toxicity led to accumulating data on Treo regimens.27,28 In fact, a recent randomized trial demonstrated reduced NRM and improved survival for RIC treosulfan compared with busulfan in combination with fludarabine in patients with AML or MDS receiving a MSD or UD HSCT.16 Nevertheless, evidence about Treo preparatory regimens in haplo-HSCT with PTCy is limited to data from a single-center analysis,18 and no comparative study is available. Here, we report the results of a large registry study conducted on a homogeneous cohort of AML patients receiving haplo-HSCT with PTCy following TBF or Treo regimen. We did not observe any significant outcome difference between the 2 conditioning platforms. Notably, engraftment following the Treo regimen was not different to that following TBF, despite the latter being widely regarded as a higher intensity protocol. Indeed, our finding is in accordance with results of the previously mentioned randomized trial in which the authors did not observe any graft failure in the Treo-Flu arm compared with 4% of patients receiving Bu-Flu.16 We observed a trend toward reduced incidence of grade II–IV aGVHD at 180 days in the Treo group, which did not exceed 20% at 180 days. This result is remarkable as ≈90% of the patients included in our study had received a PBSC graft. Furthermore, GVHD was the cause of death in 10% of Treo patients compared with ≈30% of deaths in the TBF group. The incidence of aGVHD in the Treo group was substantially lower than that observed in recent reports on haplo-HSCT with PTCy following different conditioning regimens,29-31 in which the grade II–IV aGVHD rate exceeded 30% in recipients of a PBSC graft. Indeed, an early report on Treo conditioning in haplo-HSCT with PTCy showed a reduced aGVHD rate,18 which is similar to our result. However, the low incidence of aGVHD reported in that study might be, at least in part, explained by a significant early increase in circulating regulatory T cells, possibly related to use of sirolimus instead of calcineurin inhibitors (CNI) for GVHD prophylaxis. More recent EBMT registry analyses in patients receiving MSD and UD HSCT23,32,33 reported reduced incidence of aGVHD in Treo compared with busulfan-based regimens. It is conceivable that the immunosuppressive properties of Treosulfan combined with a reduced tissue injury and subsequent lower release of proinflammatory cytokines could temper the incidence of aGVHD,11 although intercenter variability in terms of immunosuppressive drugs administered in addition to PTCy for GVHD prophylaxis should be taken into account. Our finding, if confirmed in larger series, might be of particular relevance in the context of PBSC haplo-HSCT. We observed no significant difference in terms of NRM between the 2 regimens. Notably, the 2-year NRM rate in the Treo group was as low as 14%, with most of the patients receiving higher doses of treosulfan (42 mg/m2) and in about half of the patients included in the analysis a second alkylator (mostly thiotepa) was added to the conditioning platform. The pivotal Treo-Flu versus Bu-Flu randomized trial that provided for treosulfan at the reduced dose of 30 g/m2 reported a 2-year NRM rate of 12%, which was significantly different to that of the control arm (Bu-Flu: 20%) thus leading to a survival advantage for the Treo group.16 In our study, the comparator group performed remarkably well, with an NRM rate as low as 19%; this rate compares favorably with that observed in previous reports of TBF haplo-HSCT in AML.34 This difference could be explained by the stringent inclusion criteria of the present study, which provided for clearly defined doses of alkylators (ie, excluding patients receiving a dose of busulfan >9.6 mg/kg or thiotepa >10 mg/kg). Furthermore, the matched-pair analysis was stratified by conditioning intensity in order to yield fully comparable groups. It is noteworthy that the only cases of fatal SOS/VOD were in patients who received TBF; this finding confirms previous reports of low incidence of SOS/VOD-related deaths in patient receiving treosulfan as part of the conditioning regimen.17,32,35 This could be particularly relevant when selecting a conditioning for patients at very high risk of developing SOS/VOD, considering that the haplo-HSCT with PTCy platform provides for a built-in alkylator (cyclophosphamide) with known toxicity to sinusoidal endothelial cells.36 Importantly, reduced toxicity should not come at the expense of increased relapse. In our cohort, relapse rates following Treo and TBF were superimposable. TBF is widely regarded as the paradigm of a conditioning regimen with powerful antileukemic potential and has been shown to provide the lowest relapse rates among chemo-based regimens for AML.34,37,38 Nonetheless, potent antileukemic activity of treosulfan has been previously shown by several groups in the context of MSD and UD15,39 and we confirm that this holds true for haplo-HSCT with PTCy. The relapse rate following a Treo regimen in our cohort was lower than that reported in the trial by Beelen et al;16 we assume that this difference could be explained by the higher dose of treosulfan (42 g/m2) received by most patients in our study; however, we cannot rule out a possible stronger graft-versus-leukemia effect in patients receiving a haplo-HSCT in view of the broader HLA disparity as previously demonstrated.40 It should be highlighted that the vast majority of patients included in the study had intermediate risk AML and were in first remission at the time of HSCT; further, about half of patients within the Treo group received an additional alkylator, thus making it difficult to assess the specific antileukemic effect of Treo in comparison to TBF. Finally, a noteworthy result of the present analysis is that OS with either Treo or TBF resulted in ≈75% at 2 years, an extremely encouraging result, which should be taken as a benchmark for future research on haplo-HSCT in AML.
NRM (95% CI) |
RI (95% CI) |
LFS (95% CI) |
OS (95% CI) |
GRFS (95% CI) |
CRFS (95% CI) |
|
|---|---|---|---|---|---|---|
| TBF | 19% (13-27) | 16% (9-24) | 65% (55-74) | 73% (63-80) | 54% (43-63) | 28% (19-38) |
| Treo | 14% (8-21) | 18% (11-27) | 68% (58–77) | 76% (67-83) | 53% (43-63) | 39% (29-49) |
| HR (95% CI) | 1.4 (0.74-2.65) | 0.89 (0.44-1.8) | 1.14 (0.71-1.83) | 1.19 (0.7-2.03) | 1.04 (0.73-1.49) | 0.81 (0.58-1.14) |
| P-value | 0.31 | 0.75 | 0.59 | 0.53 | 0.83 | 0.23 |
- CI = confidence interval; CRFS = cGVHD-free, relapse-free survival; GRFS = GVHD-free, relapse-free survival; HR = hazard ratio; LFS = leukemia-free survival; NRM = nonrelapse mortality; OS = overall survival; TBF = thiotepa, busulfan, fludarabine; Treo = treosulfan-based regimen.
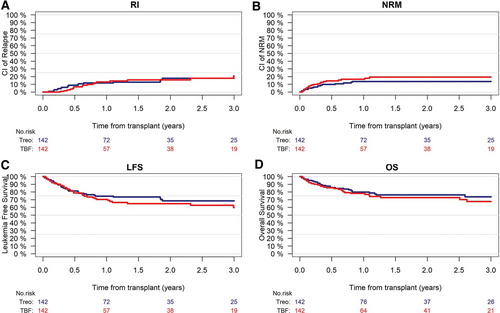
RI (A), NRM (B), LFS (C), and OS (D) according to the conditioning group (Treo vs TBF). RI: P = 0.9; NRM: P = 0.4; LFS: P = 0.6; OS: P = 0.5. LFS = leukemia-free survival; NRM = nonrelapse mortality; OS = overall survival; RI = relapse incidence; TBF = thiotepa, busulfan, fludarabine; Treo = treosulfan-based regimen.
This study carries the inherent limitations of its retrospective design. Allocation to the conditioning regimen was not randomized, and the nature of the analysis made it impossible to identify the reason for choosing a specific protocol. It could be hypothesized that older age favored the choice of Treo over TBF as the median age of the global population was significantly higher in the Treo group. Similarly, it is not possible to assess the reason for adding a second alkylator in patients receiving Treo, and to analyze the relative impact on outcome of the specific agent (melphalan or thiotepa) used. Although these limitations are present, the matched-pair analysis has allowed the comparison of groups fully balanced with respect to the main patient characteristics.
In conclusion, we did not find a significant difference between the 2 conditioning in the present study; Treo and TBF represent 2 valid alternative regimens for haplo-HSCT with PTCy for AML in remission. Additional data are needed to better clarify the impact of the addition of a second alkylator to treosulfan-fludarabine backbone. The path toward tailoring conditioning design to patient characteristics should move forward, and large randomized studies are required to identify which patients are likely to benefit the most from each regimen.
AUTHOR CONTRIBUTIONS
FSa, MMo, AN, and ML designed the study, the synopsis of which was approved by the acute leukemia working party of the European Society for Blood and Marrow Transplantation (EBMT). ML performed all the statistical analysis. FSa wrote the first draft of the article. FSa, AN, ML, and MMo interpreted the data, and edited the article. All coauthors contributed data to the EBMT registry, reviewed the article, and approved the final version.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
The authors declare no sources of funding.