The Insulin Tolerance Test in Morbidly Obese Patients Undergoing Bariatric Surgery
Abstract
Objective: To assess the effect of massive weight loss in relation to insulin resistance and its correlation to changes in glycemic homeostasis and lipid profile in severely obese patients.
Research Methods and Procedures: A prospective clinical intervention study was carried out with 31 morbidly obese women (body mass index: 54.2 ± 8.8 kg/m2) divided into three groups according to their glucose tolerance test: 14 normal, 8 impaired glucose tolerance, and 9 type 2 diabetes. All subjects underwent an insulin tolerance test with intravenous bolus of 0.1 U insulin/kg body weight before silastic ring vertical gastroplasty Roux-en-Y gastric bypass surgery, and again at 2, 4, 6, and 12 months postoperatively. Fasting plasma glucose, hemoglobin A1c, and lipid profile were also evaluated.
Results: A reduction of 68 ± 15% in initial excess body weight was evident within 1 year. Along with weight loss, the following statistically significant changes were found: an increase in the insulin-sensitivity index (Kitt) and a decrease in fasting plasma glucose and hemoglobin A1c, most notably in the type 2 diabetes group. An overall improvement in lipid profile was observed in all three groups.
Discussion: Bariatric surgery was an effective therapeutic approach for these obese patients because it reduced both weight and insulin resistance, along with improving metabolic parameters. Significant correlations were found between insulin resistance and metabolic improvements. Weight loss after bariatric surgery induced an improvement in metabolic fitness, related to the reduction in insulin resistance over a range of glucose tolerance statuses from normal to diabetic.
Introduction
Obesity is an insulin-resistant state par excellence (1) and the metabolic comorbidity is part of a multifaceted syndrome called the insulin-resistance syndrome (2). Our recognition of potential morbidities in severely obese subjects has increased dramatically over time (3) (4). The positive impact of weight loss on the restoration of metabolic fitness has been detected in prior studies of severely obese patients (5). However, the traditional methods of diet and exercise have been unsuccessful in securing significant and lasting weight loss (6) (7). Bariatric surgery is the most radical treatment for obesity and is typically offered only to those with body mass index (BMI) ≥40 kg/m2 or BMI ≥35 kg/m2 with severe comorbidities (8) (9). Data on bariatric surgery have shown improvement in metabolic parameters (10) (11) and marked improvement in glycemic control for type 2 diabetes patients (12) (13) (14). However, little data are available in regard to insulin resistance in severely obese patients (15) (16) (17) (18) (19). Some studies showed a decrease in resistance after bariatric surgery with conflicting results about complete (16) (18) or incomplete (17) (19) reversal of the insulin-resistant state associated with obesity.
Insulin resistance can be measured by using the glucose clamp technique (20), which is considered to be the reference method for an accurate assessment of in vivo insulin sensitivity (21). However, this method is laborious, expensive, and unsuitable for repetition with the same patients in large prospective studies (22). In this study, an intravenous insulin tolerance test (ITT) was performed. Approximately 12 years ago, the ITT was proposed as a simple and inexpensive alternative to more sophisticated techniques (23). The ITT is used currently to estimate insulin sensitivity in diabetic patients at different stages of the disease and with several comorbidities (24) (25) (26) (27). Safety and reproducibility of the ITT were recently discussed (28) (29).
Because of our interest in studying the insulin sensitivity when morbidly obese patients lose massive amounts of weight, we performed ITTs prospectively in a surgical series of patients. We observed the correlation between the reduction of insulin resistance and the improvement in metabolic parameters (glucose homeostasis and lipid profile) over a range of glucose tolerance (normal to diabetic).
Research Methods and Procedures
Thirty-one morbidly obese women (age, 39.8 ± 10.0 years) were classified according to American Diabetes Association criteria: 14 with normal glucose tolerance (NGT), 8 with impaired glucose tolerance (IGT), and 9 with type 2 diabetes (DM). All patients gave informed written consent after the local Research Ethical Committee had approved the study protocol. The patients were recruited from the obesity outpatient clinic of Hospital das Clínicas, University of Campinas (Unicamp), to undergo bariatric surgery. The operation performed was silastic ring vertical gastroplasty with Roux-en-Y gastric bypass (SRVG-RGB) (30), which is based on a combination of restrictive plus malabsorptive mechanisms. The gastroplasty consists of a 25 mL pouch constructed vertically on the lesser curvature of the stomach and completely divided ∼2.0 cm from the gastrojejunostomy. A silastic ring band of 6.2 cm was placed loosely around the pouch at a point ∼2.0 cm from its distal point. Reconstruction was by Roux-en-Y with an efferent limb measuring 120 cm and a pancreaticobiliary limb of 50 cm from the ligament of Treitz (Figure 1).
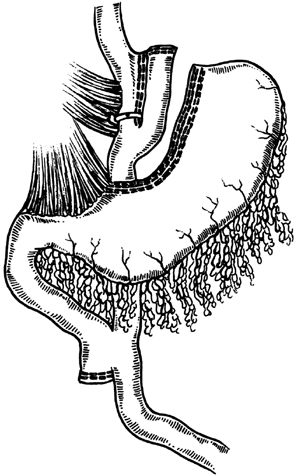
SRVG-RGB technique.
The patients had no clinical evidence of endocrine, cardiac, hepatic, or renal disease. Antihypertensive medications were being taken by 17 of the 22 patients with arterial hypertension (medications included 10 alpha-methyldopa, 4 captopril, and 3 diuretics). No patients were taking estrogen for either contraception or hormone replacement. Nine patients were postmenopausal. One-year follow-up in all subjects has been achieved with interim evaluation performed weekly for the first month, monthly to the sixth month, then quarterly for the next year.
All subjects had anthropometric and laboratory parameters checked at baseline, and at 2, 4, 6, and 12 months after surgery. After a 12- to 14-hour overnight fast, blood specimens were obtained for plasma glucose, triglycerides, glycosylated hemoglobin (HbA1c), and serum total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol. Two patients were using insulin at bedtime combined with metformin (1 g) daily. Two patients were only taking metformin (2 g) daily. Five diabetic patients had only a restricted diet. Patients who were using insulin had their treatment stopped the day before the test but were closely monitored for their glycemic status. Four patients who were taking an oral antidiabetic agent (metformin) stopped the treatment 2 days before testing. At each visit, an ITT, consisting of a bolus of regular human insulin (0.1 U/kg body weight equivalent to 5.22 ± 0.44 U/m2 of body surface) was performed. Blood samples were collected 10 and 5 minutes before, and 3, 6, 9, 12, and 15 minutes after insulin injection. Glucose was injected after an additional 15 minutes to stop the fall of plasma glucose. Plasma glucose t1/2 was calculated from the slope of least-square analysis of plasma glucose concentrations from 3 to 15 minutes after insulin injection, when plasma glucose declined linearly. Kitt represents the percent decline in plasma glucose concentration per minute and is calculated according to the formula: Kitt = (0.693/t1/2) × 100, in which t1/2 represents the half-life of plasma glucose decay (21). Lower insulin-sensitivity index (Kitt) scores mean higher degrees of insulin resistance.
Both correlation and curve regression analyses assessed the improvement changes in Kitt in relation to other variables. One-way ANOVA was also used to compare values during follow-up. Statistical methods are cited in the tables and figures as well as in the results section for clarity. A p < 0.05 was required for statistical significance.
Results
The baseline characteristics of the patients are presented inTable 1. Patients presented with a mean body mass index (BMI) of 54.2 ± 8.8 kg/m2, values similar between groups (p > 0.05). Weight loss after surgery was expressed as percentage of excess body weight lost (EBWL). Patients showed an EBWL average of 67.4 ± 13.4% at the end of 1-year follow-up, reaching a mean BMI of 35.0 ± 5.2 kg/m2 (Figure 2). There were no differences between the groups in EBWL (NGT, 68.7 ± 13.7%; IGT, 65.4.1 ± 8.9%; DM, 67.0 ± 17.0%; p > 0.05, 1-way ANOVA).
| Age (years) | 39.8± 10.0 (range 20–57) |
| Body weight (kg) | 139.5± 23.6 |
| BMI (kg/m2) | 54.2± 8.8 (range 41–75) |
| IEBW (kg) | 76.6 ± 23.4 |
| Hypertension* | 71% |
| Dyslipidemia† | 61.3% |
| Glucose tolerance | 14 NGT, 8 IGT, 9 DM |
| Menstrual status | 71% premenopause |
| 29% postmenopause | |
| Current smoking | 16.1% |
| Familial history for | |
| Obesity | 90.3% |
| Type 2 diabetes | 71% |
| Hypertension | 71% |
- Data are means ± SD.
- * Blood pressure > 160/95 mm Hg or treated.
- † Total cholesterol > 200 mg/dL or HDLcholesterol < 35 mg/dL or triglycerides > 200 mg/dL.
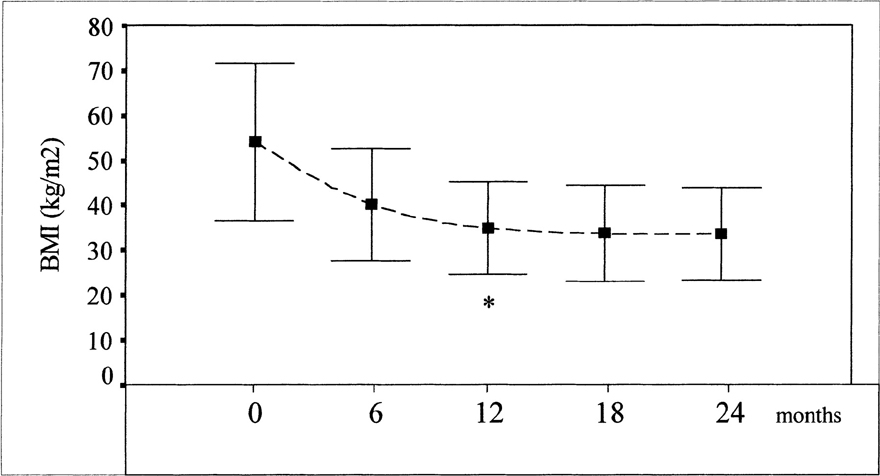
Changes in BMI after bariatric surgery. *After 12 months there is no significant difference among BMI means (ANOVA, p > 0.05). A logarithmical curve is the best-fit in this model (r = −0.73, p < 0.001).
Fasting glucose and HbA1c decreased at 1 year in all three groups (p < 0.01, 1-way ANOVA; Table 2), with normalization of parameters of glucose metabolism in all patients at 12 months. These findings were observed at the same time as the dosages of antidiabetic medication were being reduced. The two insulin-treated patients had their insulin requirements decreased before hospital discharge after surgery and discontinued around the sixth week. Patients who were taking metformin had their treatment discontinued around the sixth month after surgery. At 1 year, all patients were classified as nondiabetic, based on fasting glucose criteria.
| NGT (n = 14) | IGT (n = 8) | DM (n = 9) | ||||
|---|---|---|---|---|---|---|
| Basal | 1 Year | Basal | 1 Year | Basal | 1 Year | |
| BMI (kg/m2) | 54.1 ± 8.9 | 34.3 ± 4.9† | 55.5 ± 9.6 | 35.7 ± 4.5† | 53.2 ± 8.7 | 35.5 ± 6.5† |
| Glucose (mM) | 5.1 ± 0.5* | 4.4 ± 0.3† | 5.9 ± 0.5* | 4.4 ± 0.3† | 10.3 ± 2.3* | 4.6 ± 0.4† |
| HbA1c (%) | 4.6 ± 0.4* | 4.0 ± 0.3† | 5.1 ± 0.3* | 4.4 ± 0.5† | 7.2 ± 2.0* | 4.8 ± 0.6† |
| Cholesterol (mM) | 5.28 ± 1.02 | 4.52 ± 0.75† | 5.71 ± 1.05 | 4.50 ± 0.90† | 5.85 ± 0.96 | 5.14 ± 0.85† |
| LDL cholesterol (mM) | 3.33 ± 0.90 | 2.74 ± 0.59† | 3.98 ± 0.93 | 2.89 ± 0.80† | 3.93 ± 0.88 | 3.31 ± 0.72† |
| HDL cholesterol (mM) | 1.16 ± 0.38 | 1.33 ± 0.22† | 0.96 ± 0.21 | 1.18 ± 0.24† | 1.11 ± 0.28 | 1.34 ± 0.31† |
| Triglycerides (mM) | 3.59 ± 1.91 | 2.26 ± 0.98† | 4.03 ± 1.55 | 2.03 ± 0.75† | 3.93 ± 0.64 | 2.45 ± 0.48† |
| Kitt (%/min) | 2.86 ± 1.39* | 5.50 ± 1.68† | 2.28 ± 0.67 | 4.78 ± 1.33† | 1.77 ± 0.93* | 4.67 ± 2.34† |
- Data are means ± SD. Comparisons between groups and baseline vs. 1-year results (1-way ANOVA).
- * p < 0.01, difference between groups at baseline.
- † p < 0.05, baseline vs. 1 year.
Among the three groups, 1-way ANOVA was applied to the comparison of all clinical variables listed in Table 1, showing no significant differences (p > 0.05). The Kitt showed significant differences only between the DM and NGT groups (p < 0.05). A significant increase in Kitt was noted in the second month of follow-up in the DM group (p < 0.05). In the NGT and IGT groups, the Kitt scores were significantly higher at the fourth month compared with the baseline figures (p < 0.05). A continuous increase in Kitt was seen over a 1-year follow-up in the three groups as a whole group (r = 0.54, p < 0.001). At 1 year there were no difference in Kitt scores between the groups (p > 0.05, 1-way ANOVA;Figure 3).
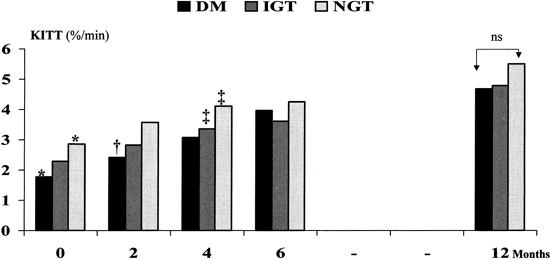
Kitt changes within follow-up. A linear increment of Kitt was observed within follow-up for the whole group (p < 0.001; r = 0.54). The following comparisons were performed by 1-way ANOVA. *Kitt significantly different between NGT vs. DM (p < 0.05); †DM group: Kitt, second month vs. baseline (p < 0.05); ‡NGT and IGT groups: Kitt, fourth month vs. baseline (p < 0.05). NS, not significant.
The simultaneous reductions in both glucose and HbA1c levels in the DM group were correlated logarithmically to the improvement in the Kitt indexes during follow-up (r2 = 0.54 and r2 = 0.46, respectively; p < 0.001). In the NGT and IGT groups, we also observed a negative correlation between glucose and Kitt (r2 = 0.12, p < 0.01; and r2 = 0.31, p < 0.001, respectively), but not to HbA1c (p > 0.05).
The effect of the bariatric surgery was not limited to glucose homeostasis. Table 2 shows the effect of weight reduction on lipid parameters. There were significant reductions in triglycerides, total cholesterol, and LDL cholesterol and an increase of HDL cholesterol levels in all groups at 1 year (1-way ANOVA, p < 0.05). Considering the analysis for the group as a whole, there was a significant negative correlation between Kitt indexes and triglycerides levels (r2 = 0.15, p < 0.001) and a positive correlation with HDL cholesterol levels (r2 = 0.06, p < 0.01). The same pattern was not observed for either total cholesterol or LDL cholesterol (p > 0.05).
Discussion
Bariatric surgery is successful in treating severely obese patients (30) (31). This study showed consistent weight loss in all groups of patients. In agreement with prior studies (32) (33), no differences in weight loss were observed between groups divided by classification of glucose tolerance, reaching a 67.4 ± 13.4% average in EBWL, independent of glucose tolerance status.
Weight loss improves various metabolic parameters in obesity, particularly when glucose tolerance is abnormal (34). Weight reduction after bariatric surgery has a positive effect on metabolic parameters for those patients with and without diabetes (35). Research using insulin clamp techniques have demonstrated an increase in insulin sensitivity among a small series of severely obese patients undergoing bariatric procedures (17) (18).
The use of a Kitt as a measure of insulin resistance, as described by Bonora et al. (22), is convenient for clinical trials (36) (37) (38). A previous study conducted with a large series of patients ranging from normal glucose tolerance to diabetic, and from normal weight to obesity, demonstrated ITT as an accurate and safe technique (27).
Although there are no previous studies using ITT in patients with an average BMI > 50 kg/m2, seven subjects from our cohort were also studied with an euglycemic hyperinsulinemic clamp. Interestingly, a β-coefficient between Kitt and M-value of 0.73 was found, with a trend to statistical significance (p = 0.06; E. Muscelli et al., unpublished data, 2001).
Some studies had observed that the increased extracellular water compartment to intracellular water compartment (ECW/ICW) ratio in severe obesity (39) does not normalize after weight reduction in subjects undergoing surgical treatment (40) (41). The observation that the ECW/ICW does not normalize after weight reduction, and even increases after malabsorptive surgery, has led to the conclusion that obesity is accompanied by a primary defect in fluid regulation, and that severe obesity leads to irreversible changes in the fluid regulation (42). The fluid-overloaded state accompanying severe obesity, and the changes in body composition impacting fat mass during dynamic weight loss (43), could affect the volume of distribution of intravenous insulin during the ITT and impact the results of Kitt presented here. Finally, preoperative mean body fat accounted for 50% of the body weight in obese patients (44). After a 3-month postoperative period of rapid weight loss due to reduction of both lean body mass and body fat, a continued decrease in body fat, with no change in lean body mass, is observed (44). No data on body composition are available in this study.
A simultaneous improvement in glucose homeostasis (fasting glucose and HbA1c) related to an increase in Kitt scores was observed in the diabetic subgroup, but not in the NGT or IGT subgroups. In other words, the patients with the higher levels of fasting glucose and HbA1c experienced greater drops in these parameters than their more normal counterparts. Moreover, it was possible to withdraw all pharmacological treatment for diabetes by the sixth month after surgery, as previously observed (45) (46) (47) (48) (49) (50).
A reasonable explanation for the improvement in glucose homeostasis could be related to a complex metabolic transition observed in patients undergoing bariatric surgical procedures. Glucose and insulin kinetics may be influenced not only by the negative calorie balance, but also by a reduction in the capacity for absorbing glucose by the gut (51). Furthermore, changes in gastrointestinal hormones may contribute to the beneficial effects. Kellum at al. (52) had observed an increase in glucagon-like peptide 1 (GLP-1) in the Roux-en-Y gastric bypass. Sirinek et al. (53), observing the parallel reduction in glucose, glucose-dependent insulinotropic polypeptide, and insulin after surgery, suggested that the exaggerated glucose-dependent insulinotropic polypeptide release was partly responsible for the hyperinsulinism in the morbidly obese patients. Otherwise, hyperinsulinism seems to be a secondary event to the insulin-resistant state, but not a primary defect of severe obesity. Mason at al. (54) had discussed the role of GLP-1 during weight reduction and glucose metabolism after anti-obesity surgery. The increase in GLP-1 levels after weight reduction promotes an inhibition of hyperglucagonemia and improvement in hepatic insulin action. Moreover, the increase in GLP-1 stimulates insulin secretion, especially restoring first-phase insulin secretion that is impaired in the obese diabetic patients. Other possible mechanisms involve weight loss-induced changes in insulin action in skeletal muscle (15) and changes in insulin and C-peptide secretory dynamics (18).
These favorable effects of bariatric surgery on glucose metabolism were so impressive that some authors claim that type 2 diabetes may be considered a surgical disease in severely obese patients (50) and that “an operation proves to be the most effective therapy for adult-onset diabetes” (51). Several other authors suggest diabetes as an ancillary criteria for patient selection for obesity surgery (55) (56) (57).
In addition to the improvement in glycemic control, bariatric surgery resulted in a major improvement of the lipid profile. Lipid levels were improved, but only HDL cholesterol and triglycerides were statistically related to changes in the insulin-resistant state. This was not a surprising result considering that HDL cholesterol and triglycerides are part of the metabolic syndrome. Other possible mechanisms could account for these improvements rather than just changes in insulin sensitivity. Severely obese patients usually consume more fat and cholesterol than normal-weight subjects (58), and after our surgical intervention there is a decrease in both the total amount of fat intake due to gastric restriction and dietary counseling, and a decrease in fat absorption due to the proximal small bowel bypass.
Kitt improved rapidly postoperatively, reinforcing the benefit of modest weight loss in improving insulin resistance (59). Despite the improved Kitt after surgically induced weight loss, our subjects remained obese and had reduced Kitt values compared with our normal population, 6.4 ± 2.4%/min (B. Geloneze and M.A. Tambascia, unpublished data, 2000). Interestingly, all patients had normalization of glucose homeostasis at 1-year follow-up. Such favorable metabolic effects may explain why marked weight loss associated with bariatric surgery could prevent the development of overt diabetes in NGT patients (60).
In conclusion, weight loss is a major target in severely obese patients and can be achieved using bariatric surgery. This method leads to an improvement in insulin sensitivity in patients ranging from normal glucose tolerance to diabetic. The presence of mild type 2 diabetes did not impact the overall response to weight loss. Subjects with diabetes achieve better glycemic control and discontinuation of all pharmacological treatment. These favorable results have been confirmed in large prospective trials such as the ongoing Swedish Obese Subjects Study (61). Finally, the clinical implications of these results add to the impact of previous studies placing surgery as an ancillary therapeutic approach for severely obese patients and especially for the subgroup of type 2 diabetic patients.
Acknowledgments
This work was supported by the Funcamp (Fundação de Desenvolvimento da Unicamp—Universidade de Campinas). We thank Dr. Robert Ratner, Medlantic Research Institute, Washington, DC, for helpful comments on this manuscript.





