The protective role of resveratrol against sulfoxaflor-induced toxicity in testis of adult male rats
[Correction added on 9 July 2021, after first online publication: ORCID ID for the author Areeg M. Abd-Elrazek has been added.]
Abstract
This work was designed to explore the protective role of resveratrol (RES) against sulfoxaflor (Sulfx)-induced reproductive toxicity in adult male rats. The animals were divided into six groups: Control group, Sulfx treated groups (79.5 and 205 mg/kg/day), RES treated group (20 mg/kg/day), RES + Sulfx treated groups (20 mg/kg Res + 79.5 or 205 mg/kg Sulfx) orally for 28 consecutive days. Testicular samples were collected from all groups at the end of the treatment period. Tissue supernatants were isolated for oxidative stress and cellular energy parameters; tissue samples were prepared for histopathological examination. In addition, caspase-3 activity was calculated to assess spermatogenesis. Finally, DNA laddering assay was performed to detect DNA fragmentation as a hallmark of apoptosis. Our results showed that Sulfx treatment induced a significant increase in testicular levels of MDA, NOx, GSSG and reduced GSH level and cellular energy parameters in a dose-dependent manner compared to the control group. The results were confirmed by histopathological study which showed pathological changes in Sulfx treated groups. A significant increase in caspase 3 and DNA fragmentation was also observed. However, concomitant administration of RES to Sulfx -treated rats showed significant modulation against Sulfx-induced reproductive toxicity and attenuated the biochemical, apoptotic and histopathological changes. In conclusion, our results suggest that exposure to Sulfx at the two selected doses induces testicular toxicity and these effects can be ameliorated by supplementation of RES.
1 INTRODUCTION
The widespread use of insecticides in agriculture and pest control protocols greatly increases human exposure to this toxic chemicals.1-3 The potential risk from exposure to insecticides and their effects on human and animal health are of great concern.4 Residues of these insecticides have been detected in grains, vegetables, water bodies and soil.5 Animal studies have reported that exposure to insecticides can affect the male reproductive organs and cause reproductive toxicity.6-8
Neonicotinoids are used worldwide as agricultural insecticides for crop protection. Although neonicotinoids have low toxicity to humans and mammals compared to conventional insecticides, many studies have shown that exposure to neonicotinoids results in potential risk to humans and mammals.9 Nowadays, neonicotinoids and their metabolites have been detected in various human biological samples. Due to their low molecular weight and high solubility in water, neonicotinoids can penetrate plant tissue and remain there for a long time10 and this increases the probability of exposure to humans and non-target organisms.11 Neonicotinoids have been estimated to exert many toxic effects on both invertebrates and vertebrates, such as neurotoxicity, hepatotoxicity, nephrotoxicity and reproductive toxicity.12, 13 Several in vivo studies have demonstrated the reproductive toxicology of neonicotinoids, especially in rodents. These results showed that exposure to neonicotinoid pesticides has adverse effects on sperm fertility and embryonic development in mammals, suggesting that neonicotinoid pesticides may pose potential reproductive health risks to humans, especially children and farmers.7, 9
Sulfoxaflor (Sulfx) is an insecticide that belongs to the sulfoximine class of neonicotinoids.14 The mechanism of action of sulfoximine-based insecticides occurs by acting on the nicotinic acetylcholine receptor (nAChR) of insects as a competitive modulator,15 which causes rapid excitatory neurotransmission in the central nervous system of insects, followed by paralysis and eventually death.16 Recently, sulfoximine has been approved for use in many countries all over the world. Biologists emphasized the use of sulfoximines in threatening pollinators due to lack of knowledge about their sublethal effects.17 As a systemic insecticide, traces of Sulfx may remain in the nectar and pollen of plants after treatment, posing a health hazard to animals and humans.18 Toxicokinetics studies showed that Sulfx is rapidly absorbed and widely distributed following oral treatment in rats with little metabolization. No bioaccumulation of Sulfx or its metabolites in tissues or plasma was observed.19 Dietary toxicity studies in rats for 28 days showed that the major target organ was the liver, resulting in increased liver weight and hepatocellular hypertrophy.20 Long-term dietary administration of Sulfx (750 ppm and above) resulted in liver tumors in rodents.21 In a long-term toxicity study, rats showed enlarged testes, atrophy of seminiferous tubules, reduced sperm count in epididymitis, and secretory material in accessory sex glands in addition to tumors of preputial glands.20 Testes of rats exposed to Sulfx treatment at doses of 4.24 and 21.3 mg/kg bw/d for 24 months exhibited Leydig cell tumors.20 In addition, exposure to high-dose dietary Sulfx caused developmental toxicity as evidenced by limb contractures and reduced neonatal survival in rats.22 The proposed mechanism of action responsible for the Sulfx-induced fetal abnormalities and neonatal death in rats involves sustained activation of the fetal nAChR of rat muscles, resulting in sustained muscle contracture. This mechanism of action is not considered relevant to humans because of qualitative differences in Sulfx agonism at rat and human muscle nAChR.22 A previous study showed that exposure to Sulfx induces oxidative stress and lipid peroxidation in earthworms, as indicated by the significant increase in hydroxyl radical, cellular antioxidant enzymes, and reactive thiobarbituric acid substances (TBARS) content.23 Based on the fact that Sulfx contributes to the formation of reactive oxygen species (ROS). The use of plant products that can scavenge free radicals and upregulate endogenous antioxidant enzymes can be used to modulate Sulfx-induced toxicity.
Resveratrol (trans-3,5,4′-trihydroxystilbene, RES), is a natural polyphenolic phytoalexin and a potent antioxidant abundant in many plants, including grapes, peanuts, and red wine.24 RES has been proposed as an effective and powerful therapeutic approach due to its many biological activities including antioxidant, anti-inflammatory, anticancer, and antiviral properties.25, 26 Daily oral intake of RES to healthy adult male rats increased sperm production by stimulating the hypothalamic–pituitary-gonadal axis.27 RES has been described as an effective scavenger of hydroxyl-, superoxide-, and metal-induced radicals, as well as having antioxidant capabilities in cells producing ROS. Therefore, the protective role of RES could also be mediated by counteracting constitutive oxidative stress within testicular tubules by acting as a radical scavenger, metal chelator and enzyme regulator.28 It inhibits lipid peroxidation (LPO) of spermatocytes and increases sperm motility, viability and enhances sperm production in vivo.29 Moreover, it decreases germ cell apoptosis in mice and rats and shows protective effects against testicular toxicity induced by many anticancer drugs and environmental toxins.28, 30-32
The present study provides the first toxicological efficacy data of a short-term toxicity study induced by Sulfx at two selected doses (79.5 and 205 mg/kg body weight) on the testis of adult male rats after daily oral administration. In addition, the possible ameliorative effect of RES on Sulfx-induced testicular toxicity was investigated.
2 MATERIALS AND METHODS
2.1 Chemicals
Trans-resveratrol (CAS 92614, NEOCELL.com, United States) was purchased from a local pharmacy, it was provided as Harmoni-T micronized trans-resveratrol capsules for ingestion (100 mg/5 capsules). Sulfoxaflor (50%) was purchased from AGRIMATCO Ltd Co (Egypt) under the trade name Transform; the other components include balance (25.9%) and Kaolin (24.1%). According to Trckova et al.33 oral administration of kaolin did not exert any toxic effect in the organisms. Furthermore, due to the lack of primary toxicity, kaolin is considered an effective means to prevent or ameliorate the adverse effects of many environmental toxic agents. Other used chemicals were of analytical grades and were supplied by Sigma Chemical Co. (St. Louis, MO, United States).
2.2 Dose selection
The dose of RES was selected based on reports from previous studies that showed safety and ameliorative effects of RES against testicular damage.27, 29 The LD50 of Sulfx is reported to be 1405 mg/kg body weight in male rats.34 A previous study demonstrated that the low observable adverse effect level (LOAEL) for sulfoxaflor was reported to be 79.4 mg/kg bw/d in a 28-day dietary rat study.35 In a study of toxicity conducted according to the Organization for Economic Co-operation and Development (OECD) test guideline 407 on F344/DuCrl rats, excessive reductions in food consumption with body weight loss as well as changes in the male reproductive system were observed in rats treated with 3000 ppm of Sulfx (equivalent to 205 mg/kg bodyweight, ~1/7 th of LD50) for 28 days.36 These clinical signs determine the maximum tolerated dose (MTD) that will be tolerated by the animal for the study duration and where target organ toxicity is likely to be observed.37 The same was confirmed in our preliminary laboratory study. Therefore, two doses, that is, high dose (205 mg/kg) and LOAEL (79.4 mg/kg) were selected for subacute toxicity study.
2.3 Experimental groups and animals
A total of 36 adult male Sprague Dawley rats weighing 180–220 g were purchased from Helwan Breeding Farm, Ministry of Health, Helwan, Egypt. The animals were housed six per cage in a room under standard conditions of ventilation, temperature (25 + 2°C) and humidity (60%–70%) and subjected to a 12 h light–dark cycle throughout the experiment. Animals were provided with water ad libitum and fed the standard laboratory diet. The protocols for the animal experiments were carried out according to the national guidelines for Ethical Conduct in the Care and Use of Animals. All experimental procedures and animal maintenance were performed and approved by National Organization for Drug Control and Research, Giza, Egypt. After 7 days of acclimatization, the animals were randomly divided into six different groups, each consisting of six rats, as follows:
Group 1 (control group): each animal was given dist. water daily by gastric tube for 28 days. Group 2 (RES only): the animals received RES (20 mg/kg b.wt/day) orally by gastric tube for 28 days. Group 3 (low dose of Sulfx): the animals received Sulfx (79.5 mg/kg b.wt/day) dissolved in dist. water and given orally by gastric tube for 28 days. Group 4 (high dose of Sulfx): the animals received Sulfx (205 mg/kg b.wt/day) and administered as previously. Group 5 (RES+ L-Sulfx group): the animals received combined treatment of RES (20 mg/kg) and Sulfx (79.5 mg/kg). Group 6 (RES + H-Sulfx group): the animals received combined treatment of RES (20 mg/kg) and Sulfx (205 mg/kg).
2.4 Dissection and sampling
After the experimental period, all rats were killed by decapitation under ketamine anesthesia and were dissected. Testes from all groups were excised, weighed, and homogenized to make 10% homogenate (wt/vol) in ice-cold 0.1 M Tris (hydroxymethyl) aminomethane-HCl (Tris-HCl), pH 7.4, using an ice-chilled glass-homogenizing vessel in a homogenizer fitted with Teflon pestle (Glass-Col, United States). The homogenate was then centrifuged in a cooling centrifuge at 3000 × g at 4°C for 10 min to remove nuclei and debris. The supernatant was then kept at −80°C for further analyses. For histological assessments, sections from the testes were kept in 10% neutral buffered formalin. Another section was kept at −20°C to assess the laddered DNA fragmentation assay.
2.5 Histopathological processing
Specimens were collected from all groups subjected to our study (n = 6), which were sliced, and fixed in 10% buffered formalin. Paraffin blocks were prepared from those samples after a serial of dehydration, clearing and embedding. The paraffin-embedded material was prepared in 5-μm-thick slices, which were mounted on microscope slides, stained with hematoxylin and eosin. Finally, it was examined under optical microscopy to evaluate the morphologic aspects.38
2.5.1 Morphometric measurements
Detection of circumferences (μm)
Means of seminiferous tubules circumferences of the tested groups were determined by a lining of a cross section of seminiferous tubules (n = 6) selected on each field at a magnification of 50× (longitudinal cuts were excluded).
Total sperm cells maturation count
Counting of spermatogenic cells was performed including all stages from spermatogonia, up to spermatozoa.
Modified Johnsen spermatogenesis scoring
(1) Absence of seminiferous epithelium. (2) Absence of germinal cells, Sertoli cells only. (3) Spermatogonia only. (4) Absence of spermatozoa or spermatids, only few spermatocytes found. (5) Absence of spermatozoa or spermatids, many spermatocytes. (6) Absence of spermatozoa, late spermatids, but few early spermatids. (7) Absence of spermatozoa and late spermatids, but many early spermatids found. (8) Less than five spermatozoa per tubule, few late spermatids. (9) Slightly impaired spermatogenesis, many late spermatids, disorganized epithelium. (10) Full spermatogenesis.38
2.6 DNA fragmentation assay
DNA fragmentation was determined according to the standard protocol described by Khalaf et al.39 with minor modifications. Briefly, testicular tissues were homogenized, washed in PBS, and suspended in 100 μl lysis buffer (10 mM Tris–HCl, pH 8.0, 100 mM NaCl, 5 mM EDTA and 5% Triton X-100, 0.25% SDS) for 20 min on ice before centrifugation. Then incubated with 50 μg/ml proteinase K at 56°C overnight. After incubating overnight, DNAase-free RNAase A was added to 100 μg/ml. The samples were incubated at 37°C for 2 h, extracted with an equal volume of phenol: chloroform: isoamyl alcohol (25: 24: 1) for 1 min, and centrifuged at 10 000 rpm for 10 min. The aqueous phase was further extracted with chloroform, and centrifuged. DNA was precipitated from the aqueous phase with two volumes of cold absolute alcohol containing 0.3 M sodium acetate at 4°C overnight. The precipitate was centrifuged at 10 000 rpm for 10 min. DNA pellet was washed with 70% alcohol and dissolved in TE buffer. Electrophoresed in 1.5% agarose gel containing ethidium bromide at 80Volt. A 100-bp DNA ladder (Invitrogen, Waltham, MA, United States) was used as a molecular size marker. DNA fragments were visualized under UV light Transilluminator (Stratagene, La Jolla, CA, United States) and captured.
2.7 Oxidative stress parameters
The used apparatus in oxidative stress determination is Agilent HP 1200 series HPLC, UV detector, and quadrate pump.
Malondialdehyde (MDA) was assayed by using a modified method of karatase40 The column used was hypersil C18 (5 μm particle and 80 Ao pore size) (250 × 4.6 ID), with mobile phase 30 mM KH2PO4 and methanol (65%–35%), at 254 nm.35
Reduced and oxidized glutathione determination in testis homogenate performed according to Jayatilleke and Shaw41 method, by using a mobile phase consisting of 0.0025 M sodium phosphate buffer and methanol, C-18 column 250-mm × 3.9-mm, UV detector at 190 nm.
Nitrates and nitrites were determined using anion exchange PRP-X100 Hamilton, 150 × 4.1 mm, 10 μm, mobile phase: was a mixture of 0.1 M NaCl—methanol, (45:55), at 230 nm, UV detector.42
ATP, ADP and AMP determination in testicular tissue by using Ultrasphere ODS EC 250 × 4.6 mm column, Mobile phase phosphate buffer adjusted to pH 7.0 and acetonitrile, wave length 254 nm.43 Total adenylate energy charge (AEC) was calculated according to the equation: AEC = (ATP + 0.5ADP)/(ATP + ADP + AMP).44
2.8 Detection of Caspase 3 Activity
Caspase-3 was assayed using Glory Science Co. ELISA kits (United States), following manufacturer's instructions.
2.9 Statistical analysis
Statistical analysis of the obtained data was performed using Statistical Package Software Systems (SPSS) version 25. Significant differences among means were evaluated using one-way analysis of variance, Tukey's Posthoc multiple comparisons, estimated to compare between means of different groups where p < .05 was accepted as being significant in statistical tests. Values were expressed as means ± SE
3 RESULTS
3.1 Histopathological evaluation
Figure 1A showing that the control group exhibited normal spermatogenesis, testicular parenchyma with seminiferous tubules and interstitial tissue. RES group showed normal spermatogenesis, testicular parenchyma with seminiferous tubules and interstitial tissue. Testis treated with a low dose of sulfoxaflor (L-Sulfx group) showed hypospermatogensis, spermatocytes exfoliated into the lumen (D), seminiferous tubules atrophy (a), distorted interstitial tissue (arrows). Sulfoxaflor high dose (H-Sulfx) treated testis showing marked hyaline degeneration (*), sertoli cells only syndrome (sco), pyknotic nuclei (p), and distorted interstitial tissue (arrow). Co-administration of RES and Sulfx (79.5 mg/kg) showed normal spermatogenesis but some atrophy of seminiferous tubules (a) still found. While combined treatment with RES and Sulfx high dose caused spermatocytes exfoliated into the lumen (D), mild seminiferous tubules atrophy (a), and microvacuoles (v) (Magnification ×200).
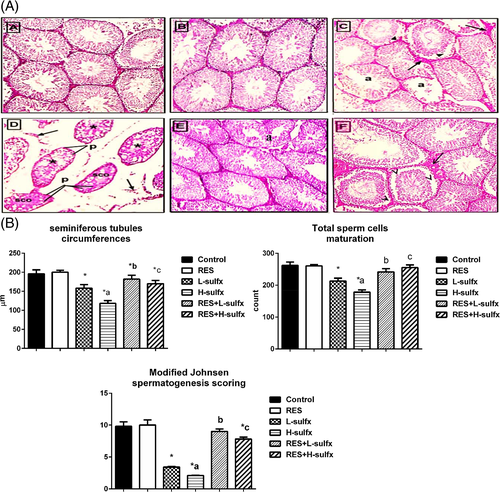
Furthermore, the data reported in Figure 1B, Table 1 represented morphometric measures, it showed that Sulfx administration at the two selected doses significantly diminished the mean of seminiferous tubules circumferences and total sperm cells maturation count as compared to the normal control in a dose-dependent manner. While co-administration of resveratrol markedly increased both the mean of seminiferous tubule circumferences, and total sperm cells maturation count indicating an enhancement in the spermatogenesis process. Regarding Modified Johnsen spermatogenesis scoring, it was clear that the normal control group showed a score of 9.8, while RES positive control showed a score of 10. On the other hand, the L-Sulfx and H-Sulfx groups reported 3.4 and 2.1 respectively. Co-administration of RES with Sulfx (79.5 and 205 mg/kg) resulted in an elevation of the score to 9 and 7.8 respectively.
3.2 Effect of RES on testicular DNA fragmentation in Sulfx-treated animals
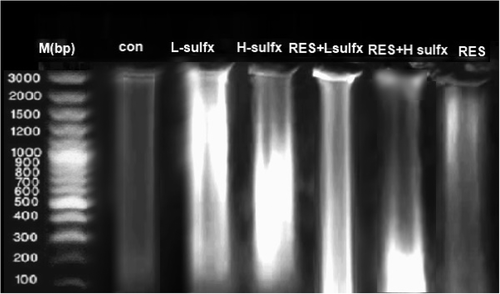
DNA laddering is used as a distinct feature for the detection of late-stage apoptosis, so, we used DNA fragmentation assay as a criterion for apoptosis. Figure 2 showed the fragmentation patterns in the testicular DNA of the control and different treated groups. As shown in figure (2) treatment with RES does not induce any DNA fragmentation (lane 7) as it has a banding pattern with neither a DNA ladder nor a smear on the gel similar to the pattern of a control group (lane 2). DNA isolated from the testis of rats exposed to Sulfx treatment at both the low and high dose showed DNA fragmentation indicated by the appearance of laddered and smeared DNA fragments (lanes 3 and 4) when compared with the intact pattern of genomic DNA of the control group. Treatment with RES before Sulfx administration at the two tested doses reduced the DNA damage as indicated by a slight smear of DNA and the less obvious fragmentation on the gel (lanes 5, 6).
3.3 Effect of RES on oxidative stress markers (MDA, GSH, GSSG, and NO) in Sulfx-treated animals
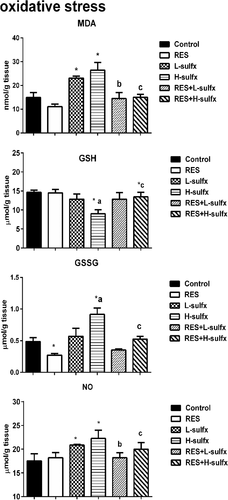
Treatment with RES alone (20 mg/kg) resulted in a statistically non-significant change in MDA and GSH levels in testis homogenate when compared with the control group (Figure 3). Animals that received Sulfx at the two tested doses induced a significant increase in LPO represented by the significant elevation in MDA (p ≤ .05) in addition to a significant increase of NO in testis homogenate as compared with the control group. Moreover, administration of Sulfx at the high dose (205 mg/kg) resulted in a significant reduction in GSH (p ≤ .05) versus elevation in GSSG comparing to the control group (Figure 3). Oral administration of RES before Sulfx at the two tested doses significantly increased the MDA and NO levels (p < .05) when compared with Sulfx treated groups. Co-administration of RES exhibited a significant improvement in GSH and GSSG (p < .05) when compared with groups treated with a high dose of Sulfx (Table 2).
| Control | RES | L-Sulfx | H-Sulfx | RES + L-Sulfx | RES + H-Sulfx | |
|---|---|---|---|---|---|---|
| Seminiferous tubules circumferences (μm) | 196.0 ± 10.4 | 200.0 ± 5.2 | 158.0 ± 9.5a | 118.2 ± 7.1a,b | 181.7 ± 10.2a,c | 169.9 ± 8.4a,d |
| Total sperm cells maturation (count) | 261.9 ± 10.4 | 260.3 ± 4.1 | 212.5 ± 9.5a | 178.1 ± 7.1a,b | 241.6 ± 10.2a,c | 155.2 ± 8.4a,d |
| Modified Johnsen spermatogenesis scoring | 9.8 ± 0.7 | 10.0 ± 0.8 | 3.4 ± 0.1a | 2.1 ± 0.02a,b | 9.0 ± 0.4c | 7.8 ± 0.3a,d |
- Note: Data expressed by means ± SEM, (n = 6).
- a Significantly different from control group.
- b Significant difference between the low and high dose Sulfx-treated groups (bp < .05).
- c Significantly different between L-Sulfx and RES + L-Sulfx treated groups (cp < .05).
- d Significantly different between H-Sulfx and RES + H-Sulfx treated groups (dp < .05).
3.4 Effect of RES on testicular tissue cell energy performance in Sulfx-treated animals
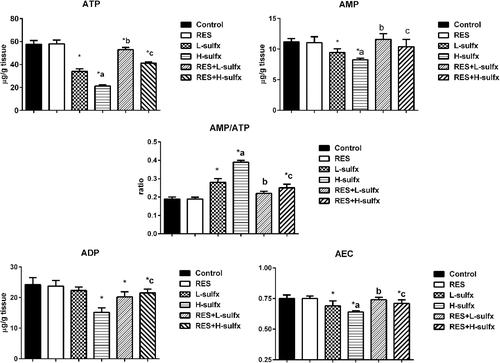
In the recent study, Sulfx administrated at the two tested doses lead to a significant decrease in cell energy indicated by decreased adenylate energy charge (AEC) in testis homogenate and increased AMP/ATP ratio when compared to the control group (Figure 4). whereas, treatment with RES before Sulfx at the two tested doses enhanced the disruption in cell energy by increasing the ATP, ADP and AMP levels, and decreasing AMP/ATP ratio significantly (p < .05) (Figure 4 and Table 3).
| Groups parameters | Control | RES | L-Sulfx | H-Sulfx | RES + L-Sulfx | RES + H-Sulfx |
|---|---|---|---|---|---|---|
| MDA (nmol/g tissue) | 15.12 ± 0.12 | 11.74 ± 0.03 | 23.35 ± 0.14a | 26.73 + 0.02a | 14.48 ± 0.17b | 15.57 ± 0.04c |
| GSH (μmol/g tissue) | 14.38 ± 0.02 | 14.89 ± 0.12 | 13.42 ± 0.07 | 9.06 ± 0.04a,d | 12.04 ± 0.14 | 11.61 ± 0.12a,c |
| GSSG (μmol/g tissue) | 0.488 ± 0.01 | 0.273 ± 0.03a | 0.571 ± 0.02 | 0.857 ± 0.01a,d | 0.453 ± 0.01 | 0.564 ± 0.25c |
| NOx (μmol/g tissue) | 17.51 ± 1.5 | 18.19 ± 1.1 | 20.87 ± 0.12a | 22.28 ± 1.73a | 18.19 ± 1.04b | 19.98 ± 1.4c |
- Note: Data expressed by means ± SEM, (n = 6).
- a Significantly different from control group.
- b Significantly different between L-Sulfx and RES + L-Sulfx treated groups (bp < .05).
- c Significantly different between H-Sulfx and RES + H-Sulfx treated groups (cp < .05).
- d Significant difference between the low and high dose Sulfx-treated groups (dp < .05).
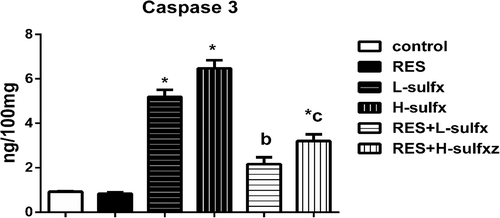
3.5 Detection of caspase 3 activity
In the recent study, Sulfx administrated at the two tested doses showed a significant decrease in caspase 3 levels in testis homogenate regarding control group, while RES co-administration with Sulfx at the two selected doses attenuated the elevated levels of caspase 3 as compared to the corresponding Sulfx treated group. The effect of RES was more pronounced in the group treated with a low dose of Sulfx as indicated by the absence of significant difference from the control group (Figure 5).
4 DISCUSSION
Sulfx is one of the recently marketed insecticides that belong to the sulfoximine class of neonicotinoids. This class has broad application in agriculture due to its neurotoxic impact on a broad range of piercing and sucking insects. Despite the intensive exposure of both animals and humans to Sulfx, limited data available on the toxicity of Sulfx to non-target organisms, particularly mammalian reproductive organs.
This report was planned to investigate the influence of two chosen doses of Sulfx on the testis of mature male rats. The study also evaluated the possible ameliorative role of the antioxidant RES against Sulfx- induced testicular toxicity. Our results revealed that daily oral intake of Sulfx for 28 consecutive days at low (79.5 mg/kg) and high dose (205 mg/kg) expert toxic influences on the male rat reproductive organs indicated by the high content of testicular MDA, GSSG, and NOx versus the reduction in GSH content in a dose-dependent manner. These data suggested elevated LPO and reflected the occurrence of oxidative stress due to Sulfx administration in the testis, in agreement with the results reported by Chakrabarti et al.45 who reported increased oxidative stress in bees exposed to Transform® (active ingredient sulfoxaflor). Other investigations correlated oxidative stress and increased production of ROS and reactive nitrogen species (NS) to neonicotinoid toxicity.46-48 Bal et al.8 reported the induction of oxidative stress and reduction in GSH level due to treatment with imidacloprid (8 mg/kg body weight) orally for 3 months. Moreover, Bal et al.49 found that exposure of adult rats to low doses of Clothianidin (2, 8, and 24 mg/kg body weight/day) for 90 days caused LPO of testicular tissue indicated by the significant increase in the level of thiobarbituric acid-reactive substances. Zhang et al.50 suggested that acetamiprid insecticide toxicity in mice may be induced by oxidative stress, as acetamiprid appeared to increase activation of p38 mitogen-activated protein kinases (MAPK) that are markers of oxidative stress, and decrease the activity of antioxidant enzymes.
Exposure to neonicotinoids potentially releases ROS and NS and leads to an imbalance between oxidants and antioxidant defense systems. Consequently, oxidative stress and LPO in the testicular tissue of adult male rats occur.51 Oxidative stress is considered one of the most common causes of testicular dysfunction, leading to infertility due to the high rate of cell division and mitochondrial oxygen consumption in testicular tissue. In addition, ROS production in the testis leads to oxidative damage and destroys steroidogenesis and spermatogenesis.52
In addition, male germ cells are rich in polyunsaturated fatty acids and have low antioxidant capacity, making them more susceptible to LPO and oxidative damage.53, 54 ROS attacks the double bonds of spermatozoa lipids membrane and induces damage to the lipid matrix in sperm membranes associated with a rapid loss of intracellular ATP.55, 56 Low ATP content exhibits a reduction in sperm motility, which may lead to infertility since a full ATP pool is required for the normal movement of spermatozoa. This can explain the decrement of the testicular adenine nucleotides (ATP, ADP, and AMP) observed in our study after treatment with Sulfx.
DNA fragmentation and caspases activation are considered as a hallmark of apoptosis.57 Biochemical features of apoptosis include activation of caspase cascades and DNA fragmentation into a 180–200 base pair long ladder. Caspases are crucial mediators of apoptosis, among them caspase-3 is a frequently activated protease that catalyzes the cleavage of several structural and regulatory proteins essential for cell survival and maintenance. Its activation increases under pesticide stress in all organisms.45 An increase in the occurrence of germ cell apoptosis is commonly reported following toxicant exposure.
Our results showed that Sulfx administration resulted in a noticeable increase in the caspase 3 activity, and testicular DNA fragmentation that reflects impaired spermatogenesis. It is well established by previous studies that exposure to different environmental pollutants associated with raised lipid peroxidation and increased apoptotic germ cells.58, 59 Oxidative stress has been shown to cause damage in lipids, DNA, and proteins of sperm and also induce apoptosis that lead to the decrease of sperm count and impairment of sperm function.60
In the recent study, histopathological examination of the testes revealed that Sulfx administration especially at the high dose caused a testicular injury. This damage usually promotes a decline in testicular morphometric parameters, such as the diameter and size of the seminiferous tubules due to degeneration of germ cells. Another morphological alteration observed was the presence of only Sertoli cells in some seminiferous tubules. As the seminiferous tubules were formed by spermatogonia and elongating spermatids in normal control and these phases were not found in Sulfx treated groups thus indicated disruption of spermatogenesis. These findings are similar to the effects induced by cadmium and diazinon that produced histopathological alterations in the seminiferous tubules, indicating disruption of spermatogenesis.61 Lonare et al.8 found that treatment of male Wister rats with imidacloprid (45 and 90 mg/kg, b.wt) for 28 days induced oxidative stress that leads to histopathological defective spermatogenesis indicated by the depletion of spermatocytes along with testicular and epididymal degenerative changes. In addition, Tokumoto et al.62 found that Clothianidin administered at a dose of 50 mg/kg body weight significantly increased DNA fragmentation in the seminiferous tubules, increased vacuolization in the seminiferous epithelia and decreased the number of germ cells in mature male quails in a dose-dependent manner as a consequence of oxidative stress.
| Groups parameters | Control | RES | L-Sulfx | H-Sulfx | RES + L-Sulfx | RES + H-Sulfx |
|---|---|---|---|---|---|---|
| ATP (μg/g tissue) | 57.50 ± 3.4 | 58.03 ± 3.2 | 33.99 ± 2.3a | 21.10 ± 1.16a,b | 52.90 ± 1.9a,c | 41.05 ± 1.1a,d |
| ADP (μg/g tissue) | 24.25 ± 2.3 | 23.8 ± 1.8 | 22.33 ± 1.1 | 15.17 ± 1.5a | 20. 22 ± 1.7a | 21.55 ± 1.2a,d |
| AMP (μg/g tissue) | 11.19 ± 0.54 | 11.05 ± 0.98 | 9.43 ± 0.61a | 8.27 ± 0.23a,b | 11.60 ± 0.91c | 10.40 ± 1.2d |
| AMP\ATP | 0.19 ± 0.01 | 0.19 ± 0.009 | 0.28 ± 0.02a | 0.39 ± 0.0a,b | 0.22 ± 0.012c | 0.25 ± 0.02a,d |
| AEC | 0.75 ± 0.03 | 0.75 ± 0.02 | 0.69 ± 0.04a | 0.64 ± 0.01a,b | 0.74 ± 0.02c | 0.71 ± 0.03a,d |
- Note: Data expressed by means ± SEM, (n = 6).
- a Significantly different from control group.
- b Significant difference between the low and high dose Sulfx-treated groups (bp < .05).
- c Significantly different between L-Sulfx and RES + L-Sulfx treated groups (cp < .05).
- d Significantly different between H-Sulfx and RES + H-Sulfx treated groups (dp < .05).
Various plant products can be used to protect against neonicotinoid-induced oxidative stress by scavenging free radicals and modulating antioxidant defense systems such as vitamin C, curcumin, and resveratrol.51 RES is a natural phytoalexin and a powerful antioxidant agent, found in many dietary sources, especially grapes. Previous in vivo and in vitro studies have shown that RES act as a strong inhibitor of ROS production and enzyme regulator63 that protected germ cells by reducing LPO and increasing antioxidant enzyme release.64
In the present study, co-treatment with RES significantly lowered the testicular MDA, GSSG, and NO levels while enhancing GSH content as compared to Sulfx treated rats. Moreover, it significantly decreases the testicular DNA fragmentation and the degeneration of seminiferous tubules thus improves the spermatogenesis process as a whole. This evident protective role of RES may be due to its impact as a free radical scavenger, and its ability to protect against oxidative stress induced by Sulfx. The suggested three different antioxidant mechanisms of resveratrol as a natural antioxidant are: (i) competition with coenzyme Q to decrease the oxidative chain complex, where ROS generation takes place. (ii) Scavenging O2− radicals formed in the mitochondria. (iii) Inhibition of LPO induced by Fenton reaction products.65 RES has also been shown to increase sperm production,27 reduce apoptosis in germinal cells, prevents DNA damage that occur as a consequence of the rise of ROS levels and protect against environmental toxins.66 The anti-apoptotic influence of RES has been established in many experimental models.67, 68 It has been shown to protect germ cells against apoptosis emerging from physiological injury.63
Juan et al.27 found that RES has a therapeutic effect on the testis in adult rats by exerting a marked increase in the production of spermatozoa after oral treatment in rats. Eleawa et al.69 established that the protective effects of RES could also be mediated by its promising effect on apoptosis where it declined the expression of p53 and Bax genes in healthful and cadmium chloride (CdCl2)-intoxicated rats and enhanced the mRNA expression of Bcl-2 levels, thus exerting a protective effect against testicular injury. Collodel et al.28 reported that RES showed an ameliorative effect against LPO and protected sperm from ROS attacks in sperm culture media mainly in mechanical techniques, such as semen cryopreservation, in which oxidative stress is exacerbated. Moreover, RES enhanced fertility and protected spermatogenesis by reducing oxidative stress and exerting anti-apoptotic effects against testicular injury induced by many anticancer drugs.70-73 In addition, it successfully increases the activity of the antioxidant defense system, prevents LPO, and improves the pathological changes in rats after malathion treatment.74 Moreover, it was effective at partially retrieving enzyme activity and protecting cells from the oxidative effects of pesticides.75, 76
5 CONCLUSION
The findings of the present study demonstrated that oxidative stress can be considered as a mechanism of testicular damage induced by Sulfx, which leads to reproductive toxicity. The antioxidant capacity of RES could protect testis against the Sulfx induced testicular toxicity as they could scavenge ROS and suppress apoptosis triggered by Sulfx administration. Thus, individuals who are regularly exposed to insecticides should take sufficient dietary supplementation of RES for counteracting the testicular damage of Sulfx. Further studies are required to find out the effect of lower doses of Sulfx on the fertility of adult male rats and define the pathways involved in RES actions while competing Sulfx-induced testicular damage.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Noha I. Said, Areeg M. Abd-Elrazek, and Heba A. El-dash: All authors planned the study, carried out the experiments and performed the statistical analysis, summarized, discussed and interpreted the results, and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




