Thiamethoxam-induced oxidative stress, lipid peroxidation, and disturbance of steroidogenic genes in male rats: Palliative role of Saussurea lappa and Silybum marianum
Abstract
Thiamethoxam (TMX) belongs to the neonicotinoid insecticide family and may evoke marked endocrine disruption. In this study, the reproductive toxicity of TMX on male rats was assessed along with the ability of Saussurea lappa (costus roots) and/or Silybum marianum extract (SM) to alleviate TMX toxicity. Male rats were allocated to seven groups and orally treated daily for 4 weeks: Control (saline), Costus (200 mg/kg), SM (150 mg/kg), TMX (78.15 mg/kg), TMX-costus, TMX-SM, and TMX-costus-SM (at the aforementioned doses). Compared with control group, TMX administration induced reductions in testicular levels of glutathione and antioxidant activities of SOD and CAT. In addition, TMX-exposed rats showed lower serum testosterone hormonal levels as well as higher malondialdehyde and nitric acid levels were detected in TMX-administered rats. On a molecular basis, mRNA expressions of StAR, CYP17a, 3β-HSD, SR-B1, and P450scc genes were significantly down-regulated in TMX group, whereas the expression of LHR and aromatase genes was up-regulated. Moreover, TMX-induced testicular damage was confirmed by histopathological screening. Importantly, however, the administration of either costus roots or SM significantly alleviated all aforementioned TMX-induced changes, indicating the effective antioxidant activities of these plant products. Interestingly, simultaneous treatment with costus root and SM provided better protection against TMX reproduction toxicity than treatment with either agent alone.
1 INTRODUCTION
The reproductive toxicity of xenobiotics has garnered the attention of researchers over recent decades because endocrine disruptive chemicals are now abundant in the environment. Neonicotinoids are a major class of insecticides that have been broadly and effectively used in veterinary practice and to protect various crops as alternatives to older pesticide classes such as organochlorines, organophosphates, and carbamates.1, 2 Humans and nontarget organisms are frequently exposed to neonicotinoids because of their wide use, persistence in crops and long half-life in soils (>350 days for thiamethoxam [TMX]).3 Neonicotinoids exert their insecticidal activity by acting on postsynaptic nicotinic acetylcholine receptors, which play a vital role in synaptic transmission in the central nervous systems of insects and mammals, particularly in excitatory cholinergic neurotransmission.4 Additionally, neonicotinoids have been reported to affect reproductive functions in both sexes through their endocrine-disrupting potential, which can interfere with the hormonal pathways that control reproduction.5-8
TMX is a broad-spectrum second-generation neonicotinoid with potent insecticidal activity and high selectivity for insect nicotinic acetylcholine receptors.9 In general, neonicotinoids may potentially induce endocrine disruption in vertebrates.10, 11 TMX along with its metabolite, clothianidin, has endocrine disrupting capabilities. Organisms are likely to be exposed to TMX in the environment. For example, Bonmatin et al.12 analyzed the levels of TMX in soil, water, and human hair sampled from three agricultural regions of the Philippines; they found that TMX levels reached 0.2 ng/L in water, 268 μg/kg in soil, and 0.51 ng/g in hair samples. In another study, TMX was detected in the surface water of the central Yangtze River, China at 1.10 ng/L.13 In terms of reproductive effects, TMX has been shown to induce a significant increase in aromatase activity in fetoplacental cocultures with concomitant increases in both estradiol and estrone secretions.14, 15 Additionally, oral administration of clothianidin to female quails was reported to have changed the histology of ovarian granulosa cells as well as induced oxidative stress.7 Furthermore, a notable disruption in the hypothalamic–pituitary-gonadal axis was demonstrated in both male and female lizards exposed to oral TMX, alongside significant disturbances in sex steroid hormonal levels and related gene expression.5
Saussurea lappa (Costus roots) is an antioxidant-rich herbal plant belonging to the Asteraceae family. Costus root is widely used as a traditional medicine to treat cases of ulcer, convulsions, hepatitis, and arthritis because of its high content of flavonoids and polyphenol content.16-18 The significant antioxidant activity of costus extract has been reported to alleviate myocardial injury induced by isoproterenol administration in rats.17 In addition, S. lappa root provides protection against ethephon-induced testicular toxicity in rats.16
Silybum marianum extract (Silymarin [SM]), is a mixture of flavonoids extracted from the milk thistle. It has striking antioxidant properties due to its polyphenolic structure and the methoxy moiety on one of its phenolic rings.19, 20 Indeed, the antioxidant and free radical scavenging abilities of SM are suggested to be greater than those of vitamin E.20 Previous studies have reported the antioxidant capacity of SM and its ability to reverse tissue damage associated with various diseases and disorders such as hepatotoxicity,20 liver fibrosis21 and skin disorders.22
Because exposure to neonicotinoids can potentially have deleterious effects on male reproduction, it is of great importance to identify medicinal plants that could help alleviate such male reproductive toxicity. To the best of our knowledge, the male reproductive toxicity of TMX has yet to be studied in conjugation with the counter treatment of costus or SM. Consequently, in the current study, we assessed the palliative effects of single and/or combined treatments with S. lappa and S. marianum on TMX-induced changes androgen hormones, oxidative stress markers, steroidogenic gene expression, and testicular ultrastructure in male rats.
2 MATERIALS AND METHODS
2.1 Chemicals and reagents
TMX is the active substance in the insecticide “Actara 25 WG” (product code # A9584E) which was obtained from Syngenta Crop Protection AG, Switzerland. All other reagents used were of high-analytical grade. Costus roots were obtained from a local market. The dried-plant powder (230 g) was extracted three times with 95% ethanol (2 L, 25 C, 1 week). After filtration, the solvent was concentrated by a rotatory evaporator (BuchiR-215) to obtain the ethanolic extract (26.1 g) which was used in the current study. Mariagon capsules labeled as containing 140 mg of SM, were obtained from AlphAmoun Pharmaceuticals Co., Badr City, Cairo, Egypt.
2.2 Animals and ethical statement
In total, 42 adult male albino rats (9–12 weeks old and 140–180 g), were purchased from the animal unit, Faculty of Medicine, Mansoura University, Egypt. The rats were housed in metal cages with free access to water and a commercial pelleted rodent feed and maintained under standard conditions of 22–25°C temperature, 45%–55% relative humidity and a 12-h artificial light/dark cycle. The Research Ethics Committee, Faculty of Veterinary Medicine, Egypt approved the experimental design and animal handling protocols of this study (Approval number; R/28).
2.3 Experimental design
After a 2-weeks of acclimation period, rats were randomly divided into seven equal groups (n = 6 rats per group) as follows;
Control: rats received normal saline orally. TMX: rats were administrated TMX orally at a dose of 78.15 mg/kg body weight (bwt) according to Khaldoun-Oularbi et al.9 (this dose was selected as a one-twentieth of the oral TMX LD50 in rats, which is 1563 mg/kg/bwt.23 Costus: rats were administered costus roots at 200 mg/kg.18 SM: rats were administrated SM at 150 mg/kg bwt.24 Costus + TMX: rats received both the aforementioned costus root and TMX doses. SM + TMX: rats were administered both the aforementioned SM plus TMX doses and costus + SM+ TMX: rats received all of the aforementioned costus, SM, and TMX doses.
All treatments were administered daily via oral gavage for 4 weeks. Both costus and SM were administered 1 h before TMX administration. At 24-h after the last treatment, rats were euthanized by an overdose of pentobarbital (400 mg/kg bwt) via an intraperitoneal injection. They were then decapitated, and blood samples were collected to determine serum testosterone levels. The testes were also rapidly excised and divided into three portions. The first portion was directly homogenized in ice-cold 10 mM phosphate buffer (pH 7.4) to prepare a 10% (wt/vol) homogenate, which was centrifuged at 4°C for 10-min. The supernatant was then utilized for biochemical analyses. The second portion was fixed for histopathological examination of the testes. The third portion was frozen in liquid nitrogen and stored at −80°C for RNA extraction.
2.4 Gas chromatography–mass spectrometry (GC–MS) analysis
The chemical composition of costus roots was performed using Trace GC-TSQ mass spectrometer (Thermo Scientific, Austin, TX) with a direct capillary column TG–5 MS (30 m × 0.25 mm × 0.25 μm film thickness). The column oven temperature was initially held at 50°C and then increased by 5°C/min to250°C hold for 2 min. Increased to the final temperature 300°C by 30°C/min and hold for 2 min. The injector and MS transfer line temperatures were kept at 270, 260°C respectively; Helium was used as a carrier gas at a constant flow rate of 1 ml/min. The solvent delay was 4 min and diluted samples of 1 μl were injected automatically using Autosampler AS1300 coupled with GC in the split mode. EI mass spectra were collected at 70 eV ionization voltages over the range of m/z 50–650 in full scan mode. The ion source temperature was set at 200°C. The components were identified by comparison of their mass spectra with those of WILEY 09 and NIST 14 mass spectral database.
2.5 Hormone measurements
The serum testosterone levels were assayed by electrochemiluminescence assay using a Cobas e411 analyser (Roche Diagnostic) according the manufacturer's protocol. The intra- and inter-assay coefficients of variation were less than 10% and 15%, respectively. The lowest limit of detection was 0.025 ng/ml. Each assay was performed in triplicate, and the experiments were repeated at least three times.
2.6 Assessment of testicular tissue oxidant/antioxidant capacity
The testicular tissue concentration of malondialdehyde (MDA) was determined as a marker for lipid peroxidation based on a previously reported protocol.25 Nitrite/nitrate (nitric oxide [NO]) was measured spectrophotometrically by following the described method of Sastry et al.26 In addition, levels of reduced glutathione (GSH) as a nonenzymatic antioxidant biomarker were determined on the basis of the method of Sedlak et al.27 Furthermore, two enzymatic antioxidants, superoxide dismutase (SOD) and catalase (CAT), were evaluated according to the methods of Mishra et al.28 and Aebi,29 respectively.
2.7 RNA extraction and reverse transcription
Total RNA was extracted from a 50 mg rat testis sample using Trizol reagent (Direct-zol RNA MiniPrep, catalog No. R2050) according to the manufacturer's instructions. The quantity and purity of RNA were measured using a NanoDrop (UV–Vis spectrophotometer Q5000) and the integrity was evaluated by gel electrophoresis. The cDNA of each sample was synthesized using a SensiFast cDNA synthesis kit following the manufacture's protocol (Bioline, catalog no, Bio-65 053). The reaction mixture comprised a total volume of 20 μl consisting of up to 1 μg total RNA, 4-μl of 5x Trans Amp buffer, 1 μl of reverse transcriptase and up to 20 𝜇l DNase free-water. The final reaction mixture was placed in a thermal cycler and the following program was applied; primer annealing at 25°C for 10 min, reverse transcription at 42°C for 15 min followed by inactivation at 85°C for 5 min. The samples were then maintained at 4°C.
2.8 Quantitative real-time PCR analysis
Relative quantification of the mRNA levels of steroid-forming acute regulatory protein (StAR), CYP17a, Aromatose, LHR, cytochrome P450 cholesterol side-chain cleavage enzyme (P450scc), scavenger receptor class B type I (SR-B1) and 3β-HSD in rat testes samples were performed by real-time PCR using SYBR Green PCR Master Mix (2x SensiFast SYBR, Bioline, catlog No. Bio-98 002). Primer sequences of testicular steroidogenic biomarkers were designed following Shi et al.30 These primer sequences and the sizes of each amplified PCR product are shown in Table 1. The housekeeping gene β-actin was used as an internal control. The reaction mixture comprised a total volume of 20 𝜇l consisting of 10 𝜇l 2x SensiFast SYBR, 3 𝜇l cDNA, 5.4 μl H2O (d.d water), 0.8 μl of each primer. The PCR cycling conditions were as follows: 95°C for 2 min followed by 40 cycles of 94°C for 15 s, use of the annealing temperatures shown in Table 1 for 30 s, and finally 72°C for 20 s. At the end of the amplification phase, melting curve analysis was performed to confirm the specificity of the PCR product. The relative gene expression in each treatment group versus the control compared with expression of β-actin, was calculated according to the 2-ΔΔCt method, previously described by Pfaffl.31
| Gene | GenBank accession number | Oligonucleotide sequence | Annealing temperature (C0) | Size (bp) |
|---|---|---|---|---|
| StAR | NM_031558 | f5′-GGGCATACTCAACAACCAG-3′ r5′-ACCTCCAGTCGGAACACC-3′ |
58 | 111 |
| CYP17a | NM_012753 | f5′-CTCTGGGCACTGCATCAC-3′ r5′-CAAGTAACTCTGCGTGGGT-3′ |
58 | 114 |
| Aromatase | NM_017085 | f5′-GCCTGTCGTGGACTTGGT-3′ r5′-GGTAAATTCATTGGGCTTGG-3′ |
58 | 142 |
| LHR | NM_012978 | f5′-CATTCAATGGGACGACTCTA-3′ r5′-GCCTGCAATTTGGTGGA-3′ |
55 | 130 |
| 3B-HSD | M38178 | f5′-TGTGCCAGCCTTCATCTAC-3′ r5′-CTTCTCGGCCATCCTTTT-3′ |
56 | 145 |
| SR-B1 | AY451993 | f5′-ACAGGTCCCAGGGCTCAG-3′ r5′-CGTGCGGTTCATAAAGG-3′ |
58 | 156 |
| P450SCC | J05156 | f5′-CTTTGGTGCAGGTGGCTAG-3′ r5′-CGGAAGTGCGTGGTGTTT-3′ |
56 | 115 |
| β-actin | NM_031144 | f5′-TCGTGCGTGACATTAAAGAG-3′ r5′-ATTGCCGATAGTGATGACCT-3′ |
56 | 134 |
2.9 Histopathological screening
Testes specimens were fixed for 24 h at room temperature in 10% neutral-buffered formalin. They were then dehydrated in alcohol, cleared in xylene, embedded in paraffin wax, and sectioned to a thickness of 5-μm. The paraffin sections were subsequently stained with hematoxylin and eosin (H&E) and examined with a light microscope. Images were obtained at an initial magnification of ×400 (Nikon Eclipse E200-LED, Tokyo, Japan).32
2.10 Statistical analysis
Data are displayed as the means ± SEM. Data were analyzed using one-way analysis of variance (ANOVA) and post hoc Duncan's multiple comparison test. p values <.05 indicated statistical significance between different groups.
3 RESULTS
3.1 GC–MS profile of costus roots extract
The costus roots extract was analyzed by GC–MS to show the main components as well as their retention times and relative percentage of the total peak area (Figure 1 and Table 2). The data demonstrated that the extract contained 13 components with retention times ranging from 4.78 to 24.91 min.
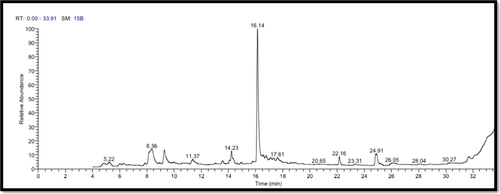
| Chemical name | Phytochemical compounds | RT (min) | Peak area % | Chemical formula | Molecular weight |
|---|---|---|---|---|---|
(S,E)-2,5-Dimethyl-4-vinylhexa- 2,5-dien-1-yl acetate |
Carvyl acetate | 4.78 | 1.90 | C12H18O2 | 194 |
| cis-p-Mentha-2,8-dien-1-ol | Citral geranial | 5.22 | 1.11 | C10H16O | 152 |
4-Hydroxy-4-methylhex-5-enoic acid, tert-butyl ester |
5-Oxoundecanoic acid | 6.02 | 0.96 | C11H20O3 | 200 |
1,6-Octadiene-3-OL, 3,7-dimethyl- |
Linalool | 6.28 | 0.91 | C10H18O | 154 |
| 2-Cyclohexen-1-one, 3,5,5-trimethyl- | Isophorone | 8.15 | 4.30 | C9H14O | 138 |
| 4-Allylanisole | Estragole | 8.36 | 10.20 | C10H12O | 148 |
| Benzaldehyde, 4-(1-methylethyl)- | cis-Anethole | 9.27 | 4.87 | C10H12O | 148 |
| CYCLOOCTENONE, DIMER | Phenethyl octanoate | 11.37 | 1.93 | C16H24O2 | 248 |
| Ascaridole epoxide | Ascaridole epoxide | 14.06 | 0.75 | C10H16O3 | 184 |
| trans-á-Ionone | trans-á-Ionone | 14.23 | 4.29 | C13H20O | 192 |
cis-5,8,11,14,17-Eicosapentaenoic acid |
Eicosapentaenoic acid | 14.36 | 1.20 | C20H30O2 | 302 |
| 7-epi-cis-sesquisabinene hydrate | Eudesm-4(14)-en-11-ol | 14.94 | 1.05 | C15H26O | 222 |
Formic acid,3,7,11-trimethyl-1,6,10- dodecatrien-3-yl ester |
Roughanic acid | 15.78 | 0.80 | C16H26O2 | 250 |
| (−)-Spathulenol | Caryophyllene oxide | 16.14 | 47.54 | C15H24O | 220 |
7-(1,3-Dimethylbuta-1,3-dienyl)-1,6, 6-trimethyl-3,8-dioxatricyclo [5.1.0.0(2,4)]octane |
Saussurea lactone | 17.11 | 1.02 | C15H22O2 | 234 |
| Retinaldehyde | Retinal | 17.61 | 1.32 | C20H28O | 284 |
| Hexadecanoic acid, methyl ester | Methyl palmitate | 22.16 | 3.11 | C17H34O2 | 270 |
Methyl 10-trans, 12-cis-octadecadienoate |
Methyl linoleate | 24.91 | 3.70 | C19H34O2 | 294 |
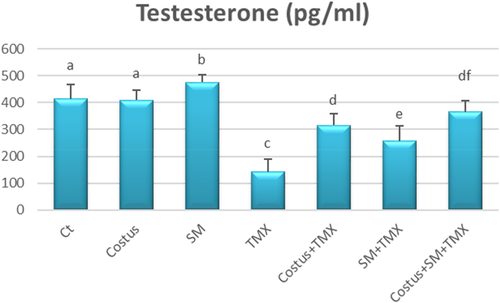
3.2 Variations in testosterone levels
In comparison with control TMX-exposed rats exhibited a significant decline in serum testosterone levels (p < .05). However, there were no significant differences in the testosterone hormone levels of the control, SM, and costus groups. Nevertheless, combined treatment of TMX with either SM or Costus (i.e., SM + TMX or costus + TMX) markedly increased the testosterone levels (p < .05) relative to the levels in rats treated with TMX only. Interestingly, the combined treatment of costus + SM+ TMX restored testosterone concentrations to normal levels (Figure 2).
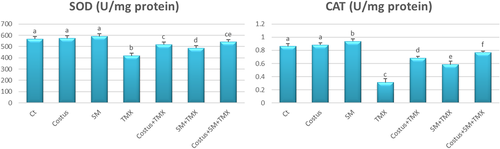
3.3 Testicular oxidative status and antioxidant biomarkers
The enzymatic antioxidant activities of treatment groups are shown in Figure 3. There was no significant difference in SOD levels among the costus, SM, and control groups, but there was a significant rise (p < .05) in CAT activities only in SM group relative to the control. However, TMX-exposed rats displayed notable reductions in SOD and CAT activities (p < .05) compared with control rats. In contrast, rats treated with costus + TMX and SM + TMX showed marked increases (p < .05) in the activities of both enzymes compared with rats treated with TMX only. Interestingly, TMX + costus + SM treatment produced a marked rise (p < .05) in SOD levels relative to TMX + SM as well as a significant increase in CAT (p < .05) when compared with the levels observed following treatment with either SM or costus only.
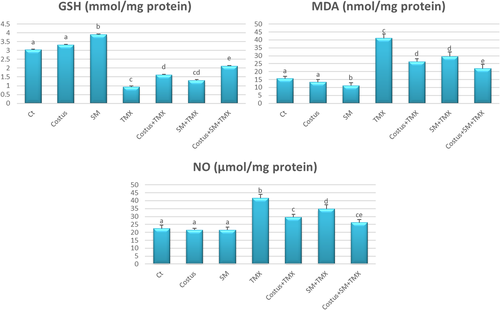
There were no significant differences in the levels of nonenzymatic oxidative stress parameters, that is, MDA and GSH between the control and costus groups; however, the SM group exhibited a significant increase in GSH levels (p < .05) and decrease in MDA (p < .05) compared with the control group. Contrastingly, rats in the TMX group showed higher MDA level (p < .05) and lower GSH level (p < .05) than those in the negative control group. Moreover, combined treatment of costus + TMX, SM + TMX or costus + SM + TMX significantly increased GSH level (p < .05) and decreased MDA levels (p < .05) compared with the TMX group (Figure 4).
Testicular NO levels did not differ significantly among control, SM, and costus groups. However, compared with control rats, TMX-intoxicated rats showed significantly higher testicular NO levels (p < .05). The SM + TMX and costus + TMX combination treatment were each associated with significant decreases (p < .05) in NO levels when compared with the effects of the TMX only treatment. Furthermore, the costus + SM + TMX treatment significantly decreased NO levels (p < .05) in rats relative to levels observed in TMX only and TMX + SM groups (Figure 4).
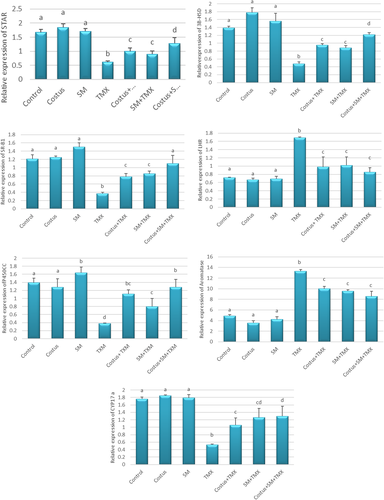
3.4 Expression of testicular steroidogenic genes
Notably, the mRNA expression of selected testicular steroidogenic genes was altered in testicular tissues of TMX-exposed rats. Compared with control group, StAR, CYP17a, 3β-HSD, SR-B1, and P450scc genes were significantly downregulated in the TMX group, whereas LHR and aromatase genes were significantly up-regulated. However, the ameliorative effects of costus and SM were notable in TMX + costus and TMX + SM groups: StAR, CYP17a, 3β-HSD, SR-B1, and P450scc were up-regulated whereas LHR and aromatase were down-regulated compared with the respective expression of these genes in rats exposed to TMX only. Furthermore, the combined treatment of both SM + costus + TMX produced further significant improvements in the mRNA expression of the tested genes (Figure 5).
3.5 Testicular histopathology
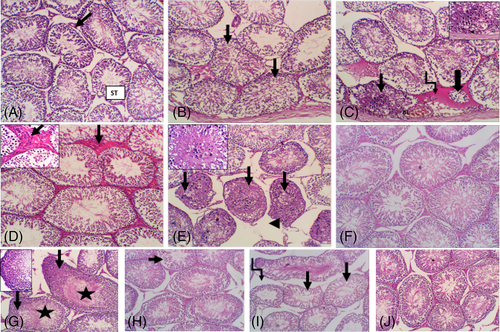
To evaluate potential pathological changes, histopathological examinations of testis were performed in each treatment groups compared with the control. Testis of control group showed regular outline of testicular seminiferous tubules (ST) (Figure 6(A)). TMX exposed group resulted in diffuse, severe testicular degeneration with irregular, disorganized in some ST (Figure 6(B)). The altered tubules showed epithelial cells disorganization with lumen filled with detached germ cells and absence of luminal mature sperm. Desquamation of cells into the tubule lumen forming intraepithelial empty spaces (Figure 6(C)). Additionally, the interstitium showed hyalinization of interstitial connective tissue and dilated blood vessels (Figure 6(D)). Other ST showed marked testicular necrosis with complete obliteration of tubular lumen (Figure 6(E)). In contrast to TMX, the group exposed to SM showed regular outline of ST and interstitial connective tissue (Figure 6(F)). Furthermore, the costus exposed group had hyperplasia of germ cells with numerous luminal spermatozoa (Figure 6(G)). Mild lytic necrosis in some ST with few luminal spermatozoa were seen in SM + TMX-exposed group (Figure 6(H)). Moderate irregularity of the ST with degenerative changes and intertubular vacuoles were also observed in costus + TMX-exposed group (Figure 6(I)). However, normal arrangement of ST and interstitial tissue were detected in TMX + SM + costus exposed group (Figure 6(J)).
4 DISCUSSION
Our results revealed that TMX evoked a significant decrease in serum testosterone levels in rats while significantly upregulated the expressions of LHR and aromatase genes and downregulated the expression StAR, CYP17a, 3β-HSD, SR-B1, and P450scc genes, all relative to the negative control. These findings are in accordance with previous studies that revealed notable disruptions in testosterone biosynthesis in response to other neonicotinoids such as acetamiprid,33 and imidacloprid.10, 11, 34 Moreover, exposure of male lizards to TMX and clothianidin resulted in notable decline in plasma testosterone level accompanied by significant upregulation of testicular cyp17 and cyp19 genes expression.5 Testosterone produced entirely by Leydig cells in the testicular tissue. It is the principal androgenic hormone that plays a crucial role in spermatogenesis, maturation of spermatozoa, and secondary sexual functions. Accordingly, impairment of testosterone biosynthesis can result in significant reproductive dysfunction.10 Membrane-bound low-density lipoprotein receptor (LDL-R) and SR-B1 are responsible for recognition and transport of LDL and HDL, respectively from blood to testes.11 Further, the StAR transports cholesterol to the inner mitochondrial membrane where it is metabolized to pregnenolone via P450scc. Pregnenolone is then converted into testosterone, a process catalyzed by CYP17, and 3b-HSD enzymatic activities.35 In this context, our results indicate that TMX has the ability to disrupt mRNA expression of steroidogenesis markers necessary for testosterone synthesis in rat testes. Moreover, these results coincide with TMX-induced alterations to testes biochemical parameters and decreased serum testosterone. We speculate that the mechanisms underlying TMX-induced decrease in testosterone hormonal levels may endorsed for many reasons. First, TMX may directly damage the Leydig cells' structure and function;36 indeed, this deleterious effect was confirmed, by our testicular histopathological screening results. Second, the marked inhibitory action on steroidogenic (3b-HSD and 17b-HSD) enzymatic activities or downregulated expression of testicular StAR protein may be involved in suppression of testosterone biosynthesis.37 Finally, a previous study revealed that imidacloprid substantially decreased serum LDL-cholesterol and testicular total cholesterol in male mice,11 which suggests that the injurious effects of neonicotinoids on lipid components in testes may strongly affect the androgen synthesis.
Leydig cell LHr binds to LH and enhances the expression of StAR through cAMP-stimulated extracellular signal-regulated kinase. We found low-testosterone levels and StAR expression in TMX-exposed rats suggesting that TMX disrupted LHr expression and hindered the signal transfer from LHr to StAR, which is consistent with the results of a previous study.38 Cytochrome P450 aromatase is an enzyme that maintains the testosterone/estrogen balance through its role in the irreversible bioconversion of testosterone into estrogens and the regulation of various physiological functions.39 Upregulation of P450 aromatase can be accompanied by changes to the structure and function of male phenotypes, as previously observed in response to glyphosate exposure.39
Oxidative stress caused by overgeneration of both reactive oxygen species (ROS), and reactive nitrogen species is implicated in neonicotinoid-induced injury to cellular molecules such as lipids, DNA, and proteins.40 In the present study, testicular oxidative damage was induced in TMX-exposed rats as evidenced by marked suppression of antioxidant enzymatic activities of SOD and CAT with diminished GSH levels. In addition, high levels of testicular MDA and NO were detected following exposure to TMX. Similar findings of TMX-evoked oxidative damage and induction of free radicals in hepatic and cardiac tissues have reported.41 A significant oxidative/antioxidant imbalance has been demonstrated in male reproductive organs following exposure to other neonicotinoids as imidacloprid,34 acetamiprid42 and thiacloprid.43 SOD and CAT are vital enzymes to the removal of oxyradicals through scavenging the superoxide anion (O2−).44 Further, GSH is an important endogenous antioxidant molecule that plays a central role in detoxification process, acts as a cosubstrate in antioxidant enzymatic reactions mediated by glutathione peroxidase and glutathione-S-transferase, and contributes in scavenging oxyradicals, which was previously reported following chronic exposure to imidacloprid.44,45 The reduced levels of GSH observed here may be attributed to its utilization for conjugation process and/or its antioxidant role in scavenging the free radical products.46 Furthermore, suppression of SOD, CAT activities indicates that TMX may significantly exhaust of the endogenous antioxidant system because of oxidative stress in testicular cells, which was confirmed by histopathological changes observed in this study.
MDA, another marker of oxidative damage and ROS generation, accumulates because of lipid peroxidation and the deleterious effects of free radicals on cellular lipid components.47 The mitochondrial membrane of sperm is more vulnerable to lipid peroxidation, because it is abundant in polyunsaturated fatty acids and low in antioxidants.4 Consistent with a previous study,46 TMX increased lipid peroxidation in male rats in the present study due to the destructive effect of ROS on lipid molecules, which was confirmed by our histopathological examination. In addition to generating ROS and causing oxidative stress, neonicotinoids can generate NO and induce nitrosative stress as previously shown in rats injected by imidacloprid, in which NO levels in brain, and hepatic tissue were significantly elevated.41, 45 Similarly, we found that NO levels were significantly increased in the testicular tissue of TMX-exposed rats which is consistent with the effect of acetamiprid on NO levels in testes of male mice.48
In the present study, pretreatment with costus roots markedly improved the testicular antioxidant capacity while reducing the effect of TMX on lipid peroxidation and NO content. The potent antioxidant effect of Costus roots has been reported in other studies,17, 18, 49 they are attributed to the two main polysaccharides in the plant extract, SLT-3, SLT-4, which help to decline the ROS generation, inhibit peroxidation of lipids and enhance antioxidant enzyme activity.50 Furthermore, costus can act as an electron donor during conversion of reactive radicals into more stable unreactive species.51 Thus, costus-treated group in our study showed decreased MDA levels that are attributable to the palliative influence of costus against the effects of ROS on lipid macromolecules. In a previous study, dehydrocostus lactone isolated from costus markedly downregulated of inducible nitric oxide synthase enzyme expression with decreased NO production.18 Therefore, in our study, decreased NO content may have been caused by suppression of inducible nitric oxide synthase. SM is a natural phytochemical with potent antioxidant properties that acts by scavenging ROS without causing considerable toxicity.52 Supporting previous studies,19, 52 we found that SM restored the oxidant/antioxidant balance, which may be explained by its ROS scavenging ability and boosting the antioxidant enzymatic activities.
Administration of costus and/or SM produced significant ameliorative effects on testicular dysfunctions in comparison with TMX-exposed rats evidenced by elevated testosterone level, upregulated the expression profile of steroidogenic (StAR, CYP17a, 3β-HDS, SR-B1, and P450scc) markers, down-regulated LHr and aromatase and improved histopathological picture. The ameliorative effects of SM may be due to its flavonoid content and probable antioxidant properties.53 Abedi et al.54 also found that because of its antioxidant properties, rats received SM showed significant increases in the secretion of LH, FSH, GnRH, and testosterone as well as the number of spermatids and spermatozoa cells. Similarly, Al Ahmed55 reported that S. marianum green extract significantly increased testes weight, sperm activity and testosterone levels during in vitro fertilization of mice.
Costus extract improves testicular function as follows: (1) protecting the regenerated Leydig and Sertoli cells, (2) normalizing serum levels of reproductive hormones, (3) decreasing the TMX-induced testicular DNA damage, (4) maintaining or reversing spermatogonia proliferation (5) decreasing spermatogonia cell cycle arrest and apoptosis, (6) improving spermatozoa cell density and quality, (7) decreasing deterioration and abnormalities of spermatozoa.16 The beneficial effects of costus roots may be attributable to its numerous active antioxidant compounds, including flavonoids, steroids, and chlorogenic acid56, 57 which improve scavenging and decreasing the generation of free radicals in testicular tissue due to its richness in polyunsaturated fatty acids. Furthermore, antioxidants are known to progress steroidogenesis by enhancing Leydig cell function and thereby increasing androgen production and augmenting spermatogenesis.36 Indeed, costus extract is used to alleviate several disorders and as an aphrodisiac owing to its ability to improve the semen quality.48
5 CONCLUSION
In summary, we speculated that TMX apparently induced testicular damage in male rats by inducing oxidative and nitrosative stress alongside with noticeable disruption in expression of genes involved in testosterone biosynthesis. Administration of costus extract and/or SM alone or in combination, however, protects male rats against TMX reproductive toxicity by triggering endogenous antioxidant defenses and reversing disruption of steroidogenic gene expression. Moreover, co-administration of both agents prompted better protection than use of either agent alone.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All authors confirm that all data underlying the findings are included within the manuscript without restrictions.




