In vitro investigation of the genotoxic and cytotoxic effects of thiacloprid in cultured human peripheral blood lymphocytes
Abstract
Thiacloprid, a neonicotinoid insecticide, is widely used for controlling various species of pests on many crops. The potential genotoxic effects of thiacloprid on human peripheral blood lymphocytes (PBLs) were investigated in vitro by the chromosome aberrations (CAs), sister chromatid exchanges (SCEs), and cytokinesis-block micronucleus (MN) assays. The human PBLs were treated with 75, 150, and 300 μg/mL thiacloprid in the absence and presence of an exogenous metabolic activator (S9 mix). Thiacloprid increased the CAs and SCEs significantly at all concentrations (75, 150, and 300 μg/mL) both in the absence and presence of the S9 mix and induced a significant increase in MN and nucleoplasmic bridge formations at all concentrations for 24 h and at 75 and 150 μg/mL for 48-h treatment periods in the absence of the S9 mix; and at all concentrations in the presence of the S9 mix when compared with the control and solvent control. Thiacloprid was also found to significantly induce nuclear bud (NBUD) formation at 300 μg/mL for 24 h and at 150 μg/mL for 48-h treatment times in the absence of the S9 mix and at the two highest concentrations (150 and 300 μg/mL) in the presence of the S9 mix. Thiacloprid significantly decreased the mitotic index, proliferation index, and nuclear division index for all concentrations both in the absence and presence of the S9 mix. © 2012 Wiley Periodicals, Inc. Environ Toxicol 29: 631–641, 2014.
INTRODUCTION
Neonicotinoids are the most important and fairly new chemical class of insecticides that are widely used for agricultural and household pest control (Casida and Quistad, 1998; Yamamota and Casida, 1999; Tomizawa and Casida, 2003). Neonicotinoids are remarkably potent neurotoxic insecticides that act as agonist on the nicotinic acetylcholine receptor (nAChR; Tomizawa and Yamamoto, 1993). These systemic insecticides have significant toxicity to insects but low toxicity to mammals, because they preferentially bind to the insect nAChR over the mammalian nAChR (Liu and Casida, 1993; Tomizawa and Casida, 2003). However, more recently, it was also reported that neonicotinoid-containing insecticides may have stronger side effects on humans due to the fact that they can activate and/or modulate nicotinic receptors of vertebrates (Li et al., 2011).
Thiacloprid is the first chloronicotinyl neonicotinoid insecticide and it was registered in 2000 as trade name Calypso (Elbert et al., 2001; Erdelen, 2001). Today, the use of this insecticide for controlling various species of pests on many crops is very common (Elbert et al., 2000). Because the use of thiacloprid has become increasingly widespread throughout the world, the assessment of its possible genotoxic and cytotoxic effects on living organisms is very important.
A literature survey showed that a few studies have been carried out on the potential genotoxic/cytotoxic (Zang et al., 2000; Feng et al., 2004, 2005; Karabay and Oguz, 2005; Demsia et al., 2007; Kocaman and Topaktaş, 2007) and carcinogenic (Green et al., b; Pastoor et al., 2005) effects of some neonicotinoid insecticides. The available studies indicate that only one study addressed the cytogenetic effect of thiacloprid. Şekeroğlu et al. (2011) reported that thiacloprid significantly decreased the mitotic index (MI) and binuclear cell numbers and significantly increased the chromosome aberrations (CAs; 112.5 mg/kg, for 24 h) and also caused a significant increase in micronucleated binuclear cells (22.5 mg/kg/day, for 30 days) in rat bone marrow cells, in vivo. However, the genotoxic and cytotoxic potentials of thiacloprid have not yet been investigated for the in vitro tests.
It is known that genotoxicity assessment of a particular compound is the evaluation for its ability to induce any change in the chromosome structure and number such as gene mutations, chromosomal rearrangements, or deletions and loss or gain of a whole chromosome. CAs, sister chromatid exchanges (SCEs), and micronucleus (MN) formation in human peripheral blood lymphocytes (PBLs) are among the most widely used and well-established cytogenetic markers for determination of the genotoxicity of compounds (Carrano and Natarajan, 1988).
The structural CA formation is resulted from DNA-level damage that a high level of CA in peripheral lymphocytes is associated with an increased risk of cancer and also predicts genotoxic risk of potentially mutagenic and carcinogenic chemicals (Norppa et al., 2006; Bonassi et al., 2000, 2004, 2005, 2007).
SCEs involve the exchange of DNA segments between two sister chromatids in a single chromosome during cell proliferation and are regarded as a manifestation of damage to the genome (Tucker et al., 1993; Sonoda et al., 1999; Helleday, 2003). An increased of SCE frequency in PBLs in the presence of compound indicates its genotoxicity (Albertini et al., 2000).
MN can be formed from acentric chromosomal/chromatid fragments or whole chromosomes/chromatids fail to be segregated to the daughter nuclei during mitotic cellular division, because they did not attach properly with the spindle during the segregation process in anaphase (Fenech, 2007; Fenech and Bonassi, 2011; Fenech et al., 2011). Thus, both clastogenic and aneugenic effects (leading to structural and numerical CAs, respectively) can be determined with the cytokinesis-block micronucleus (CBMN) assay (Kirsch-Volders et al., 1997, 2003; Norppa and Falck, 2003). In the CBMN assay, because cells are blocked in the binucleated (BN) stage after inhibition of cytokinesis by cytochalasin B, it is also possible to measure nucleoplasmic bridges (NPBs), which represent DNA misrepair and/or telomere end fusions (Thomas et al., 2003; Fenech, 2007). NPBs occur in cells exposed to DNA-breaking agents (Fenech, 2002; Fenech and Crott, 2002) and therefore represent clastogenic effect of the agent. In addition, nuclear buds (NBUDs), MN-like bodies attached to the nucleus by a thin nucleoplasmic connection, have also been validated in this system as a biomarker of gene amplification (Fenech and Crott, 2002; Fenech, 2007). Finally, MN and other nuclear anomalies such as NPBs and NBUDs are biomarkers of genotoxic events and chromosomal instability and an increased MN, NPB, and NBUD frequency in PBLs implies cancer risk in humans in vivo (Bonassi et al., 2011).
The present study focuses on the possible genotoxic and cytotoxic/cytostatic effects of thiacloprid on cultured human PBLs using in vitro CA, SCE, and CBMN assays in the absence and presence of an exogenous metabolic activation system (S9 mix).
MATERIALS AND METHODS
Test samples and chemicals
The study was carried out using human peripheral blood samples from four healthy, nonsmoking volunteer donors aged 21–23 years old (two males and two females). All donors had no previously known exposure to high concentrations of genotoxicants. All volunteers gave informed consent to participate in the study and signed consent forms.
Thiacloprid (Fluka 37905, CAS No: 111988-49-9) was used as the test substance for the in vitro tests. Test substance was purchased from Sigma-Aldrich. The chemical structure of thiacloprid is shown in Figure 1. The test substance was dissolved in 50% ethanol supplied by Merck (Darmstadt, Germany), which was also tested as solvent control. 5-Bromodeoxyuridine (B-5002, St. Louis, MO), colchicine (C-9754, St. Louis, MO), and cytochalasin B (C-6762, St. Louis, MO) were purchased from Sigma. In the cultures without metabolic activation, the positive control was mitomycin-C (MMC; Sigma M-05030) at 0.25 μg/mL for treatments. For cultures with metabolic activation, cyclophosphamide monohydrate (CP; 28 μg/mL, Sigma C-0768) was used as a positive control at 28 μg/mL for the in vitro tests. Giemsa and all other chemicals were purchased from Merck (Darmstadt, Germany). All test solutions were freshly prepared before each experiment.
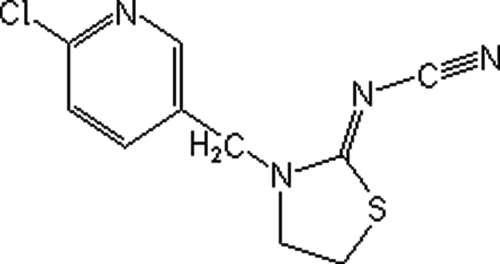
The chemical structure and formula of thiacloprid (C10H9ClN4S; [3-(6-chloro-3-pyridinylmethyl)-2-thiazolidinylidene]cyanamide; molecular weight: 252.72).
In Vitro Assay (Without S9 Mix)
The CA and SCE tests were performed using the methods developed by Evans (1984) and Perry and Thompson (1984) with minor modification. This study was conducted according to IPCS guidelines (Albertini et al., 2000).
Lymphocyte cultures were set up by adding 0.2 mL of whole blood from four healthy donors to 2.5 mL of chromosome medium PB Max (Gibco, cat. no. 12552-013) supplemented with 10 μg/mL of bromodeoxyuridine. Cultures were incubated at 37°C for 72 h. The test concentrations were chosen as 75, 150, and 300 μg/mL based on the top concentration that resulted in ∼50% (LD50) reduction in MI (300 μg/mL). The cells were treated with 75, 150, and 300 μg/mL concentrations of thiacloprid that dissolved in 50% ethanol for 24 (thiacloprid was added 48 h after initiating the culture) and 48 h (thiacloprid was added 24 h after initiating the culture). A control (i.e., untreated control), a solvent control (50% ethanol, 4 μL/mL), and a positive control (MMC, 0.25 μg/mL) were also used. To arrest the cells in metaphase, the cells were exposed to 0.06 μg/mL colchicine 2 h before harvesting. The whole blood was centrifuged at 2000 rpm for 5 min, and the supernatant was removed. Then the cells were treated with a hypotonic solution (0.4% KCl) for 15 min at 37°C to lyse red blood cells and centrifuged at 1200 rpm for 10 min. The supernatant was removed and replaced with 10-mL cold fixative consisting of methanol: glacial acetic acid (3:1 v/v) at room temperature (22°C ± 1°C). The cell suspension was then centrifuged again at 1200 rpm for 10 min. The supernatant was removed, and the cell pellet was washed further two times in 10 mL of cold fixative. Finally, the centrifuged cells were dropped onto clean slides. The staining of the air-dried slides was performed following the standard methods using 5% Giemsa stain for CA analysis (i.e., with 5% Giemsa in Sorensen Buffer, pH = 6.8, 15 min), and the modified Fluorescence Plus Giemsa method (FPG) for SCE analysis (Speit and Haupter, 1985). For FPG staining, 1-day-old slides were covered with Sorensen Buffer (pH = 6.8). Then, slides were irradiated with 30 W, 254-nm UV lamp at 15 cm distance for 30 min. After irradiation, the slides were incubated in 1× SSC (standard saline citrate) at 58–60°C for 60 min and then stained with 5% Giemsa prepared with Sorensen buffer for 20 min.
For the CBMN assay, 0.2 mL of fresh blood was used to establish the cultures, and the cultures were incubated for 68 h. The cells were treated with 75, 150, and 300 μg/mL concentrations of thiacloprid for 24 and 48 h (thiacloprid was added 44 and 20 h after initiating the culture, respectively) treatment periods. Cytochalasin B (final concentration of 6 μg/mL) was added after 44 h of incubation in order to block cytokinesis and obtain BN cells. After an additional 24-h incubation at 37°C, the cells were harvested by centrifugation, and the pellet was resuspended in a hypotonic solution of 0.4% KCl for 5 min at 37°C. The cells were fixed in a cold fixative (methanol: glacial acetic acid: 0.9% NaCl, 5:1:6 v/v/v). After centrifugation, the cells were fixed further two times with methanol-glacial acetic acid (5:1 v/v). Slides were prepared by dropping and air-drying. The slides were stained with 5% Giemsa stain solution (diluted with Sorensen buffer, pH = 6.8) for 15 min (Fenech, 2000; Kirsch-Volders et al., 2003).
In Vitro Assay (with S9 Mix)
Some chemical substances require metabolic activation to exhibit its genotoxicity. Therefore, in this study, exogenous metabolic activation system [cofactor-supplemented postmitochondrial fraction (S9) prepared from livers of rats treated with enzyme inducing agent 3-methylcholanthrene] was used to determine the genotoxic effects of metabolite(s) of thiacloprid.
Generally, the same procedure for the CA, SCE, and CBMN assays described earlier was used to assess the indirect genotoxic effect of thiacloprid with minor modifications depending on the methodology of the metabolic activation. The lymphocytes were cotreated with 75, 150, and 300 μg/mL concentrations of thiacloprid and 0.5-mL S9 mix for 3 h. Thiacloprid and S9 mix were added 48 h after initiating the culture. For every culture of lymphocytes with the tested compound, a control, a solvent control (50% ethanol, 4 μL/mL), and a positive control (cyclophosphamide monohydrate, 28 μg/mL) were also performed. The test chemical and S9 mix were removed from the culture by centrifugation 4 min at 2500 rpm. The pellet of lymphocytes was washed twice with 2.5 mL RPMI 1640 medium (Biochrom AG, F 1215) and resuspended in fresh complete medium (chromosome medium PB Max). The cultures were incubated for a total of 72 h at 37°C for the CA and SCE assays and 68 h at 37°C for the CBMN assay.
Preparation of S9
The albino male rats (Rattus norvegicus var. albinos) weighing 200 g were pretreated with 80 mg/kg concentration of 3-methylcholanthrene (dissolved in sunflower oil) for 5 days. For the preparation of S9 fraction and the S9 mix, the method described by Maron and Ames (1983) was used.
Microscopic Evaluation
For each donor, a total of 100 well-spread metaphases were investigated (a total of 400 metaphase spreads for four donors) in order to score the CAs at each concentration and treatment period that showed structural and/or numerical CAs. The CA was classified according to ISCN (International System for Human Cytogenetic Nomenclature; Paz-y-Mino et al., 2002). Gaps were not considered as CA according to Mace et al. (1978). Percentage of cells with structural CAs and percentage of cells with CAs have been calculated following the scoring of CAs.
The scoring of SCE was performed according to the IPCS guidelines (Albertini et al.2000). To score SCE, 25 well-differentiated second-division metaphase cells were analyzed per donor (a total of 100 second-division metaphase for each concentration) and the frequency of SCE per cell was recorded.
In the CBMN assay, MNi, NPBs, and NBUDs were scored in BN cells according to the scoring and identification criteria of Fenech et al. (2003) and Fenech (2007). To determine MN and other nuclear anomalies, 1,000 BN cells were analyzed for each donor (4,000 BN cells were scored per concentration).
In this study, the scoring of CAs, SCEs, MNi, NPBs, and NBUDs was performed blindly on the coded slides at 1000 magnification (Nikon, Eclipse E200, Japan).
MI, Proliferation Index, and Nuclear Division Index
In the CA assay, to determine cytotoxicity, the mitotic index (MI) was calculated from the number of metaphases in 3000 cells analyzed per culture for each donor (12,000 cells per concentration).
In the SCE assay, a total of 400 cells (100 cells from each donor) were scored for the proliferation index (PI). PI was calculated according to formula as follows: PI = [(1 × M1) + (2 × M2) + (3 × M3)]/N, where M1, M2, and M3 represent those metaphases corresponding to first, second, and third divisions, and N is the total number of metaphases scored (Lamberti et al., 1983).
Moreover, in the CBMN assay, cytostaticity was calculated by using the NDI. To this aim, 1000 lymphocytes per donor were analyzed. The numbers of cells with one to four nuclei were determined in 1000 cells. NDI was calculated using the following formula: NDI = [(1 × M1) + (2 × M2) + (3 × M3) + (4 × M4)]/N, where M1 through M4 represent the number of cells with one to four nuclei and N is the total number of cells scored (Eastmond and Tucker, 1989; Fenech, 2000, 2002).
Statistical Analysis
One-way ANOVA was used for the statistical significance of all parameters. The comparisons between groups were made using a post hoc analysis, LSD test. Concentration-response relationships were determined from the correlation and regression coefficients for all parameters (CA, SCE, MN, NPB, NBUD, MI, PI, and NDI).
RESULTS
The effects of thiacloprid on CAs and MI, in the absence and presence of the S9 mix, are summarized in Table 1. Thiacloprid increased the percentage (%) of cells with structural CAs and % cells with CAs significantly for all concentrations and treatment periods when compared with the control and solvent control in the absence and presence of the S9 mix (P < 0.001). The increase of % cells with CAs was concentration-dependent only in the presence of the S9 mix (r = 0.999, P < 0.05). As shown in Table 1, both in the absence and presence of the S9 mix, the chromatid-type aberrations were more common than the chromosome-type aberrations. However, the aberrations were significantly lower in comparison with the respective positive control. In addition, thiacloprid generally did not induce the numerical CAs in human PBLs.
| Test Substance | Treatment | Structural CA | Polyploid Cells | % Cells with Structural CA ± SD | % Cells with CA ± SD | MI ± SD | |||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | S9 | Conc. (μg/mL) | B' Type | B'' Type | |||||
| Control | − | − | − | 17 | 1 | − | 4.50 ± 0.57 | 4.50 ± 0.57 | 3.75 ± 0.32 |
| Ethanol (50%) | 24 | − | 4 μL/mL | 17 | 1 | 1 | 4.50 ± 1.00 | 4.75 ± 0.50 | 3.71 ± 0.29 |
| MMC | 24 | − | 0.25 | 100 | 17 | 1 | 25.00 ± 1.41a3b3 | 25.25 ± 1.25a3b3 | 1.01 ± 0.11a3b3 |
| Thiacloprid | 24 | − | 75 | 38 | 5 | − | 10.00 ± 1.63a3b3c3 | 10.00 ± 1.63a3b3c3 | 3.31 ± 0.23a1b1c3 |
| 150 | 58 | 5 | − | 13.75 ± 0.95a3b3c3 | 13.75 ± 0.95a3b3c3 | 2.44 ± 0.12a3b3c3 | |||
| 300 | 61 | 8 | − | 16.00 ± 0.81a3b3c3 | 16.00 ± 0.81a3b3c3 | 1.88 ± 0.15a3b3c3 | |||
| Ethanol (50%) | 48 | – | 4 μL/mL | 17 | 4 | − | 5.25 ± 0.95 | 5.25 ± 0.95 | 3.19 ± 0.15 |
| MMC | 48 | − | 0.25 | 890 | 98 | − | 81.50 ± 3.00a3b3 | 81.50 ± 3.00a3b3 | 1.02 ± 0.10a3b3 |
| Thiacloprid | 48 | − | 75 | 45 | 5 | 1 | 11.50 ± 1.29a3b3c3 | 11.75 ± 1.70a3b3c3 | 2.67 ± 0.25a3b2c3 |
| 150 | 45 | 13 | − | 13.25 ± 1.89a3b3c3 | 13.25 ± 1.89a3b3c3 | 1.33 ± 0.20a3b3c1 | |||
| 300 | 85 | 12 | − | 22.25 ± 2.50a3b3c3 | 22.25 ± 2.50a3b3c3 | 0.63 ± 0.14a3b3c1 | |||
| Control | 3 | + | − | 16 | 2 | 1 | 4.00 ± 1.41 | 4.25 ± 0.95 | 4.71 ± 0.38 |
| Ethanol (50%) | 3 | + | 4 μL/mL | 16 | 1 | 1 | 4.25 ± 0.95 | 4.50 ± 0.57 | 4.70 ± 0.57 |
| CYP | 3 | + | 28 | 54 | 19 | − | 15.75 ± 1.25a3b3 | 15.75 ± 1.25a3b3 | 4.28 ± 0.22 |
| Thiacloprid | 3 | + | 75 | 31 | 9 | 1 | 9.75 ± 0.95a3b3c3 | 10.00 ± 0.81a3b3c3 | 4.14 ± 0.50a1b1 |
| + | 150 | 31 | 9 | 2 | 10.00 ± 2.30a3b3c3 | 10.50 ± 1.73a3b3c3 | 3.99 ± 0.35a1b1 | ||
| + | 300 | 37 | 14 | − | 11.75 ± 2.75a3b3c2 | 11.75 ± 2.75a3b3c2 | 3.39 ± 0.13a3b3c2 | ||
- All data are expressed as mean ± SD; n = 4.
- A total of 400 cells were scored per concentration in the CA assay and 12,000 cells were scored for the MI.
- Abbreviations: MMC, mitomycin C; CYP, cyclophosphamide; B', chromatid-type aberration; B'', chromosome-type aberration.
- a Significant from control.
- b Significant from solvent control (ethanol 50%).
- c Significant from positive control (MMC, CYP).
- a1b1c1P < 0.05.
- a2b2c2P < 0.01.
- a3b3c3P < 0.001.
MI decreased significantly at all concentrations of thiacloprid for the 24- and 48-h treatment periods when compared with the both controls in the absence of the S9 mix. The test compound also significantly decreased the MI at 300 μg/mL concentration for 48-h treatment period when compared with the positive control, MMC. Similarly, in the presence of the metabolic activator, thiacloprid caused a statistically significant reduction in the MI when compared with the control and the solvent control (75, 150, and 300 μg/mL). Furthermore, thiacloprid also decreased the MI to the same extent as (75 and 150 μg/mL) or more than (300 μg/mL) the positive control, CP, in the presence of the S9 mix (Table 1). Thus, it can be said that the highest concentration of thiacloprid showed a higher cytotoxic effect than CP and also MMC (for 48-h treatment period). However, no concentration-dependent effect was observed both in the absence and presence of the S9 mix.
The observed frequencies of SCE and PI in the PBLs are given in Table 2. Thiacloprid induced the SCE frequency significantly at all concentrations (75, 150, and 300 μg/mL) when compared with both the control and solvent control in the absence and presence of the S9 mix. The SCE increase was in a concentration-dependent manner only 48-h treatment in the absence of the S9 mix (r = 0.999, P < 0.05). Thiacloprid decreased the PI at all concentrations studied for both in the absence and presence of the S9 mix when compared with the control and solvent control. The decrease in PI occurred in a concentration-dependent manner only in the presence of the metabolic activation system (r = −0.997, P < 0.05). In addition, at the highest concentration (300 μg/mL) of thiacloprid decreased the PI as much as the positive control for both in the absence and presence of the S9 mix (Table 2).
| Test Substance | Treatment | Min–Max SCE | SCE/Cell ± SD | M1 | M2 | M3 | PI ± SD | ||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | S9 | Conc. (μg/mL) | |||||||
| Control | − | − | − | 1–12 | 4.00 ± 0.29 | 86 | 152 | 162 | 2.19 ± 0.15 |
| Ethanol (50%) | 24 | − | 4 μL/mL | 1–12 | 4.30 ± 0.14 | 76 | 201 | 123 | 2.11 ± 0.07 |
| MMC | 24 | − | 0.25 | 13–90 | 49.25 ± 4.54a3b3 | 234 | 155 | 11 | 1.44 ± 0.08a3b3 |
| Thiacloprid | 24 | − | 75 | 2–15 | 6.73 ± 0.78a1b1c3 | 111 | 209 | 80 | 1.92 ± 0.08a2b1c3 |
| 150 | 3–15 | 7.53 ± 0.66a1b1c3 | 179 | 180 | 41 | 1.65 ± 0.21a3b3c1 | |||
| 300 | 4–17 | 8.09 ± 0.24a2b2c3 | 251 | 138 | 11 | 1.40 ± 0.06a3b3 | |||
| Ethanol (50%) | 48 | − | 4 μL/mL | 2–12 | 4.81 ± 0.14 | 101 | 159 | 140 | 2.09 ± 0.05 |
| MMC | 48 | − | 0.25 | 35–149 | 89.55 ± 4.23a3b3 | 315 | 85 | 0 | 1.21 ± 0.12a3b3 |
| Thiacloprid | 48 | − | 75 | 1–17 | 7.71 ± 0.39a2b1c3 | 132 | 186 | 82 | 1.87 ± 0.09a3b2c3 |
| 150 | 2–19 | 9.32 ± 0.54a3b2c3 | 245 | 146 | 9 | 1.41 ± 0.07a3b3c1 | |||
| 300 | 5–18 | 12.05 ± 1.21a3b3c3 | 364 | 34 | 2 | 1.09 ± 0.03a3b3 | |||
| Control | 3 | + | − | 1–8 | 3.90 ± 0.14 | 51 | 176 | 173 | 2.30 ± 0.04 |
| Ethanol (50%) | 3 | + | 4 μL/mL | 1–13 | 4.28 ± 0.05 | 70 | 234 | 96 | 2.06 ± 0.05 |
| CYP | 3 | + | 28 | 10–47 | 20.08 ± 0.78a3b3 | 121 | 265 | 14 | 1.73 ± 0.10a3b3 |
| Thiacloprid | 3 | + | 75 | 1–10 | 4.86 ± 0.75a1c3 | 75 | 240 | 85 | 2.02 ± 0.12a3c3 |
| + | 150 | 2–12 | 6.65 ± 0.33a3b3c3 | 105 | 219 | 76 | 1.92 ± 0.10a3b1c2 | ||
| + | 300 | 3–16 | 7.62 ± 0.44a3b3c3 | 134 | 224 | 42 | 1.77 ± 0.04a3b3 | ||
- All data are expressed as mean ± SD; n = 4.
- A total of 100 cells were scored per concentration for the SCE assay, 400 cells were scored for the PI.
- Abbreviations: MMC, mitomycin C; CYP, cyclophosphamide.
- a Significant from control.
- b Significant from solvent control (ethanol 50%).
- c Significant from positive control (MMC, CYP).
- a1b1c1P < 0.05.
- a2b2c2P < 0.01.
- a3b3c3P < 0.001.
The effects of thiacloprid on MN and other nuclear anomalies (NPBs and NBUDs) are presented in Table 3. Thiacloprid induced a statistically significant increase in micronucleated binuclear cells (MNBN ‰) when compared with the control and solvent control at the all concentrations used (75, 150, and 300 μg/mL) both in the absence and presence of the S9 mix. However, binuclear (BN) cells could not be detected sufficiently in the highest concentration of thiacloprid (300 μg/mL) for 48-h treatment period in the absence of the S9 mix. In addition, the MN formation increased linearly as thiacloprid concentration increased for both in the absence (r = 0.999, P < 0.05 and r = 1.000, P < 0.001 for the 24 and 48 h, respectively) and presence (r = 1.000, P < 0.001) of the S9 mix.
| Test Substance | Treatment | Distribution of BN Cells According to No. Micronuclei | Micronucleated BN Cells ‰ ± SD | BN Cells with NPBs ‰ ± SD | BN Cells with NBUDs ‰ ± SD | NPB/MN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | S9 | Conc. (μg/mL) | 0 | 1 | 2 | 3 | >3 | |||||
| Control | − | − | − | 3984 | 16 | 0 | 0 | 0 | 4.00 ± 0.81 | 0.50 ± 0.57 | 1.50 ± 0.57 | |
| Ethanol (50%) | 24 | − | 4 μL/mL | 3982 | 18 | 0 | 0 | 0 | 4.50 ± 0.57 | 0.75 ± 0.50 | 1.25 ± 0.50 | |
| MMC | 24 | − | 0.25 | 3881 | 109 | 10 | 0 | 0 | 29.75 ± 4.27a3b3 | 3.50 ± 0.57a3b3 | 5.00 ± 0.81a3b3 | |
| Thiacloprid | 24 | − | 75 | 3971 | 28 | 1 | 0 | 0 | 7.25 ± 0.50a1b1c3 | 1.50 ± 0.57a1c3 | 2.00 ± 0.81c3 | 0.20 |
| 150 | 3968 | 28 | 4 | 0 | 0 | 8.00 ± 0.81a2b1c3 | 2.00 ± 0.81a2b1c2 | 2.50 ± 1.00c2 | 0.25 | |||
| 300 | 3961 | 30 | 8 | 1 | 0 | 9.75 ± 0.95a3b3c3 | 2.25 ± 0.95a2b2c1 | 3.50 ± 1.73a1b2c1 | 0.23 | |||
| Ethanol (50%) | 48 | − | 4 μL/mL | 3985 | 15 | 0 | 0 | 0 | 3.75 ± 0.95 | 0.75 ± 0.50 | 1.25 ± 0.50 | |
| MMC | 48 | − | 0.25 | 3746 | 222 | 30 | 2 | 0 | 63.50 ± 3.10a3b3 | 3.75 ± 0.95a3b3 | 10.25 ± 1.70a3b3 | |
| Thiacloprid | 48 | − | 75 | 3958 | 38 | 4 | 0 | 0 | 10.50 ± 1.91a3b3c3 | 1.75 ± 0.50a1b1c3 | 2.00 ± 1.15c3 | 0.16 |
| 150 | 3939 | 53 | 8 | 0 | 0 | 15.25 ± 1.50a3b3c3 | 2.50 ± 0.57a3b2c1 | 3.25 ± 0.95a1b1c3 | 0.16 | |||
| 300 | a | a | a | a | ||||||||
| Control | 3 | + | − | 3991 | 9 | 0 | 0 | 0 | 2.25 ± 0.50 | 0.50 ± 0.57 | 0.75 ± 0.95 | |
| Ethanol (50%) | 3 | + | 4 μL/mL | 3989 | 11 | 0 | 0 | 0 | 2.75 ± 0.95 | 0.25 ± 0.50 | 1.25 ± 0.50 | |
| CYP | 3 | + | 28 | 3960 | 35 | 4 | 0 | 1 | 10.00 ± 1.41a3b3 | 3.00 ± 0.81a3b3 | 4.00 ± 0.81a3b3 | |
| Thiacloprid | 3 | + | 75 | 3982 | 17 | 1 | 0 | 0 | 4.50 ± 1.00a2b1c3 | 1.25 ± 0.95b1c2 | 1.75 ± 0.50c3 | 0.27 |
| + | 150 | 3982 | 15 | 5 | 0 | 0 | 5.00 ± 0.81a1b2c3 | 1.50 ± 0.57a1b1c2 | 2.25 ± 0.95a2c2 | 0.30 | ||
| + | 300 | 3976 | 23 | 1 | 0 | 0 | 6.00 ± 0.81a3b3c3 | 2.00 ± 0.81a2b2c1 | 3.00 ± 0.81a3b2 | 0.33 | ||
- All data are expressed as mean ± SD; n = 4.
- A total of 4,000 cells were scored per concentration for the MN and other nuclear anomalies in binuclear cells.
- Abbreviations: MMC, mitomycin C; CYP, cyclophosphamide.
- a Insufficient binuclear cells.
- a Significant from control.
- b Significant from solvent control (ethanol 50%).
- c Significant from positive control (MMC, CYP).
- a1b1c1P < 0.05.
- a2b2c2P < 0.01.
- a3b3c3P < 0.001.
The results of the present study indicate that thiacloprid statistically significantly increased the BN cells with NPBs (‰) when compared with the control and the solvent control at all concentrations for 24 and 48 h (except 300 μg/mL: due to the excessive cytostaticity, BN cells could not be determined sufficiently) treatment periods in the absence of S9 mix; however, the NPBs were significantly lower in comparison with the positive control, MMC. The increase of NPBs formation was concentration-dependent only for the 48-h treatment (r = 1.000, P < 0.001). In the presence of the S9 mix, the test compound induced a concentration-dependent increase in BN cells with NPBs (‰) when compared with the controls at the all concentrations (r = 1.000, P < 0.001; Table 3). Furthermore, a statistically significant correlation was observed between MNBN ‰ and BN cells with NPBs ‰ for 48-h treatment period in the absence of the S9 mix (r = 1.000, P < 0.001) and also in the presence of the S9 mix (r = 1.000, P < 0.001).
Thiacloprid also induced a statistically significant increase in BN cells with NBUDs (‰) when compared with the control and solvent control at 300 μg/mL for 24 h and at 150 μg/mL for 48-h treatment periods in the absence of the metabolic activator. In addition, NBUD formation increased at 150 μg/mL when compared only with control and at 300 μg/mL when compared with both control and solvent control in the presence of the S9 mix (Table 3). Furthermore, these increases were concentration-dependent, both in the absence (for 24 h: r = 1.000, P < 0.001 and for 48 h: r = 1.000, P < 0.001) and in the presence (r = 0.997, P < 0.05) of the metabolic activator. It was also found that a significant correlation exists between MNBN ‰ and BN cells with NBUDs ‰ both in the absence (for 24 h: r = 0.999, P < 0.05 and for 48 h: r = 1.000, P < 0.001) and presence (r = 0.997, P < 0.05) of the S9 mix.
Finally, cytostatic effects of thiacloprid were measured by NDI. Thiacloprid decreased the NDI significantly for all concentrations and treatment periods when compared with the control groups both in the absence and presence of the S9 mix (Table 4). In addition, at the highest concentration (300 μg/mL) for 24 h and two higher concentrations (150 and 300 μg/mL) for 48-h treatment periods, thiacloprid caused a significant reduction in the NDI to the same extent as positive control, MMC, in the absence of the S9 mix. Similarly, in the presence of the S9 mix, at all concentrations (75, 150, and 300 μg/mL) of thiacloprid reduced the NDI to the same extent as the positive control, CP (Table 4). In addition, the decrease of the NDI was concentration-dependent in the presence of the S9 mix (r = −0.997, P < 0.05).
| Test Substance | Treatment | Distribution of cells according to No. Nuclei | NDI ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | S9 | Conc. (μg/mL) | 1 | 2 | 3 | 4 | ||
| Control | − | − | − | 3253 | 661 | 38 | 48 | 1.22 ± 0.02 |
| Ethanol (50%) | 24 | − | 4 μL/mL | 3429 | 507 | 40 | 24 | 1.16 ± 0.01 |
| MMC | 24 | − | 0.25 | 3808 | 189 | 2 | 1 | 1.04 ± 0.01a3b3 |
| Thiacloprid | 24 | − | 75 | 3437 | 526 | 17 | 20 | 1.15 ± 0.01a3c3 |
| 150 | 3450 | 523 | 8 | 19 | 1.14 ± 0.00a3c3 | |||
| 300 | 3802 | 188 | 5 | 5 | 1.05 ± 0.01a3b3 | |||
| Ethanol (50%) | 48 | − | 4 μL/mL | 3567 | 395 | 23 | 15 | 1.12 ± 0.02 |
| MMC | 48 | − | 0.25 | 3870 | 129 | 1 | 0 | 1.03 ± 0.01a3b3 |
| Thiacloprid | 48 | − | 75 | 3723 | 272 | 4 | 1 | 1.07 ± 0.00a3b3c1 |
| 150 | 3831 | 169 | 0 | 0 | 1.04 ± 0.00a3b3 | |||
| 300 | 3949 | 51 | 0 | 0 | 1.01 ± 0.00a3b3 | |||
| Control | 3 | + | − | 3106 | 878 | 10 | 6 | 1.22 ± 0.03 |
| Ethanol (50%) | 3 | + | 4 μL/mL | 3211 | 778 | 7 | 4 | 1.20 ± 0.06 |
| CYP | 3 | + | 28 | 3479 | 520 | 1 | 0 | 1.13 ± 0.03a2b1 |
| Thiacloprid | 3 | + | 75 | 3385 | 595 | 14 | 6 | 1.16 ± 0.01a1 |
| + | 150 | 3451 | 541 | 5 | 3 | 1.14 ± 0.02a2b1 | ||
| + | 300 | 3552 | 444 | 3 | 1 | 1.11 ± 0.02a3b2 | ||
- All data are expressed as mean ± SD; n = 4.
- A total of 4000 cells were scored per concentration for the NDI.
- Abbreviations: MMC, mitomycin C; CYP, cyclophosphamide.
- a Significant from control.
- b Significant from solvent control (ethanol 50%).
- c Significant from positive control (MMC, CYP).
- a1b1c1P < 0.05.
- a2b2c2P < 0.01.
- a3b3c3P < 0.001.
DISCUSSION
Thiacloprid is one of the most widely used neonicotinoid insecticides with high potential for human exposure. However, information on the genotoxic effects of thiacloprid is rather limited. And, this study presents the first in vitro evidence for the genotoxic and cytotoxic/cytostatic effects of thiacloprid using CA, SCE, and CBMN assays in human PBLs.
The results of the present study showed that thiacloprid significantly increased the CAs, structural CAs, and the frequency of SCEs at all concentrations (75, 150, and 300 μg/mL) when compared with both the control and solvent control in the absence and presence of the S9 mix.
In general, thiacloprid induced a significant increase in MN and NPB formations when compared with the controls at all concentrations in the 24 h and at 75 and 150 μg/mL in the 48-h treatment periods in the absence of the S9 mix and also at the all concentrations in the presence of the S9 mix. However, in the CBMN assay, because thiacloprid depressed to nuclear division, binuclear cells could not be sufficiently observed in the highest concentration (300 μg/mL) of this compound for 48-h treatment period in the absence of the S9 mix. Therefore, MN, NPB, and NBUD formation could not be evaluated under this condition. When compared with the control groups, thiacloprid was also found to significantly induce NBUD formation at the highest concentration for 24 h and at 150 μg/mL for 48-h treatment times in the absence of the S9 mix and at the two highest concentrations in the presence of the S9 mix.
These results are in agreement with previous report on the in vivo CA and MN formation of thiacloprid. Şekeroğlu et al. (2011) reported that a commercial formulation of thiacloprid significantly increased the CA (112.5 mg/kg, for 24 h) and also frequency of MN in BN cells (22.5 mg/kg/day, for 30 days) in rat bone marrow cells. In addition, results of this study support previous findings obtained by Kocaman and Topaktaş (2007) for a commercial formulation of acetamiprid (like thiacloprid, acetamiprid belongs to the chemical subclass of neonicotinoid known as cyanoamidine), which significantly induced structural CA, SCE, and MN formation (25–40 μg/mL) in human PBLs in vitro.
The significant increase in the CAs, SCEs, MNi, and other nuclear anomalies following exposure of thiacloprid in the present study supports the clastogenic and/or aneugenic potential of this compound. The results of the CA assay showed that thiacloprid induced the more structural CAs (especially chromatid breaks) than the numerical CAs, meaning that thiacloprid as a clastogen can lead to the formation of CA by breaking the phosphodiester backbone of DNA. The breaking effects of thiacloprid on the phosphodiester backbone of DNA were unknown. However, Giray et al. (2001) reported that formation of DNA damage by insecticides may be due to the generation of reactive oxygen species (ROS). It was also showed that several pesticides induce ROS formation that may cause changes or losses of nucleotide bases and hence the production of DNA single-strand breaks (Lioi et al., 1998; Banerjee et al., 2001; Muniz et al., 2008). Thus, based on the results of this study, thiacloprid and/or its metabolite(s) may act on DNA and also proteins with the production of ROS that may cause DNA single-strand breaks.
The CBMN assay was originally developed for measuring MN; however, it can also be used to measure NPBs and NBUDs, which represent DNA damage events (Fenech, 2006). Results of this study showed that thiacloprid caused a significant increase in the formation of NPBs in human, and PBLs indicate a possible chromosomal rearrangement due to its exposure. In addition, Thomas et al. (2003) suggested that the NPB/MN ratio provides an important fingerprint to distinguish clastogenic agents from aneugenic ones. For example, the induction of MN frequency in the absence of NPBs could be indicative of an aneugenic effect. In this study, thiacloprid significantly increased both the MN and NPB formation in the same cultures that represent a clastogenic effect of this compound, because MN would be the result of chromosome/chromatid breaks, which could generate NPB. However, results of the CBMN assay showed that the NPB/MN ratio varied between 0.16 and 0.33 depending on the tested concentration in the absence and presence of the S9 mix. These results indicate that thiacloprid and/or its metabolite(s) also have an aneugenic potential (chromosome/chromatid lagging because of dysfunction of mitotic apparatus) in human PBLs, due to the fact that the NPB/MN ratios are close to 0. Therefore, it can be said that MN can be formed not only from acentric chromosomal/chromatid fragments but also from malsegregated whole chromosomes/chromatids.
In the present study, thiacloprid induced a significant increase in NBUD formation at the higher concentrations (150 and 300 μg/mL) when compared with the controls. NBUDs can be formed by exclusion of amplified DNA (Lindberg et al., 2007), from remnants of broken anaphase bridges (Gisselsson et al., 2000) or by retraction of micronuclei (Lindberg et al., 2007). In this study, NBUDs were evaluated in short-term (68 h) lymphocytes culture. According to the Mladinic et al. (2009), the development of NBUDs is not possible in the short-term cultivation period. Therefore, gene amplification theory cannot be used to explain the formation of NBUDs in the present study. Furthermore, because of the fact that the frequency of NPB/MN ratio (0.16–0.33) values is very low, the NPBs are not likely for to be the source of NBUDs. However, a statistically significant correlation was observed between MN and NBUD formation, regardless of the metabolic activation. Thus, it may suggest that the origin of NBUDs could be explained by the mechanism of micronuclei retraction.
It is unclear whether thiacloprid itself or one or more of its metabolites is responsible for the genotoxicity of this compound. However, in our study, thiacloprid caused a significant increase in the CAs, SCEs, MNi, and other chromatin instabilities such as NPBs and NBUDs both in the absence and presence of the metabolic activation system. Thus, it can be said that both itself and/or the metabolite(s) of thiacloprid are effective on the induction of DNA damage in human PBLs.
In the present study, peripheral lymphocytes exposed to all concentrations of thiacloprid showed significant decreases in MI, PI, and NDI both in the absence and presence of the S9 mix when compared with both controls. The observed significant inhibition of MI and PI in the human PBLs illustrates the cytotoxicity of thiacloprid. Inhibition of NDI also illustrates the cytostatic effect of this compound. A reduction of MI was observed in this study, which is consistent with the study of Şekeroğlu et al. (2011), showing that a commercial formulation of thiacloprid reduces MI at 112.5 mg/kg for 24 h and 22.5 mg/kg/day for 30 days in rat bone marrow cells. In addition, similar to the results of this study, Kocaman and Topaktaş (2007) reported that acetamiprid significantly decreased the MI, PI, and NDI (25–40 μg/mL) in human PBLs in vitro. It was reported that the substances caused cytotoxicity by inducing the chromosomal aberrations and DNA double-strand breaks (Armstrong et al., 1992; Galloway et al., 1998; Hillard et al., 1998; Vock et al., 1998; Kirkland and Müller, 2000). In this study, thiacloprid showed a cytotoxic/cytostatic effect in human PBLs most probably due to the increased levels of DNA damage and that this effect resulted probably from the inhibition of DNA synthesis and cell proliferation. Furthermore, Madle et al. (1993) reported that the mitotic selection of the cells that had CAs is capable of decreasing the MI.
Taken as a whole, these findings suggest that thiacloprid most probably has a genotoxic effect by inducing the CAs, SCEs, MNi, NPBs, and NBUDs formation and has a cytotoxic effect by decreasing the MI and PI and also has cytostatic effect by decreasing the NDI at the tested concentrations (75, 150, and 300 μg/mL) in human PBLs both in the absence and presence of metabolic activation system. However, the tested concentrations of thiacloprid in this study may be geno- and cytotoxic to humans as a result of acute or subacute exposure. It is well known that human beings especially farm laborers are exposed to pesticides for a long time. Therefore, further in vivo studies, especially subchronic or chronic treatment of test substance to test animals, are needed to draw firm conclusion regarding the genotoxicity of thiacloprid.




