Hierarchical Porous PbO2 Electrode for Electro-Degradation of Various Contaminants
Abstract
Persistent organic contaminants in water pose imminent threats to aquatic ecosystems and human health, yet conventional water treatment systems are not able to remove them effectively. Electrochemical oxidation is a promising treatment alternative for the mineralization of persistent organic compounds. Herein, a novel hierarchical porous PbO2 electrode is fabricated via direct electrodeposition on a templated fluorine-doped tin oxide surface. Physical and electrochemical characterization confirm the superior properties (e.g., enhanced electrochemical active surface areas) of the produced electrode. In addition, compared with macroporous PbO2 and PbO2 electrode films, the hierarchical porous PbO2 electrode shows significantly improved degradation performance against a variety of pollutants, including sodium dodecyl sulfate, rhodamine B, and sodium diclofenac. Overall, it is demonstrated that the hierarchical porous PbO2 electrode can be utilized for efficient electrochemical oxidation of organic contaminants.
1 Introduction
Synthetic organic pollutants, including persistent and emerging contaminants, can have severe adverse effects on aquatic ecosystems and human health.[1] For example, the extensive usage of surfactants has raised environmental concerns, as their presence in wastewater can lead to the destruction of lipid lamellar structures and progressive loss of cell bioactivity.[2, 3] Moreover, the surge in healthcare medication needs has substantially boosted the utilization of pharmaceutical products, leading to elevated concentrations of pharmaceutical compounds in wastewater that pose adverse effects on the health of living organisms.[4] To address these challenges, the commonly used wastewater treatment technologies, including adsorption,[5] membrane separation,[6] biological treatment methods,[7] and others,[1] have been adopted. However, physiochemical treatment methods such as adsorption often struggle to remove certain organic pollutants from water effectively and selectively.[5] Biological treatments offer a solution to these challenges, but they are often hampered by high operational costs and prolonged pre-preparation cycles.[7] In contrast, advanced oxidation processes (AOPs) represent an efficient water treatment approach, capable of rapidly oxidizing and completely degrading organic pollutants.[8] AOPs generate highly potent reactive oxygen species (ROS), including hydroxyl free radicals (OH.), which oxidize organic contaminants and convert them to harmless, small inorganic molecules, e.g., CO2 and H2O.[4] Many studies have applied AOPs to degrade organic pollutants in water,[9-13] and demonstrated outstanding performance toward a wide range of organic contaminants, including per- and poly-fluoroalkyl substances, pharmaceutical waste, and others.[8]
Among various AOPs, electrochemical oxidation attracts increasing interest due to its versatility, chemical-free nature, and the ability to use renewable energy and, therefore, minimize carbon footprint.[14] In an electrochemical oxidation process, oxidative degradation of a target contaminant occurs near the anode through direct electron transfer from the contaminant molecule to the anode or by reacting with ROS formed from oxidation of water.[1, 15] Therefore, the properties of electrode materials, such as the affinity of the target contaminant to the electrode surface, active surface area, and electrical conductivity, significantly influence the performance of the electrochemical oxidation process.[1, 14, 15]
Based on the affinity of ROS to the anode surface, anodes can be categorized as either active or nonactive.[14] Nonactive anodes, such as boron-doped diamond (BDD), doped-tin dioxide (SnO2), and lead dioxide (PbO2), have relatively low affinity for OH.[16] The loosely bound OH. can directly react with organic pollutants, leading to their mineralization. Nonactive electrodes are, therefore, excellent electrode choices for organic pollutant degradation in water. While BDD has demonstrated strong stability and high efficiency,[13, 17] its wide application is limited by the prohibitive cost and restriction on suitable substrate.[14, 18] Similarly, undoped SnO2, being a semiconductor, cannot be directly employed as an anode due to its high resistivity and limited service life, which necessitates doping with various elements to improve the electrochemical properties of SnO2 electrodes.[16] In contrast, PbO2 possesses favorable properties, such as relatively high electrical conductivity and cost-effective fabrication methods, making it an important material for anodic applications in wastewater treatment.[19] However, conventionally electrodeposited PbO2 electrodes typically have low surface area and, hence, a small number of active sites.[19] This limits electrochemical oxidation performance, particularly when pollutant concentrations are low.[19, 20] To overcome this limitation, the development of porous PbO2 electrodes has emerged as an effective approach for enhancing electrochemical oxidation performance due to their increased surface area.[21-25] Hierarchically porous structures, distinguished by their multiscale porosity spanning from the micrometer to nanometer range,[26] have demonstrated remarkable efficiency in applications such as lithium-ion batteries and supercapacitors.[27] This enhanced performance is attributed to improved mass transport, larger surface area, greater accessibility to active sites, and more efficient diffusion of reactants and products through interconnected pore channels, compared to their macroporous counterparts.[28-30] Consequently, we hypothesize that the integration of hierarchical porosity into PbO2 electrodes will offer significant advantages in electrochemical performance.
In this study, we developed a novel fabrication method to produce hierarchically porous structured PbO2 (HP-PbO2) on fluorine-doped tin oxide (FTO) by using self-assembled polystyrene (PS) nanoparticles with two different sizes as the template. The surface morphology, crystal structure, chemical composition, water contact angle, and electrochemical properties of the synthesized electrodes were thoroughly characterized. The performances of these electrodes for the electrochemical degradation of various types of pollutants in water, including surfactants (sodium n-dodecyl sulfate, SDS), pharmaceutical drugs (diclofenac sodium salt, DSS), and dye pollutants (rhodamine B, RhB), were also assessed. The electrooxidation performance of HP-PbO2 was also compared to both macroporous PbO2 (MP-PbO2) and PbO2 film prepared without a template (F-PbO2) counterparts. The remarkable efficiency and versatility of the HP-PbO2 electrode make it a promising candidate for practical industrial wastewater treatment on a large scale.
2 Results and Discussion
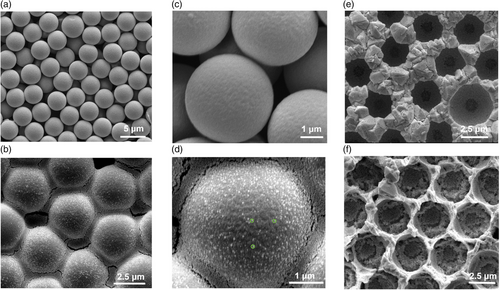
The hierarchical structure was achieved by strategically self-assembling PS spheres of two different sizes (4 μm and 50 nm) as templates. After electrodeposition and template removal, a highly ordered hierarchical PbO2 structure was obtained (Figure 1). Utilizing the commercially available FTO as the substrate not only saved significant effort in constructing an intermediate layer, but also prevented the loss of electrode functionality that could occur with the oxidation of traditional titanium-based substrates.[31] The NH2-functionalized PS spheres (50 nm) were assembled around the larger COOH-functionalized PS spheres (4 μm) through carbodiimide-assisted coupling and electrostatic forces.[32] The controlled evaporation of the solvent facilitated the crystallization of the PS particles into an optimally packed arrangement.[33] In comparison, 4 μm PS spheres alone were used in the assembly of the template for the MP-PbO2 electrode. The scanning electron microscopy (SEM) images of both templates revealed a close-packed assembly of PS spheres with long-range order (Figure 2a–d). In the hierarchical template, the 4 μm PS spheres acted as a core template, with the 50 nm spheres covering their surface (Figure 2b,d). The space between the 4 μm spheres was filled with 50 nm spheres. Following the electrodeposition of PbO2, the polymer template was removed. The SEM images of the electrodes obtained using no template (F-PbO2), the template formed with 4 μm PS spheres alone (MP-PbO2), and the template formed with both 4 μm and 50 nm PS spheres (HP-PbO2) are shown in Figure 2e,f and S1,Supporting Information. The F-PbO2 electrode exhibited a nonporous surface with tightly packed, polyhedron-shaped, PbO2 crystals of micrometers in size (Figure S1, Supporting Information). The MP-PbO2 electrode, in contrast, was highly porous with micron-sized pores closely matching the packing structure of the template; between the micron-sized pores left behind from the removal of the 4 μm PS spheres, PbO2 crystals had similar morphology as those found on the F-PbO2 electrode (Figure 2e). Similarly, the HP-PbO2 electrodes exhibited a uniform, ordered, close-packed array of macropores resulting from the 4 μm PS spheres; in addition, a large number of mesopores, presumably derived from the removal of the 50 nm PS spheres were clearly visible between the larger voids (Figure 2f).
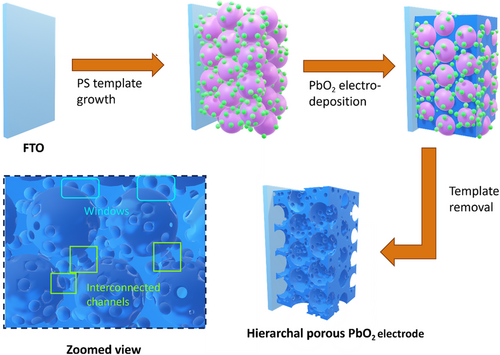
We then assessed the chemical and crystal structure of the synthesized HP-PbO2/FTO electrode. Powder X-ray diffraction(PXRD) measurements (Figure 3a) revealed prominent diffraction peaks at 25.5, 31.9, and 49.1°, corresponding to the (1 1 0), (1 0 1), and (2 1 1) crystal facets of β-PbO2, respectively.[34, 35] Diffraction peaks were also observed at 28.6 and 30.1°, which can be attributed to α-PbO2.[36] The peaks at 26.9 and 37.8° were present due to the FTO substrate.[37] Interestingly, the F-PbO2 electrode exhibited a similar PXRD spectrum as that of the HP-PbO2, with identical peak positions. These results suggest that the utilization of PS templates has no influence on the crystalline structure of the PbO2. X-ray photoelectron spectroscopy (XPS) survey scans confirmed the presence of Pb and O, as evidenced by characterized peaks located around 140, 410, 529, and 642 eV, corresponding to the binding energies of Pb 4f, Pb 4p, O1s, and Pb 4d,[20, 38-40] respectively (Figure S2, Supporting Information). The high-resolution Pb 4f spectrum exhibited a pair of split peaks with binding energies of 137.3 and 142.1 eV (Figure S3, Supporting Information), corresponding to Pb 4f7/2 and Pb 4f5/2 ionizations, respectively, suggesting the presence of Pb (IV) in the electrode. Collectively, the results obtained from PXRD and XPS provide strong evidence that the HP-PbO2 electrode possesses the appropriate chemical composition.
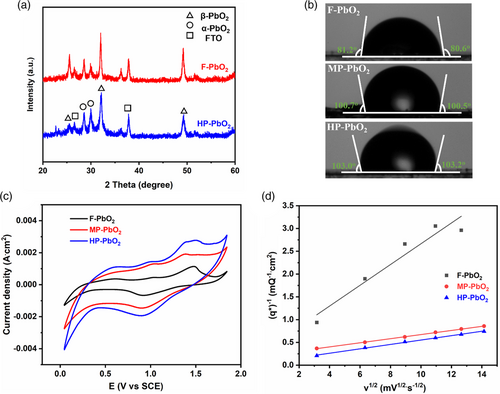
The surface wettability of the electrodes was examined by measuring the water contact angle on the electrode surface. Previous reports have shown the hydrophobic surface is advantageous for the generation of free OH• radicals and the suppression of the oxygen evolution reaction.[41] The contact angle measurements revealed that F, MP, and HP-PbO2 electrodes exhibited contact angles of around 81, 101, and 103°, respectively (Figure 3b). The increased hydrophobicity can be attributed to the porous structure of the MP and HP-PbO2 electrode: air trapped in the pores enhances the interfacial tension between the electrode surface and the liquid medium.[23]
We further investigated the electrochemical properties of the PbO2 electrodes. The oxygen evolution overpotential (OEP) of F, MP, and HP-PbO2 was determined by linear sweep voltammetry (LSV) measurements in 0.1 m Na2SO4. As shown in Figure S4, the OEP values for F, MP, and HP-PbO2 were determined to be 1.69, 1.73, and 1.76 V, respectively, relative to the saturated calomel electrode (SCE). A high OEP is beneficial for the electrochemical oxidation of contaminants as it reduces the unwanted oxygen evolution reaction and hence enhances energy efficiency for the degradation of the target contaminant.[34] HP-PbO2 exhibited the highest electrochemical activity among the three electrodes, as evidenced by the cyclic voltammetry (CV) area, which followed the order of HP-PbO2 > MP-PbO2 > F-PbO2 (Figure 3c).[42] To explore the number of active sites on the electrode's surface, we conducted CV measurements for the three electrodes, covering the potential range from 0.6 to 0.8 V versus SCE, at various scan rates (v) ranging from 10 to 200 mV s−1 (Figure S5, Supporting Information). The number of active sites is directly related to the voltammetric charges (q*), which can be calculated from the area under the CV curves. The total voltammetric charge (qT*) represents the overall quantity of active sites on the electrodes and can be obtained using Equation (S1), Supporting Information. As depicted in Figure 3d, a linear correlation between (q*)−1 and v1/2 was observed for all three electrodes. Based on these correlations, we determined the qT* values for F, MP, and HP-PbO2 to be 2.59, 4.52, and 14.32 mC cm−2, respectively. These results suggest that the porous structure of the MP and HP-PbO2 greatly increased the number of active sites on the electrode. The qT* of HP-PbO2 was 5.5 and 3.2 times that of F and MP-PbO2, respectively, suggesting that the mesopores contribute greatly to the number of active sites.[34]
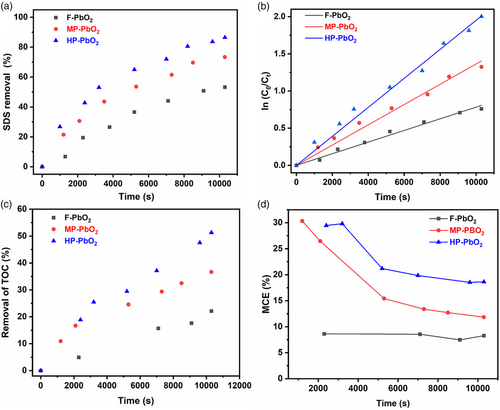
The electrodes were next compared for their performance in SDS degradation. SDS was selected as a representative pollutant due to its widespread use in various products such as detergents, shampoos, and household cleaners.[43] Figure 4 presents SDS degradation kinetics of the three electrodes. The electrochemical degradation rate of SDS followed the order of HP-PbO2 > MP-PbO2 > F-PbO2, consistent with their respective electrochemical properties discussed earlier. In ≈3 h, the F, MP, and HP-PbO2 electrodes removed 53.2, 73.4, and 86.5% SDS, respectively. The reaction kinetics can be described by a pseudo-first-order rate law (Figure 4b) with the rate constants of 7.83 × 10−5, 1.36 × 10−4, and 1.95 × 10−4 s−1 for F, MP, and HP-PbO2, respectively. The enhanced SDS removal rate observed with HP-PbO2 can be attributed to its greater surface area[23] and enhanced mass transfer[34] resulting from the interconnected pore channels[26, 32] present in the electrode (Figure 1). Moreover, a slight increase in the electrode potential was observed, rising from ≈2.4 to 2.7 V (Figure S6, Supporting Information). Because SDS degradation can lead to the formation of undesirable intermediate products, changes in total organic carbon (TOC) concentration were also monitored to assess the extent of SDS mineralization. As shown in Figure 4c, the rates of TOC removal followed a similar trend as the SDS removal: HP-PbO2 exhibited the highest TOC removal rate, followed by MP-PbO2 and then F-PbO2, with 51.3, 36.7, and 22.1% removal, respectively, in three hours of reaction time. The mineralization current efficiency (MCE), as calculated using Equation (S2), Supporting Information, ranged from 18.6 to 29.9% for HP-PbO2 (Figure 4d), which was consistently higher than those achieved by MP-PbO2 (11.8 to 26.5%) and F-PbO2 (7.5 to 8.7%) throughout the reaction time. The specific energy consumption of HP-PbO2 electrode was determined to be around 97 kWh kg−1 (Equation (S3) and Figure S7, Supporting Information). After the use of electrode, the Pb ion concentration was measured to be 0.016 mg L−1 (Figure S8, Supporting Information). These findings highlight the superior electro-degradation performance of HP-PbO2 in the context of SDS degradation, emphasizing the crucial role played by its interconnected porous structure.
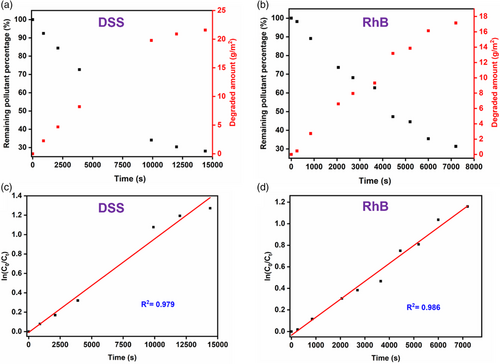
To assess the broad applicability of the HP-PbO2 electrodes for the advanced electrochemical oxidation of other contaminants, we evaluated its performance for degradation of two other organic contaminants: a pharmaceutical compound DSS, and an organic dye RhB. As hypothesized, the HP-PbO2 electrode demonstrated excellent performance in degrading both pollutants (Figure 5a,b). For DSS, a 72.0% removal efficiency and a 21.6 g m−2 degradation amount were achieved within just 4 h of treatment. Additionally, a considerable 47.7% TOC removal rate was observed. Similarly, for RhB, the HP-PbO2 electrode exhibited impressive degradation efficiency, with a 68.6% removal ratio and a 17.2 g m−2 degradation amount achieved within 2 h of operation. The reaction kinetics of both pollutants also fit well with the pseudo-first-order kinetic model (Figure 5c,d). The ability of HP-PbO2 electrodes to effectively degrade a diverse range of pollutants underscores the versatility and potential of this novel electrode material for various environmental remediation applications.
3 Conclusion
This study successfully fabricated a novel HP-PbO2 electrode via a facile one-step electrodeposition method on a PS sphere-templated FTO surface. The fabricated electrode was characterized by a variety of physical and electrochemical analyses. Compared with the MP and F-PbO2 counterparts, the HP-PbO2 anode showed significantly higher electrochemical removal efficiency against various organic compounds. The superior electrochemical oxidation performance of the HP-PbO2 electrode indicates that this material is a promising anode material for the removal of waterborne persistent organic contaminants. This study advocates for the broad application of electrochemical oxidation in removing waterborne organic pollutants and propels the development of anode materials.
4 Experimental Section
Chemicals
Polystyrene latex particles (1% w/v) of 50 nm in diameter were purchased from Lab261 (Palo Alto, CA); polystyrene latex particles (4% w/v) of 4 μm in diameter were purchased from Invitrogen. Fluorine-doped tin oxide (FTO) glasses were purchased from Yingkou OPV Tech New Energy Co., Ltd (Yingkou, China). Sodium n-dodecyl sulfate (SDS, 99%, water <2%) was obtained from Alfa Aesar. Lead (II) nitrate (Pb(NO3)2, ≥99%), sodium fluoride (NaF, ≥99%), nitric acid (HNO3, 70%), rhodamine B (RhB, ≥95%), diclofenac sodium salt (DSS, ≥98%), sodium sulfate (Na2SO4, ≥99%), tetrahydrofuran (THF), and acetone were purchased from Sigma-Aldrich. Ultra-corrosion-resistant Grade 2 titanium sheets were purchased from McMaster-CARR. Deionized (DI) water was purchased from PTI Process Chemicals, Inc. All chemicals used in experiments were used as received unless otherwise mentioned.
Preparation of the PS Template and Electrode
Hierarchically porous PbO2 electrodes (HP-PbO2) were prepared by electrochemical deposition on FTO using PS opal templates formed by PS latex particles of nanometer and micrometer sizes. The PS template was prepared following a protocol modified from a previous report.[32, 44] First, PS opal templates (1 × 1 cm2 in size) were constructed on the FTO substrates using the solvent evaporation method.[44] The FTO substrates were first sonicated for 5–10 min each in THF, acetone, and DI water. Each substrate was dried with nitrogen gas and then treated with air plasma for 10 min. PS particle solutions of sizes 4 μm and 50 nm were sonicated before use and subsequently combined at 0.4 and 0.12% w/v, respectively. One drop of a 1% SDS solution was then added to facilitate the dispersion of the particles, and the combined suspension was sonicated again for 30 min. ≈1 mL of the PS particle suspension was added to each small vial. In each vial, a FTO substrate was laid at a 45° angle. The vials were kept in an oven set at 55 °C for 24 h or until the liquid in each vial had completely evaporated, leaving behind a thin PS opal template on the FTO glass for subsequent electrodeposition.
The electrodeposition of PbO2 was conducted using the galvanostatic method following a previously reported procedure.[45] Briefly, 8.2 g of Pb(NO3)2 and 21 mg of NaF were added to 50 mL of 0.1 m HNO3. The solution was sonicated until all solids were completely dissolved. The standard three-electrode system utilized for electrodeposition used the PS template on the FTO substrate as the working electrode, a titanium counter electrode, and a silver chloride electrode as the reference electrode. During the electrodeposition of PbO2, the current was maintained at 20 mA cm−2 for 1200 s; the distance between the working and counter electrodes was maintained at 2 cm. After PbO2 electrodeposition, the PS template was removed by submerging the working electrode in fresh acetone three times for 20–30 min each time. The electrode was then gently blown with nitrogen and kept at 55 °C until completely dried. Based on the raw materials price, the preparation cost of each HP-PbO2 electrode is around $7.8 every 1 cm2 (Table S1, Supporting Information). MP-PbO2 was constructed in the same way using only 4 μm PS particles in the template fabrication process, while F-PbO2 was synthesized on pure FTO without using any PS template entirely.
Electrode Characterization
The XPS measurements of PbO2 electrodes were conducted on a PHI Quantera XPS instrument, which employed a focused monochromatic Al Kα X-ray (1486.7 eV) source for excitation. The XPS survey scan spectra were recorded in the 1100–0 eV binding energy range with a step size of 0.5 eV and a pass energy of 140 eV. High-resolution scan spectra were obtained with a step size of 0.1 eV and a pass energy of 26 eV. In each measurement, low-energy electrons and Ar+ ions were employed for specimen neutralization. SEM analysis was performed using an FEI Quanta 400 FESEM operated at 30.00 kV. Prior to analysis, each sample was coated with a 5 nm gold film using a Denton Desk V Sputter. The contact angle was measured utilizing a drop shape analyzer (DSA 100, KRÜSS GmbH, Hamburg, Germany).
Electrochemical Measurements
All the electrochemical measurements were conducted with the electrochemical workstation using a three-electrode configuration. LSV curves were obtained in 0.1 m Na2SO4 at a scan rate of 20 mV s−1. Cyclic voltammograms (CV) tests were performed in 0.1 m Na2SO4 at a scan rate from 10 to 200 mV s−1. Electrochemical impedance spectroscopy was conducted at open circuit potential from 1 MHz to 100 Hz with a 5 mV voltage in 0.1 m Na2SO4 using the same three-electrode system.
Electrochemical Degradation Experiments
All electrochemical degradation experiments were conducted in a 50 mL beaker utilizing a three-electrode setup, with a PbO2 electrode described above as the working electrode (working area of 1 cm2), a titanium sheet as the counter electrode (the same working area), and a silver chloride electrode as the reference electrode. The distance between the working and counter electrodes was maintained at 2 cm. Using the galvanostatic method, the current was maintained at 20 mA cm−2 throughout the experiment at room temperature. For pollutant degradation, a 50 mL sample of 60 mg L−1 pollutants (50 mg L−1 for RhB) in 0.1 M Na2SO4 was degraded using the synthesized PbO2 electrodes. The concentration of SDS at different time intervals was dyed using the Stains-All working solution, and then measured using Agilent Cary 60 UV–vis spectrometer. The detailed method can be found in the Supporting Information. The concentration of RhB at different time intervals was measured using Agilent Cary 60 UV–vis spectrometer according to a previous report.[46-48] The concentration of DSS at different time intervals was measured using HPLC.
Acknowledgements
This work was supported by the Bill & Melinda Gates Foundation INV-035863. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. This work was partially supported by the Welch Foundation Grant C-1716 and NSF ERC on Nanotechnology-Enabled Water Treatment (EEC-1449500).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Yifan Zhu: conceptualization (equal); data curation (lead); investigation (lead); writing—original draft (lead); Michelle T. Chen: data curation (lead); writing—original draft (equal); Yuren Feng: data curation (supporting); methodology (supporting); writing—original draft (supporting); writing—review editing (supporting); Qing Ai: conceptualization (supporting); data curation (supporting); Yiming Liu: data curation (supporting); writing—review editing (supporting); Yunrui Yan: data curation (supporting); Qilin Li: funding acquisition (lead); writing—review editing (lead); Jun Lou: conceptualization (lead); funding acquisition (lead); project administration (lead); supervision (lead); writing—review editing (lead).
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supporting information of this article.




