Atomic Active Centers Anchored Photocatalysts for CO2 Reduction to Renewable Ethylene/Ethane
Abstract
Photocatalytic CO2 reduction to ethylene/ethane offers a sustainable and cost-effective approach to simultaneously produce these high-demand chemicals while reducing atmospheric CO2 level. Lately, atomic active centers (AACs) anchored photocatalysts are comprehensively investigated for their exceptional photocatalytic performance regarding the photosynthesis of ethylene/ethane. This review provides a critical summary of the incorporation of various AACs, such as Cu, Au, Ag, Mo, Ni, P/Cu, and CuAu, onto different photocatalyst supports, including metal oxide, carbon nitride, red phosphorous, covaleng organic framework , and bimetallic sulfide together with their crucial role in raising the rates of ethylene/ethane production and selectivities. Furthermore, this review offers a comprehensive summary/analyses of preparation routes, chemical composition, atomic configurations, coordination structures, optical characteristics, structure–performance relationship, reaction intermediates, reaction pathways, photocatalysis mechanism, and photocatalytic performances of the as-prepared AAC anchored photocatalysts using state-of-art in situ characterization techniques. This review also emphasizes the challenges in the process of photocatalytic CO2 conversion to target products, the role of anchored AACs, limitations, potential applications, and future outlooks for AACs anchored photocatalysts.
1 Introduction
The high level of carbon dioxide (CO2) emission is one of the primary factors contributing to the global warming phenomenon. The continuing increase of these emissions, which mainly emerged from the employment of fossil fuels in various industrial sectors, will render it impossible to meet the greenhouse gas emissions-cutting targets set by the Paris climate change agreement utilized by the UN in 2015.[1, 2] Therefore, visible light photocatalytic conversion of CO2 into value-added products and renewable chemicals, known as artificial photosynthesis, is regarded as a prominent green/sustainable strategy to reduce the elevated CO2 level in the atmosphere and contribute to the climate change mitigation efforts, as it simulates the natural photosynthesis in plants through utilizing solar light, CO2 and photocatalysts to initiate the process of photocatalytic CO2 reduction to various renewable chemicals/fuels.[3-9] Photocatalytic reduction of CO2 (PRCO2) has two key pathway products: the first are compounds with one carbon (C1), such as carbon monoxide (CO), methane (CH4), formic acid (HCOOH), and methanol (CH3OH), which are the more common products resulting from PRCO2.[10-13] The second set of products are compounds with two (C2) or more than two carbons (C2+) products, such as ethanol (C2H5OH), acetic acid (CH3COOH), ethylene (C2H4), ethane (C2H6), and propane (C3H8).[6, 14-18] The C2 products are more economically beneficial and have higher added value than the C1 products, especially for ethylene and ethane compounds. Ethylene and ethane are considered amongst the most intensively utilized chemicals in various industries around the globe, such as polymer and chemical manufacturing, agriculture, food, and fuel industries.[19-21] However, ethylene and ethane are mainly produced from steam cracking and crude oil/natural gas refining processes at high-temperature and high-pressure conditions, resulting in high levels of greenhouse gases.[22, 23] Alternatively, green and eco-friendly photocatalyst-based production routes for ethylene and ethane can provide a more sustainable and cost-effective strategy for concurrently manufacturing these high-demand chemicals and minimizing the levels of atmospheric CO2 towards a better global carbon cycle.[24, 25]
Photocatalytic CO2 reduction to ethylene/ethane is regarded as a significant challenge owing to the multiproton/multielectron transfer and the slow kinetics of C–C coupling processes required to realize such a reaction. Moreover, the complexity of the reaction mechanism renders it more challenging to obtain high yield and high selectivity for ethylene/ethane.[26, 27] One representative challenge is the competition between the C–C coupling and the hydrogenation of formed intermediates. Consequently, the rational design of photocatalysts that can balance between the appropriate amount of hydrogenation and the C–C coupling for CO2 conversion is crucial for overcoming these barriers and reaching highly efficient and selective production levels of such renewable chemical/fuel.[28, 29] Various strategies have been utilized in synthesizing high-performance photocatalysts for ethylene/ethane production, including the use of nanoparticles,[30] heterojunctions,[26, 31] metal-organic frameworks (MOFs),[24] supramolecular structures,[16] porphyrinic triazine frameworks (PTFs),[27] and atomic vacancies.[28, 32] However, the quest for achieving enhanced production rates and selectivity performance has motivated researchers to investigate novel classes of photocatalysts to address these challenges. Recently, atomic active centers (AACs) anchored photocatalysts have been extensively explored in multiple photocatalytic applications since it was first proposed by Zhang et al. in 2011.[33] The remarkable properties of AACs anchored photocatalysts, such as unique optical/electronic properties, full coverage of support surface, low cost, highly active centers, and well-defined coordination structure of the AACs anchored on the surface, make the AACs anchored photocatalysts the groundbreaking engineering strategy for photocatalytic CO2 conversion over the last decade.[34-36] This is because AACs can facilitate electron transfer/CO2 activation, reduce the energy barriers and provide precise control over the photocatalytic process, resulting in higher production/selectivity towards the C2 products.[37, 38] Additionally, AACs play a crucial role in promoting the C–C coupling and hydrogenation of CO2 intermediates by serving as highly active centers for the adsorption and activation of CO2. This results in their photoreduction to CO and other intermediates, which can then undergo C–C coupling to form the C2 products.[39, 40] The distinguished electronic structures of AACs assure the optimum alignment of energy levels, promoting the dimerization of CO molecules or the coupling interactions between CO and other intermediates. AACs also facilitate the hydrogenation process of these intermediates by efficiently transferring protons and electrons, supporting the stepwise reduction process required to produce ethylene/ethane.[41-43] Although there are numerous reviews on the utilization of various AACs (e.g., Au,[44] Pt,[45] Ru,[46] Co,[47] Cu,[17] Ni[48]) onto different photocatalysts, such as TiO2,[49] graphitic carbon nitride,[50] covalent organic frameworks (COFs),[51] metal organic frameworks (MOFs),[52] for photocatalytic CO2 conversion into C1, C2 and C2+ products, a comprehensive review specifically focusing on the employment of AACs anchored photocatalysts for CO2 photoreduction to the valuable C2 product (e.g., ethylene/ethane) is currently lacking to the best of our knowledge.
In this review, we exclusively highlight the significant role of AACs in raising the photocatalytic activity/selectivity/stability of various photocatalysts for CO2 conversion to form ethylene/ethane at ambient conditions. The complete exposure of active centers in AACs anchored photocatalysts allows the utilization of each atom to its full potential, reducing the occurrence of unfavorable competing side reactions. This results in higher yield and selectivity of products and maintains the improved catalytic activity/stability over long periods. This review also sheds light on the correlation between atomic-scale engineering approaches to anchoring AACs over photocatalysts and the activity/selectivity of photocatalysts. Also, this review reveals deep insights into the reaction mechanism of photocatalytic CO2 reduction to ethylene/ethane in realistic conditions using in situ spectroscopy and microscopy characterization techniques. Finally, our review not only contributes to the fields of CO2 photoreduction reactions by achieving high activity and high product selectivity but also paves novel avenues to the preparation of sustainable high-performance photocatalysts. Particularly, the synchronization between the electronic and structural properties of AACs anchored photocatalysts will provide a powerful platform for the exploration of next-generation composite photocatalysts for efficient and selective CO2 conversion processes.
2 Fundamentals of AACs Anchored Photocatalysts for CO2 Photoreduction to Ethylene/Ethane
2.1 Roles on AACs for Raising Photocatalytic Ethylene/Ethane Evolution
The photocatalytic conversion of CO2 to value-added ethylene/ethane products is mainly hindered by several key factors. First, a significant energy barrier (−1.9 V vs RHE) is required to break the thermodynamically stable CO2 molecules and produce the •CO2− intermediate, which is the initial product in the PRCO2 process.[53] This highly negative potential indicates the substantial energy input required for achieving effective CO2 activation (Equation 1). Once the •CO2− is formed, a further reduction step to form the CO, which is a common intermediate in the subsequent reductions to hydrocarbons (e.g., ethylene and ethane), also requires a considerable amount of energy (−0.10 V vs RHE; Equation (2)).[32, 39, 54] Additionally, the wide variety of possible gaseous/liquid products that can possibly emerge from the PRCO2 and the complexity of generating C2 products, especially ethylene/ethane, through slow kinetics of C–C coupling and hydrogenation processes pose great challenges to achieving high yield and high selectivity for these products (Figure 1).[16, 24, 29]
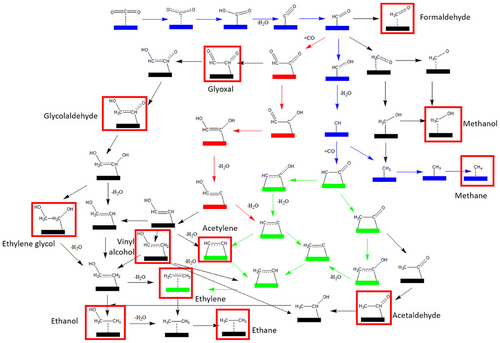
And remarkably raise the efficiency/selectivity/robusteness of photocatalytic CO2 conversion to renewable chemical/fuel. Contrary to the traditional photocatalysts that often consist of nanoparticles/clusters, AACs anchored photocatalysts exhibit the presence of AACs highly scattered on the surface of the support catalyst. This distinctive structure has equipped them with outstanding catalytic performance. One of the main merits of AACs is the maximized atomic utilization as the high exposure of AACs over the support surface allows each atom to be exposed and act as catalytic centers to interact with CO2 molecules. This is in remarkable contrast to the conventional nanoparticle/cluster scattered photocatalysts, which often get buried in the bulk of photocatalysts. This obviously raised atomic utilization not only improves the efficiency of overall photocatalytic CO2 reduction but also reduces the amounts of metals required to prepare photocatalysts, resulting in reduced economic costs.[56-59]
Moreover, the characteristics of distinctive electronic structures of AACs anchored photocatalysts can regulate the electronic densities distributed around the active centers to affect the accumulation of photoexcited charge carriers around them, arousing raised CO2 adsorption/activation, reducing the energy barriers for the thermodynamically disadvantageous reactions along with forming favorable intermediates for the subsequent desired reactions over these centers.[60, 61] For instance, Cao et al. reported that anchoring Au single atoms (SAs) on Cd1−xS photocatalysts aroused a remarkable enhancement for CO2 photoreduction compared to anchoring Au nanoclusters over the same catalyst. This is because adding Au SAs manipulates the electron density of the surface through the strong hybridization of Au 5 d and S 2p orbits, which accelerates the photogenerated electron transfer to the surface of the photocatalyst, resulting in the availability of more electrons to the PRCO2, reduced energy barriers and raised overall efficiency. Also, introducing the Au SAs to the Cd1−xS system aroused the formation of the H3CO* intermediate, which is advantageous for the successive reaction. However, the Au nanoclusters prompt the formation of HCO* intermediate for the same reaction.[62] Therefore, incorporating SAs is regarded as an innovative strategy to raise the formation rates of desired intermediates required to achieve higher production yields of C2H4 and C2H6.[30, 59, 63]
Furthermore, the regulable coordination environments of AACs anchored photocatalysts are another merit, as the AACs usually coordinate with the surrounding atoms of various supports (e.g., metal oxides, 2D materials, and MOF) in a well-defined order. These coordination environments can stabilize the AACs, prevent their agglomeration, and create highly active catalytic centers for controlling the hydrogenation of the PRCO2 process.[64, 65] For instance, Yang et al. have reported that the hydrogenation pathway of CO2 reduction intermediates over Cu–N4 active centers is completely different from the hydrogenation route over Cu–N3 active centers. They revealed that the Cu–N4 coordination structure prefers to catalyze the photoreduction and hydrogenation of CO2 to CH3OH via the formate pathway and achieving 95.5% selectivity, whilst the Cu–N3 coordination structure induces the CO2 reduction to CO with 94.3% CO selectivity. These results give prominence to the importance of tuning the coordination environment for raising the desired products yields/selectivity.[66, 67] Besides, the unsaturated coordination of AACs anchored photocatalysts facilitates the adsorption/activation of CO2 and promotes the rates of sluggish C–C coupling process and the hydrogenation competitive steps (Figure 2a–b). Consequently, through controlling these coordination environments, we can achieve the rational design of AACs anchored photocatalysts, which can enhance the interaction with CO2 reduction intermediates and improve the selectivity toward ethylene/ethane.[66, 68] Additionally, for the C2 products to be formed, the CO2 molecules ought to be adsorbed and activated over a single active site. The resulting C1 intermediates then need to migrate to other active sites with other C1 intermediates for the C–C coupling process to occur, which significantly reduces the selectivity of the produced C2 products.[69, 70] However, the highly exposed adjacent AACs dispersed over the photocatalyst support can reduce the migration distance for the C1 intermediates, owing to the availability of plenty of adjacent active sites in the AACs anchored photocatalysts, which promotes the C–C coupling and increases selectivity for ethylene/ethane.[68]

Finally, AACs can act as efficient light-absorbing centers owing to their unique properties. These properties extend the light-absorption abilities of photocatalysts compared to traditional nanoparticles/clusters, generating more photoexcited electrons/holes and raising the PRCO2 efficiency. Also, the built-in electric field in AACs can facilitate charge carrier separation, promote the transfer of electron/hole to the reactant and reduce the charge recombination rates, which are major challenges in photocatalysis. This efficient separation can provide more electrons for CO2 reduction and enhance the overall reaction rate.[61, 67]
Overall, AACs anchored photocatalysts have been extensively investigated for the photocatalytic conversion of CO2 to value-added chemicals, providing an atomic-scale understanding of the structure–performance relationship of photocatalysts and how they can be engineered to achieve higher efficiencies. But the complexity of reaction pathways, sluggish kinetics, high energy barriers, and intermediate competition are restricting the massive utilization of AACs in PRCO2 to form renewable C2 hydrocarbons, that is, ethylene and ethane. Thus, further investigations are still required to explore these obstructions and fill the existing gap toward achieving higher selectivity/efficiency/robustness for photocatalytic ethylene/ethane evolution.
2.2 Routes on Anchoring AACs onto Photocatalysts
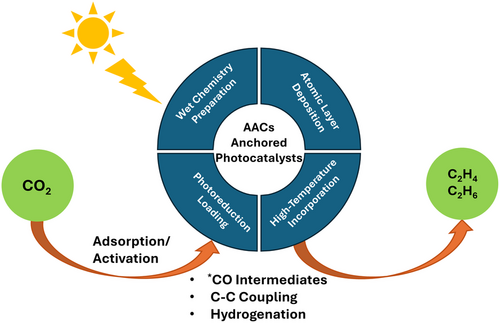
Effective routes for anchoring AACs are of prime importance for exploring highly efficient photocatalysts to transform CO2 molecules into renewable hydrocarbons. Achieving precise control over the engineering process can raise the feasibility, reproducibility, stability, and overall photocatalytic performance. This accurate control is also propitious for: i) tailoring catalytic centers at the atomic level to enable selective interactions with reactants; ii) achieving maximum atomic utilization by ensuring that all the atomic active centers are available for reaction; and iii) achieving higher catalytic activities and selectivity for the desired products. A variety of techniques have been developed to ensure the optimum scattering of AACs over photocatalysts. The most common routes reported for photocatalytic CO2 conversion into ethylene/ethane are: i) wet chemistry preparation, for example, wet impregnation and up-bottom ion-cutting (solvothermal); ii) high-temperature incorporation, for example, hydrothermal, calcination, and thermal polymerization; iii) atomic layer deposition; and iv) photoreduction loading.
First, wet impregnation and up-bottom ion-cutting are regarded as the facile wet chemistry preparation routes for the incorporation of AACs into materials. These routes are easily scalable for industrial application and can be adapted to a wide range of elements. Typically, the process involves the interaction between the photocatalysts and salt precursors, followed by calcination at ambient conditions to assure strong bonding with the support surface if required. For example, one research[68] reports implanting Au AACs on the red phosphorous (RP) surface using the wet impregnation technique. This involves mixing RP with certain amounts of HAuCl4 as a precursor for Au AACs in methanol, followed by sonication and stirring. Subsequently, the photocatalyst was freeze-dried and calcined at 125 °C for 2 h in the H2 atmosphere to acquire the Au/RP photocatalyst. Another study reports anchoring Mo AACs over TpBpy COF through mixing Mo(CO)6 with TpBpy COF in a toluene solution followed by heating at 110 °C for 6 h under an N2 atmosphere.[71] On the contrary, the up-bottom ion-cutting method is quite similar to the wet impregnation method but with some differences. For instance, CuAu diatomics (DAs) have been anchored on TiO2 photocatalyst using this technique through: i) mixing TiO2, PVP, KBr, and L-ascorbic acid in an oil bath at 80 °C for 10 min; ii) adding CuCl2·2H2O and HAuCl4 solutions to the mixture; iii) continuing the heating process at 80 °C for another 3 h to successfully construct the Cu5Au1–TiO2. After that, 100 mg of the as-prepared Cu5Au1–TiO2 was scattered in a solution of FeCl3·6H2O and 0.1 m HCl bubbled with Ar gas. Then, the suspension mixture was etched for 7 h in an oil bath at 50 °C to obtain the E7–Cu5Au1–TiO2 photocatalyst.[61] However, it is challenging for these approaches to achieve full control of loading AACs, which affects the reproducibility of the catalyst. Besides, they involve using hazardous materials.
Second, the high-temperature incorporation routes (e.g., hydrothermal treatment, calcination, and thermal polymerization) involve heating the precursors and supports to high temperatures in an inert or reducing atmosphere to facilitate the precursor decomposition and the subsequent scattering of AACs over the catalyst surface. These routes are beneficial for improving the stability of the as-prepared photocatalyst by creating a strong interaction between the support surface and anchored AACs. This can result in the creation of robust AACs anchored photocatalysts with improved temperature stability and long-term catalytic robustness. For example, one of the above references reports the process of anchoring Cu AACs onto phosphorous-modulated carbon nitride photocatalyst (Cu AACs/PCN) through a two-step thermal polymerization.[64] Two other studies outline the preparation of Cuδ+ active centers/CeO2-TiO2 and Cu1/TiO2 photocatalysts through calcination.[59, 63] The Cu AACs/PCN photocatalyst is created by dissolving CuCl2·2H2O and melamine in the HCl solution. The product is then separated, dried, and undergoes a sequence of treatments, including the first thermal polymerization step, where it is calcined with NaH2PO2·H2O at 500 °C for 4 h in the N2 atmosphere. After that, the formed product is ground with LiCl and KCl, and calcined again at 550˚C for another 4 h in an N2 atmosphere, followed by leaching using H2SO4 and boiling water to obtain the final catalyst.[64] In addition to this, the Cuδ+/CeO2–TiO2 photocatalyst is prepared by adding Cu+2 and Ce+3 solution into the MIL-125-NH2 precursor, followed by calcination at 450 °C for 3 h in air.[63] Nevertheless, the Cu1/TiO2 photocatalyst is fabricated by adding a calculated amount of CuCl2·2H2O to a colloidal solution of SiO2@TiO2 nanoparticles and stirring for 3 h at room temperature. The resulting product is then centrifuged, washed, and dispersed in water. PVP is added to the mixture, and after its adsorption, the product is collected by centrifuge and redispersed in an ethanol and water mixture solution. Aqueous ammonia and TEOS are added to the mixture, and after 4 h, the product is separated, washed, and dried at 80 °C. The resulting powder is then calcined at 950 °C for 2 h in air. The SiO2 nanoparticles are etched using a NaOH solution to acquire the final Cu1/TiO2 product.[59] Besides, one study[72] reports the synthesis of CA/Ni1CuNP N–C photocatalysts by adding Cu+2 and Ni+2 solutions to ZIF-8 precursor followed by calcination at 800–950 °C under N2 atmosphere. Then, a heterojunction with layered doubled hydroxide (LDH) (i.e., CoAl-LDH (CA)) is acquired using ultrasonication. Furthermore, there are two reports for preparing Cu1/W18O49 and Ag/CIS photocatalysts using hydrothermal treatment at 200 °C for 12 h and 180 °C for 24 h, respectively.[56, 58] On the contrary, only one reference reports the combination of both hydrothermal and calcination treatment to prepare P/Cu SAs@CN catalyst. In this report, melamine and NaH2PO2 were mixed in hydrothermal treatment at 80 °C for 6 h. The acquired product was dispersed in 1.0 M Cu(NO3)2·3H2O ethanol solution as the copper precursor, followed by drying at 80 °C and calcination of the resulting powder at 550 °C for 2 h.[65] Yet, the increased energy consumption associated with these methods is making them less energy efficient compared to other methods.
Third, one unique study reports the utilization of an atomic layer deposition route to integrate Cu AACs onto TiO2 nanosheets, assuring an even scattering of Cu AACs across the support surface. The process involved the use of a viscous-flow stainless-steel tube reactor system with N2 as a carrier gas at 300 °C, employing Cu(hfac)2 and formaldehyde as precursors.[67] On the contrary, the complexity and equipment requirements necessary for this method still challenge its applicability in large-scale applications.
Finally, a photoreduction loading route was employed in one report to anchor Cu AACs and Cu–Au alloy nanoparticles on TiO2. P25 TiO2 nanoparticles were scattered in ethanol, deoxygenated with N2, and varying amounts of Cu(NO3)2·3H2O solution were added. The mixture was then irradiated using a 300 W mercury lamp for 30 min. Then, specific quantities of HAuCl4 were added, and the suspension was further irradiated for an additional 30 min to complete the loading.[57] This incorporation method can be limited by the different reactivity of the multiple AACs, resulting in difficulties in controlling the output of the process. Finally, Figure 3 exhibits a schematic diagram of various preparation routes for acquiring AACs anchored photocatalysts for ethylene/ethane evolution.
3 AACs Anchored Photocatalysts for Raising Ethylene/Ethane Evolution
Currently, a range of AACs anchored photocatalysts have been extensively explored for the photocatalytic evolution of ethylene/ethane through CO2 photoreduction. These AACs are categorized into four classes of i) Cu-based AACs, ii) Au- or Ag-based AACs, iii) Mo- or Ni- based AACs, and iv) dual AACs, respectively. Particularly, Cu AACs have been successfully integrated into various photocatalysts, including metal oxides such as TiO2,[57, 59, 67] W18O49,[56] and CeO2–TiO2,[63] as well as 2D materials like phosphorous-modulated carbon nitride (PCN).[64] For Au- or Ag-based AACs, there are two studies reporting their anchoring on 2D RP and bimetallic sulfide like CuInS2 (CIS), respectively.[58, 68] For Mo- or Ni-based AACs, there are two studies that investigated their incorporation into TpBpy COF and nitrogen-doped carbon NC, respectively.[71, 72] For dual AACs, the synergistic effects in dual AACs anchored photocatalysts have been studied to reveal the origin of raised performance in photocatalytic ethylene/ethane evolution. This includes the utilization of metallic dual AACs (e.g., CuAu DAs) and nonmetallic/metallic dual AACs (e.g., P/Cu), which have been incorporated onto TiO2 and CN photocatalysts, respectively.[61, 65] Besides, we have summarized the composition, routes on loading AACs, reaction conditions, and results on performances of all the AACs anchored photocatalysts for CO2 reduction to ethane/ethylene (Table 1). This diverse range of AACs anchored photocatalysts will be critically reviewed in the following sections:
| Photocatalyst | Routes on loading | Reaction condition | Rates/product [μmol g−1 h−1] | Selectivity | Reference year |
|---|---|---|---|---|---|
| Cu0.8-Au0.2/TiO2 | photoreduction | 2 mg catalyst, 0.15 mL water, CO2 bubbling (90 KPa), 300 W Xenon lamp irradiation with (1.5 AM) filter | 369.8/ethylene | 11.9% | [57] |
| Cuδ+/CeO2−TiO2 | calcination | 10 mg catalyst, 30 mL H2O, CO2 atmosphere (0.1 MPa), Xe lamp (320 nm<λ<850 nm) | 4.5/ethylene | 47.5% | [63] |
| Cu1/TiO2 | calcination | 40 mg catalyst, moist CO2 bubbling, 100 W Xenon lamp | 64.2 ppm g−1 h−1/ ethane | not mentioned | [59] |
| Cu1/TiO2 | atomic layer deposition | 20 mg catalyst, 20 mL H2O, TEOA, CO2 atmosphere, Xe lamp (full spectrum) | 60.4/ethylene | 75.2% | [67] |
| Cu AACs/PCN | two step thermal polymerization | 5 mg catalyst, 45 mL H2O, 5 mL TEOA, [Ru(bpy)3]2+, CO2 atmosphere, 300 W Xe lamp | 30.51/ethylene | 53.2% | [64] |
| 3.6-Cu1/W18O49 | hydrothermal treatment | 5 mg catalyst dried over quartz filter paper, 200 μLH2O, CO2 bubbling, 300 W Xe lamp | 4.9/ethylene | 72.8% | [56] |
| Au1/RP | impregnation | 50 mg catalyst, 0.5 g KHCO3, 1-2 mL 6 M HCl, 300 W Xe lamp | 1.32/ethane | 96% | [68] |
| 2%Ag/CIS | hydrothermal treatment | 5 mg catalyst spread over glass fiber membrane, 2 mL H2O, CO2 bubbling, 300 W Xe lamp (λ>420 nm) | 53.8/ethylene | 98.8% | [58] |
| Mo-COF | impregnation | 10 mg catalyst dispersed over quartz glass dish, 5 mL H2O, CO2 bubbling, 300 W Xe lamp (λ>420 nm) | 3.57/ethylene | 32.92% | [71] |
| CA/Ni1CuNP N-C | calcination | 50 mg catalyst, 95 mL H2O, 5 mL TEOA, CO2 bubbling, 300 W Xe lamp (λ>420 nm) | 25.328/ethane | 50.61% | [72] |
| P/Cu SAs@CN | hydrothermal and polymerization | 25 mL catalyst suspension (20 mg L−1), 3 mL TEOA, CO2 bubbling, 300 W Xe lamp | 616.6/ethane | 33% | [65] |
| Et7-Cu5Au1−TiO2 | up-bottom ion-cutting | 20 mg catalyst +5 mL water dispersed over 45 mm diameter quartz glass, 2 mL H2O, CO2 bubbling, 25 °C, light irradiation (380 nm<λ<720 nm) | 71.6/ethylene 8.5/ethane | 68.3% (ethylene) 9.4% (ethane) | [61] |
3.1 Cu AACs Boosting Photosynthesis of Ethylene/Ethane
The aforementioned results elaborate on the synergetic effect of coloading Cu AACs and Cu–Au alloy NPs on TiO2 support, which enhances the CO2 adsorption, lowers energy barriers for the overall reaction, and promotes the slow C–C coupling for PRCO2 into ethylene.
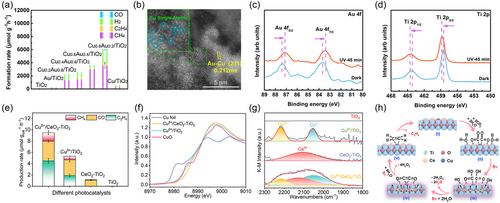
In another report, Wang et al.[63] implanted atomically scattered Cuδ+ species over CeO2–TiO2 heterostructure to acquire the Cuδ+/CeO2–TiO2 photocatalyst. The resulting Cuδ+/CeO2–TiO2 photocatalyst featured the presence of porous TiO2, which acted as a light absorber to generate charge carriers being efficiently separated over the CeO2–TiO2 heterostructure interface. This would reduce charge recombination and raise catalytic performance. In addition, the Cu and Ce active centers were explored to stimulate the adsorption/activation of CO2 molecules, and accelerate the slow kinetic C–C coupling process. These resulted in astonishing activity for CO2 conversion into ethylene in a saturated solution of CO2 irradiated for 5 h by a xenon lamp (320 nm<λ<850 nm) at room temperature. Figure 4e displays that the optimized Cuδ+/CeO2–TiO2 achieved a high ethylene production rate of 4.51 μmol g−1 h−1 with a selectivity of 47.5%, with both CO and CH4 products detected. In comparison, bare TiO2 exhibited no activity for CO2 reduction; while CeO2–TiO2 presented only a low CO yield rate of 1.08 μmol g−1 h−1. Notably, the Cuδ+/TiO2 exhibited a lower ethylene evolution rate of 1.91 μmol g−1 h−1 and a selectivity of 35.9%, compared to the as-prepared Cuδ+/CeO2–TiO2. The effect of Cu and Ce active centers on the optical characteristics of Cuδ+/CeO2–TiO2 was evaluated by steady-state PL, UV-Vis DRS, and transient photocurrent measurements. The steady-state PL peak intensity for the Cuδ+/CeO2–TiO2 sample was found to be lower than those of TiO2, CeO2–TiO2, and Cuδ+/TiO2, respectively. These results indicate that the Cu and Ce active centers significantly reduced the charge recombination, resulting in improved electron–hole separation and consequently higher activities compared to other catalysts.
Moreover, UV-Vis DRS results exhibited an upshift in the absorption peak of Cuδ+/CeO2–TiO2 to the visible light region, compared to pristine TiO2 or Cuδ+/TiO2. The efficient charge separation over the optimized Cuδ+/CeO2–TiO2 photocatalyst was further explored by transient photocurrent measurements. The results revealed more effective charge separation in Cuδ+/CeO2–TiO2 with light irradiation than those in other catalysts. Moreover, the atomic structure and coordination environment of anchored Cu AACs in the Cuδ+/CeO2–TiO2 catalyst were explored using XANES and FT-EXAFS spectroscopy. The normalized Cu K-edge XANES spectra of Cuδ+/CeO2–TiO2 exhibited an absorption edge at 8996.5 eV and a smaller shoulder at 8989.3 eV, assigned to the presence of Cu2+ atomic species (Figure 4f). Moreover, the FT-EXAFS spectra of the Cu K-edge for the Cuδ+/CeO2TiO2 exhibited a small peak at ≈3.2 Å ascribed to the CuCe bonding. This confirmed that the anchored Cu species in Cuδ+/CeO2TiO2 were atomically scattered Cuδ+ cations with no metallic copper or CuO present. Furthermore, the chemical environment of the active centers for Cuδ+/CeO2TiO2 was further explored using the CO-absorbed diffuse reflectance infrared Fourier transform spectroscopy (CO-DRIFTS) technique. The CO-DRIFTS spectra for Cuδ+/CeO2TiO2 exhibited three distinct peaks at 2217, 2055, and 2127 cm−1, corresponding to Cu2+CO, Cu+CO, and Ce3+CO bonding modes, respectively. In contrast, the Cuδ+/TiO2 sample exhibited only the Cu2+CO and Cu+CO bonding peaks, while the Ce3+CO was the sole peak observed in the CeO2TiO2 catalyst (Figure 4g). In situ DRIFTS was conducted to further track the role of CuCe dual centers in promoting the CO2 photoreduction to ethylene over Cuδ+/CeO2TiO2 with light irradiation. The analysis revealed the presence of characteristic IR bands corresponding to *CO intermediates and *COOH. Besides, the *COCO intermediate resulting from the coupling of the *CO was detected as well. These results revealed the pivotal functions of the Cu and Ce atomic centers: i) reducing the activation barriers of adsorbed CO2 molecules on Cuδ+/CeO2TiO2, and ii) boosting the formation of *COCO intermediate, which further transformed into ethylene through reduction and hydrogenation steps, as illustrated in the proposed reaction pathway mechanism (Figure 4h). In another report, Lee et al.[59] reported the integration of several transition metal (e.g., Cu, Ni, Co, and Rh) AACs over anatase TiO2 (M1/TiO2) photocatalysts for photocatalytic CO2 reduction. They found that TiO2 can stabilize the anchoring of AACs over the TiO2 surface through the electronic interaction between the TiO2 and the anchored AACs, creating spontaneous oxygen vacancies (TiO2−x) adjacent to the integrated AACs. These integrated AACs are preferred to be scattered in the Ti vacancies of TiO2. This site-preference can stabilize the anchored AACs and prevent their agglomeration. The synergetic effect of O vacancies and the incorporated AACs can further promote the photocatalytic CO2 conversion for the as-prepared (M1/TiO2) catalyst compared to bare TiO2 by eliminating the CO2 activation barriers and stabilizing the formed CO2 intermediates. This research also exhibits that the preparation route allows for the uniform scattering of metallic AACs and precise control of charge localization disturbance. The addition of various metallic AACs at a loading of 0.21% significantly improved the CO2 reduction performance of anatase TiO2 under simulated solar irradiation. The Cu1/TiO2 catalyst exhibited the highest CO2 conversion rate, producing 1416.9 ppm g−1 h−1 of CH4 and a substantial rate of 64.2 ppm g−1 h−1 of ethane, while other M1/TiO2 catalysts only produced CH4. Theoretical results revealed that the anatase TiO2 would be partially reduced to Ti3+ upon anchoring Cu AACs, with the Cu AACs preferring to be located near the Ti3+ sites, which could stabilize the added Cu AACs and create intrinsic O vacancies. A series of characterization techniques further confirmed these results. For example, the XPS Ti 2p peaks of optimized Cu1/TiO2 exhibited a movement to the lower binding energy position compared to those of anatase TiO2. These results confirmed the partial reduction of Ti4+ to Ti+3 over the TiO2 surface after integrating the Cu AACs. The coordination environment and the atomic bonding nature of the anchored Cu AACs in the as-prepared Cu1/TiO2 photocatalyst were explored by the X-ray absorption spectroscopy (XAS) technique. The X-ray absorption near-edge structure (XANES) analysis revealed the oxidation state of the Cu AACs to be (+2) with the absence of any other Cu species. Furthermore, the extended X-ray absorption fine structure (EXAFS) for the Cu1/TiO2 catalyst is in agreement with its XANES result, confirming the stabilization of Cu AACs via the Ti vacancies in the as-prepared TiO2. Considering all the aforementioned theoretical and characterization results, it is concluded that the naturally reduced anatase TiO2 stabilizes the anchoring of Cu AACs in Ti vacancies, leading to the creation of spontaneous O vacancies nearby. This synergistic effect raises the PRCO2 performance of the Cu1/TiO2 photocatalyst by reducing energy barriers and creating favorable active centers for CO2-formed intermediates, ultimately generating renewable ethane. However, this research does not provide accurate mechanistic insights into the photosynthesis of ethane.
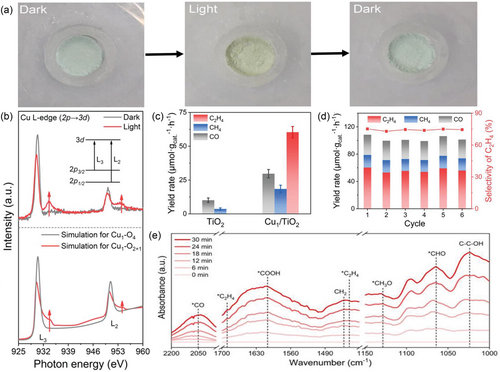
In another study, Wang et al.[67] introduced an innovative route for immobilizing photo-induced metastable asymmetric Cu AACs onto TiO2 nanosheets (Cu1/TiO2) using atomic layer deposition. With light irradiation, the photogenerated electrons migrated from TiO2 nanosheets to the Cu 3d orbits of Cu AACs, resulting in the rearrangement of the energy levels of Cu-3d orbitals. These rearrangements aroused a distortion of the previously symmetric Cu1–O4 coordination environment of the incorporated Cu AACs in the dark, resulting in a unique metastable asymmetric Cu1–O2+1 coordination structure that can revert back to the original Cu1–O4 coordination upon removal of the light stimulus. As presented in (Figure 5a), The Cu1/TiO2 photocatalyst changes color from watery blue in the dark to chartreuse in the light and back. This unique behavior is not seen in pristine TiO2 nanosheets and is not affected by temperature changes, indicating it's not driven by a photothermal effect but rather by reversible photoinduced structural changes. In order to gain insights into the fundamental properties of the Cu1/TiO2 and the metastable asymmetric Cu1–O2+1 coordination structure, a range of physicochemical characterizations were employed. For example, the HAADF-STEM images of the Cu1/TiO2 sample exhibited a uniform scattering of individual Cu atoms on the TiO2 nanosheets. Also, the electronic characteristics of the Cu1/TiO2 catalyst were investigated using XPS, XANES, and Fourier-transformed EXAFS (FT-EXAFS) techniques. The analysis of the O 1s XPS spectrum revealed the presence of oxygen vacancies. The Ti3+ oxidation state peak is attributed to structural distortions caused by incorporating Cu AACs. The Cu 2p XPS spectra show peaks indicating the presence of Cu2+ and Cu+ or Cu0 species. XANES confirms the oxidation state of Cu in Cu1/TiO2 to be ≈2+. Further analysis with FT-EXAFS in R space exhibited the presence of CuO bonding spectra. The well-fitted results confirmed the presence of atomic Cu species in a CuO4 coordination environment. The UV-Vis (DRS) results exhibited that the anchoring of Cu AACs expanded the light absorption range of the TiO2 nanosheets. Nevertheless, the steady-state photoluminescence (PL) spectra for Cu1/TiO2 presented a reduction in peak intensity compared to those of pristine TiO2 nanosheets, indicating lower electron–hole (e–h) recombination rates and improved charge separation in the Cu1/TiO2 photocatalyst. On the contrary, the photoinduced structural changes in Cu1/TiO2 with light irradiation were monitored using several state-of-art in situ techniques. The in situ XANES analysis for the corresponding Cu K-edge suffered from a blue shift with light, indicating a partial reduction of the atomic Cuδ+ species to a lower valence state (0< δ <2) when exposed to light. The in situ wavelet transform (WT)-EXAFS contour graphs demonstrated that the CuO bond was stretched after light irradiation. The combination of these results with the in situ EXAFS fitting results confirmed the destruction of the primary symmetric Cu1O4 coordination structure in the Cu1/TiO2 with light irradiation and the formation of metastable asymmetric Cu1O2+1 coordination structure intermediate. Similarly, the energy rearrangements of the Cu-3d orbitals of the incorporated Cu AACs were explored using the in situ soft-XAS technique. The results in (Figure 5b) exhibited that the peaks at 931.2 and 951.2 eV were identified as the transitions from 2p3/2 to 3 d (L3) and from 2p1/2 to 3 d (L2) states of Cu. Upon light exposure, the absorption edge of the Cu L2,3 peaks is moved to a lower energy position, revealing a partial reduction of Cu. Moreover, a new peak appeared at ≈934.7 eV, possibly originating from the rearrangement of energy levels in the Cu 3 d orbitals. This arose from the migration of photogenerated electrons from TiO2 nanosheets to the d-orbits of anchored Cu AACs. The optimized Cu1/TiO2 achieved a remarkable evolution rate of 60.4 μmol g−1 h−1 for ethylene with an outstanding 75.2% selectivity (Figure 5c). In contrast, TiO2 only produced CO and CH4, which increased in yield with the utilization of Cu1/TiO2 photocatalyst. These results reveal the role of Cu AACs over TiO2 nanosheets in promoting the C–C coupling and reducing the overall energy barriers for the value-added multicarbon synthesis. Furthermore, the ethylene-generation rate remained preserved after six catalytic cycles (Figure 5d). The reactant molecules and successive hydrogenation of the formed intermediates were tracked over the CO2 reduction pathway to ethylene using in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) measurement on Cu1/TiO2. The spectra showed rising characteristic peaks of intermediates such as *CO, *CHO, *CH3O, *COOH, and *CH2 (Figure 5e). The *C–COH intermediate which is essential for forming the C2 products, was also detected. The evolution of ethylene over the Cu1O2+1 active center with prolonged irradiation was also observed, and the calculated energy barriers of the CO2 photoreduction pathway over the Cu1O2+1 structure were found to be lower compared to the Cu1O4 structure (Figure 2a). These results corroborate the key role of the metastable asymmetric Cu1O2+1 coordination structure in exceptionally raising the photocatalytic activity of the as-prepared Cu1/TiO2 photocatalyst for ethylene evolution.
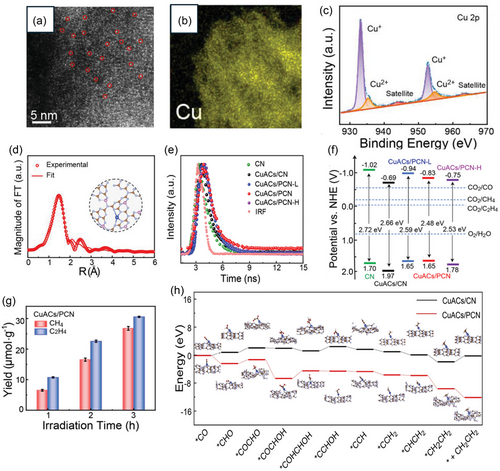
In another research, Xie et al.[64] utilized a distinct approach by integrating Cu AACs on phosphorus-modulated carbon nitride (PCN), a nonmetallic 2D photocatalyst. This route contrasts with previous reports, which primarily used metal oxides. This preparation strategy enabled the regulation of the coordination environment of anchored Cu AACs with the surrounding atoms in the as-prepared photocatalyst (Cu AACs/PCN) to create active centers with accurate CuN coordination structure (CuN4). These CuN4 centers effectively lower the energy levels of the formed intermediates in the CO2 photoreduction pathway. As a result, it raised the CO2 adsorption/activation to form *CO and accelerated the subsequent stepwise CC coupling and hydrogenation processes of the produced intermediates, ultimately resulting in the generation of ethylene. The optimal Cu AACs/PCN photocatalyst was prepared utilizing a two-step thermal polymerization process to convert the salt precursors of Cu, P, and carbon nitride into the final catalyst. In comparison, the Cu AACs/CN photocatalyst was prepared utilizing the same route but without the P precursor to reveal the role of P in raising the photocatalytic activity of Cu AACs/PCN. The P content was regulated to create two other samples, which were annotated as Cu AACs/PCNH and Cu AACs/PCNL, with higher and lower P-loading contents, respectively. Initial characterization results confirmed the successful integration of the Cu AACs into the PCN matrix. As shown in Figure 6a, the HAADF-STEM images revealed a uniform scattering of Cu AACs (circled in red) over the PCN nanosheets, with no observation of any Cu NPs/clusters/aggregation. This finding was further supported by energy dispersive X-ray spectroscopy (EDX) elemental mapping images for the as-prepared Cu AACs/PCN, which exhibited highly scattered Cu AACs throughout the PCN (Figure 6b). The intrinsic electronic state and chemical structures of the Cu AACs were explored using XPS, XANES, and FT-EXAFS. The high-resolution XPS Cu 2p spectrum was deconvoluted into four distinct peaks. Two of them at 932.7 and 952.6 eV were associated with Cu+ species; while the other two at 935.2 and 955.1 eV were attributed to Cu2+ species (Figure 6c). This revealed that the oxidation state of the Cu AACs in the Cu AACs/PCN system is between (+1) and (+2). Furthermore, the P 2p XPS spectra exhibited a single peak at 133.2 eV, indicating the presence of the PN bonding mode. The XANES absorption edge for the CuK edge in Cu AACs/PCN was found to be positioned between the absorption edges of Cu2O and CuO. This is consistent with the XPS results and indicates that the oxidation state of the Cu AACs is between +1 and +2. The fitting results of FT-EXAFS confirmed the existence of atomically scattered Cu AACs coordinated with 4 N atoms, forming the CuN4 coordination spectra measurement of all the as-prepared samples (Figure 6d). Then the impact of CuN4 active centers on the properties of Cu AACs/PCN was further explored. The TSPL of all the samples exhibited the highest average charge lifetime (τave = 3.87 ns) for Cu AACs/PCN (Figure 6e), compared to those of the other photocatalysts. These results highlight the obviously improved electron–hole separation for Cu AACs/PCN, resulting in higher catalytic performance. Figure 6f exhibited the calculated conduction band (CB) and valence band (VB) positions for the as-prepared photocatalysts against a normal hydrogen electrode (NHE) along with the measured optical bandgap energies. The results revealed that the addition of Cu AACs and P to the CN nanosheets aroused a reduction of the bandgap energy and an extension of its light absorption range. Moreover, the CB band positions confirmed that the photoreduction of CO2 to ethylene is thermodynamically feasible by all the as-prepared catalysts. Then the photocatalytic performances of all the catalysts for CO2 photoreduction were evaluated with light irradiation in the presence of [Ru(bpy)3]2+ as a photosensitizer and TEOA as a hole scavenger. The Cu AACs/PCN exhibited an impressive ethylene generation of 30.51 μmol g−1 with a selectivity of 53.2% after 3 h light irradiation (Figure 6g). Finally, the Gibbs energies all over the reaction pathway of CO2-to-ethylene conversion (Figure 6h) revealed the obviously reduced energy barriers for Cu AACs/PCN, compared to those of Cu AACs/CN. These results corroborated the key role of combining the Cu AACs and loaded P to reduce the energy barriers for CO2 reduction and boost the sluggish C–C coupling of the formed intermediates to generate value-added ethylene (Figure 6h).
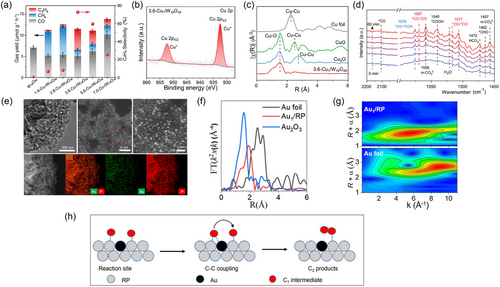
Recently, Mao et al.[56] presented an excellent ethylene production rate (4.9 μmol g−1 h−1) with high selectivity (72.8%) using Cu (I) AACs anchored W18O49 nanowires (Cu1/W18O49) (Figure 7a). The photocatalyst was prepared by a facile one-step hydrothermal approach. The intrinsic mutual conversion of the W5+ and W6+ ions in W18O49 facilitated the reduction of Cu2+ to Cu1+ cations, providing higher stability for the incorporated Cu (I) AACs in the reaction. In addition, the Cu (I) AACs reduced the energy barriers for CO2 reduction and stabilized the formed *CO intermediates. Meanwhile, the asymmetric dual active centers (Cu–W) that emerged from the interaction between Cu AACs and the W18O49 support can boost the C–C coupling of two adjacent *CO intermediates and their subsequent hydrogenation to ethylene. The oxidation states of the synthesized 3.6-Cu1/W18O49 were investigated using XPS tests. The Cu 2p XPS spectrum confirmed the presence of Cu (I) based on the appearance of the Cu 2p3/2 peak at 932 eV. Additionally, no peaks related to Cu0 or Cu2+ were observed, confirming the atomic nature of the incorporated Cu in 3.6-Cu1/W18O49 and all the Cu2+ precursors were reduced to Cu1+ within the modified W18O49 (Figure 7b). Besides, the XPS spectrum of the W 4f showed a notable 2.57 % reduction in the content of W5+ cations, providing further evidence for the involvement of W5+ centers in the reduction of Cu2+ to Cu1+. The coordination environment of the active centers over the optimized Cu1/W18O49 was explored using XANES and FT-EXAFS analyses. The normalized XANES spectra for the Cu K-edge exhibited an absorption edge close to that of Cu2O, suggesting a coordination state of ≈1 for the Cu AACs in 3.6-Cu1/W18O49. Moreover, the FT-EXAFS results presented a major peak located at ≈1.5 Å, corresponding to the CuO bonding, and the fitting results from the second shell exhibited a considerable amount of the CuW bonding path (Figure 7c). To further explore the influence of active centers on the CC coupling of the intermediates and the evolution of ethylene, in situ DRIFTS measurements were performed for the 3.6-Cu1/W18O49 under light irradiation in the CO2 atmosphere (Figure 7d). The results revealed the observation of IR bands attributed to *CHO, *COOH, and *CO intermediates, indicating the boosted CO2 adsorption/activation on 3.6-Cu1/W18O49. Furthermore, the vibrational peaks observed at 1567 and 1517 cm−1 were attributed to the *COCO intermediate, which is pivotal in the CC coupling process. Interestingly, the *CHOCO intermediate was found at 1576 cm−1, produced through the hydrogenation of *COCO intermediate. On the contrary, bare W18O49 exhibited no peaks related to the CC coupling-related intermediates, revealing the key role of Cu (I) AACs and the asymmetric dual CuW active centers in boosting the conversion of CO2 to ethylene over 3.6-Cu1/W18O49.
3.2 Au or Ag AACs Boosting Photosynthesis of Ethylene/Ethane
Apart from the Cu AACs, researchers have recently explored Au or Ag AACs anchored photocatalysts as promising candidates for photocatalytic CO2 conversion to ethylene/ethane. This research is based on the unique merits of Au or Ag AACs, such as their significant reduction capabilities, ability to enhance charge separation/transfer and promotion of CC coupling interactions. Besides, the intrinsic localized SPR of Ag and Au AACs can remarkably extend the light absorption capabilities of the as-prepared AACs anchored photocatalysts. This results in the generation of more photogenerated electrons for the multielectron PRCO2 to ethylene/ethane and further increases the overall yields. For example, Ou et al.[68] reported the regulation of the C–C coupling process in PRCO2, resulting in a remarkable selectivity of 96% for ethane as a product. This steering was achieved by utilizing highly scattered Au AACs over nonmetallic red phosphorous (RP) nanosheets as support. The intrinsic properties of the RP photocatalyst render it an excellent choice as support for CO2 photoreduction. For example, its single-element structure, which can cause a homogenous coordination environment for all of the incorporated AACs. Moreover, unlike the other common favorable centers for the AACs bonding over support materials, such as O, N, and S, the reduced electronegativity of the P elements allows for precise control of the electron density around the AACs. In addition, the rich-electron environment of RP can facilitate CO2 adsorption/activation to produce the necessary C1 intermediates for the later CC coupling interactions. Besides, the loaded Au AACs raised the photogenerated electrons transfer, and the coupling of the formed C1 intermediates to generate ethane. The Au1/RP photocatalyst was prepared utilizing a wet impregnation route followed by calcination in the H2 atmosphere to complete the anchoring of Au AACs. The morphology of the incorporated Au AACs was confirmed by various characterization techniques, such as TEM and HAADF-STEM. The HAADF-STEM images exhibit highly scattered isolated bright spots, ascribed to the anchored Au AACs over the RP (Figure 7e). Besides, the elemental mapping images revealed that the Au AACs were homogeneously scattered over the surface of RP. The chemical state of the optimized Au1/RP was further tested by XPS analysis. The P 2p XPS spectrum exhibited a main peak centering at 130 eV related to the PP bonding in the RP, and a smaller peak at 135 eV, attributed to the PO boding resulting from the oxidized surface of the RP. The Au 4f spectrum was deconvoluted into two peaks at 85 and 89 eV, corresponding to Au 4f7/2 and Au 4f5/2, respectively. This indicates that the oxidation state of the atomic Au species in Au1/RP ranged between 0 and +3. The coordination environment of anchored Au AACs in Au1/RP was verified using XANES and FT-EXAFS analyses. The absorption edge in the normalized Au K-edge XANES spectrum of Au1/RP was positioned between the absorption intensities of the Au Foil and Au2O3, revealing that the valence status of the Au AACs is between 0 and +3. The FT-EXAFS analyses of the optimized Au1/RP revealed a major peak at 1.83 Å with the absence of a characteristic peak of the AuAu bonding at >2.7 Å (Figure 7f), suggesting the absence of Au NPs in the optimized Au1/RP sample. Furthermore, the WT-EXAFS contour map of the Au foil unveiled two prominent peaks at 2.7 and 1 Å in R space, corresponding to AuAu and AuO coordination structures, respectively. Meanwhile, the contour map of Au1/RP exhibited a single peak at 1.8 Å in R-space, correlated with AuP bonding (Figure 7g). Notably, the EXAFS fitting results indicated that the immobilized Au AACs in Au1/RP adopted an AuP2 coordination structure with an average bond length of 2.34 Å. Nevertheless, the photocatalytic performance of the optimal Au1/RP photocatalyst was evaluated for PRCO2 using light irradiation without a sacrificial agent. The Au1/RP photocatalyst exhibited an outstanding production rate of 1.32 μmol g−1 h−1 for ethane and an astonishing selectivity of 96%; while RP only showed small yields of CO and CH4, highlighting the key role of Au AACs in promoting the generation of ethane. To confirm this, various AACs, such as Pt, Pd, Ir, and AuPd, were loaded on the RP and tested under the same conditions. Interestingly, only Au AACs produced ethane, confirming the key function of anchored Au AACs in the CC coupling process. In the proposed mechanism for ethane evolution over Au1/RP, the RP support is identified as the active center for adsorbing and activating CO2 molecules to generate C1 intermediates. Meanwhile, Au AACs minimize the migration distance of C1 intermediates, boosting their interaction with other C1 intermediates on a different RP active center and promoting CC coupling to generate ethane (Figure 7h).
Recently, Xu and coworkers[58] investigated the impact of Ag AACs in raising the photocatalytic performance of bimetallic sulfide support, such as CuInS2 (CIS), for CO2 photoreduction to ethylene. They reported the integration of highly scattered Ag AACs over CIS using a one-pot hydrothermal preparation route. The anchored Ag AACs induced the formation of sulfur vacancies (Vs) over the CIS surface, creating a symbiotic effect. This symbiotic effect transformed the original dual active centers of Cu and In in the bare CIS to new Vs and In dual centers in the optimized (2%Ag/CIS) photocatalyst. Besides, the similar electronic structure of Cu and In dual centers in the bare CIS resulted in high dipole–dipole repulsion, increasing energy barriers for the CC coupling and resulting in the production of only C1 products over the bare CIS. However, the unique electronic structure of the Vs and In dual centers on the 2%Ag/CIS reduced the dipole–dipole interaction, thus reducing the energy barriers for the CC coupling and ultimately boosting the selective production of ethylene. The TEM and HAADF-STEM images for the 2%Ag/CIS revealed the uniform scattering of Ag AACs over the surface of CIS, and no Ag NPs or agglomerations were observed, indicating the success of the hydrothermal preparation route for anchoring the highly scattered Ag AACs over CIS. The valence state of anchored Ag AACs in the 2%Ag/CIS was explored using XANES and EXAFS analyses. The XANES spectrum for the Ag K-edge of the 2%Ag/CIS exhibited a peak intensity located between the Ag foil and Ag2O, suggesting the partial positive charge of the loaded Ag species. Moreover, the EXAFS fitting results revealed that the Ag AACs are homogeneously scattered in 2%Ag/CIS, and each Ag atom is coordinated with three surrounding S atoms. EPR measurements were conducted to confirm the presence of structural defects. The emerged EPR signal from the Vs located at g = 2.03 exhibited the lowest intensity for the bare CIS, suggesting the low content of Vs in CIS. Interestingly, upon the addition of Ag AACs to the CIS, the signal intensity was significantly raised until it reached its peak at 2%Ag/CIS. These results highlight the key role of Ag AACs in inducing Vs formation and creating a symbiotic effect. The impact of Ag AACs and Vs upon the photo-generated electron movement was elucidated by kelvin probe force microscopy (KPFM) and in situ XPS techniques. The KPFM measurements exhibited that the contact potential difference (ΔCPD) increased from 12.11 mV for the bare CIS to 64.43 mV for 2%Ag/CIS after light irradiation, indicating that the combined Ag AACs and Vs enhanced the charge carrier transfer to the surface of 2%Ag/CIS, thereby raising the photocatalytic activity. Furthermore, the in situ XPS spectra revealed the movement in the binding energies of Cu 2p and In 3d to higher values with light irradiation; while the Ag 3d spectrum is moved to lower binding energies. These results reveal that the CIS generated electrons, which migrated to the Ag AACs. The optimized 2%Ag/CIS exhibited substantial activity for converting CO2 to ethylene using visible light irradiation without applying sacrificial agents. It achieved an ethylene production rate of 53.8 μmol g−1 h−1 with a selectivity of 98.8%. In contrast, the bare CIS only produced small amounts of lower-value C1 products. These performance results confirmed the symbiotic effect of combining the Ag AACs and the Vs on enhancing the production rate and selectivity of ethylene. Finally, the in situ DRIFTS results revealed specific intermediate and reaction products associated with the CC coupling mechanism over the 2%Ag/CIS catalyst. The identification of characteristic vibrational peaks, including *COCHO, *CH2=, *CH2 and *C2H4, confirmed the occurrence of CC coupling reactions resulting in ethylene production over the 2%Ag/CIS catalyst.
3.3 Mo or Ni ACCs Boosting the Photosynthesis of Ethylene/Ethane
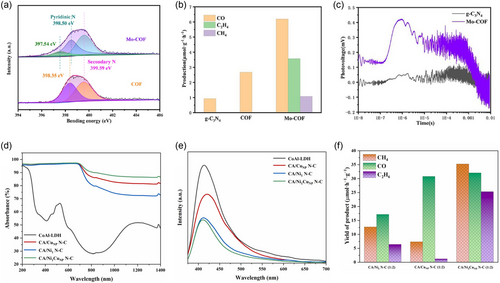
The intrinsic properties of MOFs and COFs photocatalysts, such as extended-π conjugation, visible light absorption capabilities, low recombination rates, stability, and large surface area, have resulted in extensive research on their applications in PRCO2. These exceptional properties position them as promising options as support photocatalysts to anchor multiple AACs. Moreover, anchoring AACs on MOFs and COFs supports can improve photoelectrons migration, create modulated active sites for CO2 adsorption and activation, enhance charge carrier separation efficiency, and promote the CC coupling of the formed intermediates. Consequently, this facilitates the complex multielectron CO2 reduction process to produce the desired C2 products. Kou et al.[71] successfully incorporated Mo AACs onto TpBpy COF using the impregnation method, creating an efficient Mo–COF photocatalyst. The resulting MoCOF photocatalyst showcased the presence of MoN2 active sites as catalytic centers for CO2 photosynthesis to C2H4. Characterization via TEM, elemental mapping, and HAADF-STEM images revealed the uniform dispersion of Mo AACs across the TpBpy COF without any evidence of agglomerations, clusters, or nanoparticles. The local structure of the MoN2 active sites was explored using XPS measurements, which demonstrated the Mo 3d spectrum deconvoluting into two peaks at 232.27 and 235.32 eV, corresponding to Mo in a (+6) oxidation state. The N 1s spectrum of the MoCOF exhibited an extra peak at 397.54 eV compared to the TpBpy COF N 1s spectrum, confirming the formation of MoN2 active sites (Figure 8a). The coordination structure of the optimized MoCOF was verified through XANES and EXAFS analyses, which revealed an absorption edge close to that of MoO3, indicating the Mo oxidation state to be +6, consistent with the XPS results. Furthermore, both FT-EXAFS and WT-EXAFS confirmed the coordination between the Mo AACs and the pyridinic nitrogen of the COF matrix, leading to the formation of MoN2 active sites. The presence of MoN2 sites in the MoCOF significantly enhanced its photocatalytic activity for PRCO2 to C2H4. Under visible light irradiation, the optimized MoCOF yielded 3.57 μmol g−1 h−1 of ethylene with selectivity of 32.92%, while the COF and g-C3N4 as a reference photocatalyst produced only CO under the same conditions (Figure 8b). Additionally, the influence of anchoring Mo AACs on the charge carriers kinetics of the COF was investigated using transient-state surface photovoltage (TSSPV), TSPL, and transient photocurrent measurements. The TSSPV results showed that the Mo-COF had a higher response than the reference g-C3N4 (Figure 8c), while the TSPL indicated an increased lifetime of 1.12 ns compared to 0.86 ns for the TpBpy COF. The transient photocurrent measurements showed that the Mo-COF had a higher response than the COF, suggesting improved charge carrier separation efficiencies and higher photocatalytic activities. The incorporation of Mo AACs also reduced the energy barriers for CO2 activation and promoted the CC coupling of the formed intermediates to obtain the desired C2H4 product. However, further research is needed to investigate the influence of different AACS on different MOFs and COFs over ethylene/ethane photosynthesis yields and selectivity, as research on AACs anchored to MOFs and COFs is still limited.
In addition, Pan et al.[72] investigated the synergetic effect of Ni AACs and Cu NPs on ZIF-8-derived nitrogen-doped carbon NC for enhancing the PRCO2 to ethane via the fabrication of (Ni1CuNP NC) photocatalyst through calcination, followed by the formation of heterojunction with CoAl-LDH (CA) using ultrasonic to synthesize the optimized (CA/Ni1CuNP NC) composite. The incorporation of Ni AACs into the NC resulted in the creation of NiN4 active sites with enhanced pore structures and electrical conductivity, leading to improved adsorption of reactants and intermediates, as well as enhanced charge carrier migration during the CC coupling reactions. Moreover, the inclusion of Cu NPs led to increased electron density at Ni atomic sites, providing improved charge distribution and separation, thereby promoting the formation of C2 products. Additionally, the formation of a heterojunction with CA maximized the hydrogenation of intermediates, primarily by accelerating the dissociation of water, which significantly boosted the production rate of C2H6. The coordination environment of the synthesized CA/Ni1CuNP NC has been confirmed using XPS, XANES, and EXAFS analyses. The HR-XPS Ni spectra showed peaks at 852.9 and 856.0 eV, indicating the presence of Ni0 and Ni2+ species. The N 1s XPS spectra confirmed the presence of NiN bonding at 399.36 eV, suggesting the successful construction of the Ni N4 coordination sites. Additionally, the XANES results showed that the CA/Ni1CuNP NC has an adsorption edge between that of Ni foil and NiO, and the FT-EXAFS fitting confirmed the existence of Ni AACs and Ni N4 active centers. Furthermore, the coexistence of both Ni AACs and Cu NPs in the CA/Ni1CuNP NC composite extended its light-absorption capabilities compared to CA/Ni1 NC, CA/CuNP NC, and CA photocatalysts. The UV-Vis results indicated an enhanced light absorption in the infrared (IR) region due to SPR induced by the Cu NPs in the synthesized samples (Figure 8d). Figure 8e displays the PL spectra with a remarkable reduction in intensity for the CA/Ni1CuNP NC compared to other samples, demonstrating the notable role of the Ni AACs and Cu NPs in suppressing the recombination rates and enhancing the charge carrier separation. The optimized CA/Ni1CuNP NC exhibited a notable ethane production rate of 25.328 μmol g−1 h−1 with a selectivity of 50.61% under visible light irradiation (Figure 8f). Additionally, it was observed that lower CA content led to reduced C2H6 generation, indicating the hydrogenation role of CA. The photocatalytic activities, along with the characterization results, highlighted the synergetic effect of Ni AACs and Cu NPs in enhancing the separation of photogenerated carriers and promoting the CC coupling to remarkably improve the photosynthesis of ethane.
3.4 Dual AACs Boosting Photosynthesis of Ethylene/Ethane
The anchoring of dual AACs over photocatalysts is raised as a creative design strategy for preparing the composite photocatalysts. This approach aims to address the challenges associated with the process of PRCO2 to produce valuable ethylene/ethane. The dual AACs can provide better regulation of the microstructure environment and enhance the photogenerated carrier separation, which could achieve higher production rate and selectivity for ethylene/ethane. For example, Wang et al.[65] reported the construction of PN and CuN4 active centers through anchoring metallic Cu AACs and nonmetallic P AACs onto carbon nitride (CN) nanosheets. The as-prepared P/Cu AACs@CN photocatalyst was prepared by applying hydrothermal and polymerization synthesis routes. The photogenerated charge kinetic results exhibited that both the Cu and P AACs could efficiently capture the photoexcited charge carriers to boost the charge separation over the CN structure with light irradiation. This enhanced separation resulted in the creation of a charge-enriched environment around the Cu centers. The Cu charge-enriched centers can adsorb CO2 and activate its reduction to *CO intermediate, followed by the subsequent coupling and hydrogenation of the adsorbed *CO to produce ethane. The optimal P/Cu AACs@CN photocatalyst exhibited substantial performance for PRCO2 to ethane, realizing a remarkable ethane evolution rate of 616.6 μmol g−1 h−1 and ethane selectivity of 33% after 10 h light irradiation. Notably, the Cu AACs@CN sample only exhibited 23.7 μmol g−1 h−1 yield of ethane, which was 26 times less than the measured activity of P/Cu AACs@CN. The P@CN and CN catalysts only produced minimal amounts of ethane. These findings underscore the importance of the created PN and CuN4 dual active centers in maximizing the CC coupling process for ethane production. Several characterization techniques were employed to investigate the morphological properties, microstructure, and coordination environment of the incorporated dual centers in the P/Cu AACs@CN. For example, HAADF-STEM images of P/Cu AACs@CN revealed the high scattering of bright isolated Cu AACs with no sign of any larger particles, and elemental mapping images confirmed the homogeneous scattering of Cu and P species over the CN nanosheets. Furthermore, the XPS P 2p spectrum for the P/Cu AACs@CN exhibited two peaks at 133.5 and 134.4 eV, corresponding to the PN and PN coordination bonding modes, respectively. XANES and EXAFS analyses were applied to gain insights into the valence state and coordination structure of the Cu active centers in the P/Cu AACs@CN. The XANES spectra for the Cu K edge of P/Cu AACs@CN and other Cu-based references revealed the presence of an absorption shoulder of P/Cu SAs@CN between the absorption shoulders of Cu2O and CuO, indicating that the valence state of the Cu AACs is between +1 and +2. The FT-EXAFS analysis exhibited a main peak at 1.5 Å related to the CuN bonding mode with no sign of the CuCu bonding, confirming the atomic state of the incorporated Cu species. Moreover, the WT-EXAFS contour graphs for the P/Cu AACs@CN revealed one peak located at 3.8 Å−1, also attributed to the presence of the CuN bonding. The fitting results of the FT-EXAFS revealed the coordination number of the Cu AACs to be 4 in the Cu-N4 structure. PL and TRPL techniques were applied to analyze the charge kinetics of the P/Cu AACs@CN. The reduced intensity of the PL peak for the P/Cu AACs@CN and the extended average lifetime (τave = 7.13 ns) compared to the as-prepared Cu AACs@CN, P@CN, and CN samples corroborated the superior charge separation by the PN and Cu-N4 dual centers. The transfer of photogenerated electrons and holes of the P/Cu AACs@CN was explored using in situ XPS analysis. The P 2p spectrum moves to higher binding energies after irradiation, revealing the accumulation of holes around the P AACs. Meanwhile, the Cu 2p spectrum moves to lower binding energies after light exposure, revealing the accumulation of photogenerated electrons over the Cu active centers. These findings, in conjunction with the co-existence of the vibrational band of the key intermediate *OCCOH in the in situ DRIFTS spectra, have proved the effectiveness of the inherent characteristics of the combined Cu and P AACs in altering the microstructure environment of the CN. Consequently, creating charge-enriched centers raises the charge separation and boosts the CC coupling reactions, ultimately achieving higher rates of ethane evolution.
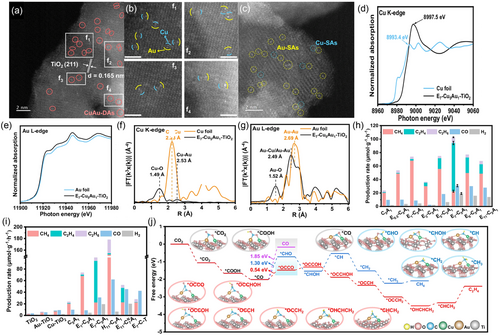
Recently, Xie et al.[61] exploited a novel strategy for preparing diatomic photocatalysts for efficient PRCO2 to ethylene. Contrary to the extensively used single atom catalysts (SACs), diatomic photocatalysts involve the incorporation of two atoms, which can maximize the utilization of atomic active centers and cooperatively alter their steric/electronic properties to regulate the reaction kinetics/pathways. They reported the anchoring of CuAu diatomic active centers over TiO2 NPs using a novel up-bottom ion-cutting preparation route. The conventional preparation strategies for bimetallic photocatalysts usually arouse the accumulation or agglomeration of atoms in preparation, resulting in a reduction in available active centers for the reaction. In comparision, this innovative up-bottom ion-cutting route can manage the vectored etching of certain atoms in an alloy, distort the alloy structure and uniformly scatter diatomic active centers over the support surface. The optimized Et7Cu5Au1TiO2 (E7-C5A1) photocatalyst was constructed after etching the Cu5Au1 alloy in the Cu5Au1TiO2 catalyst (C5A1) for 7 h. The HAADF-STEM images of the Et7Cu5Au1TiO2 photocatalyst confirmed the even scattering of diatomic CuAu over the TiO2 NPs, revealing the successful vectored etching of the Cu5Au1 alloy (Figure 9a–c). The atomic configuration and coordination structure of the diatomic CuAu in the Et7Cu5Au1TiO2 sample were further investigated using XANES, FT-EXAFS, and WT-EXAFS analyses. The XANES spectrum for the Cu K-edge of Et7Cu5Au1TiO2 is located at a higher photon energy than that of Cu foil (Figure 9d), revealing that some Cu AACs could be bonding with the O atoms of TiO2. Similarly, the Au L-edge absorption peak resembled that of the Au foil, indicating the presence of Au0 species (Figure 9e). Additionally, the FT-EXAFS spectra for the Cu K-edge and Au L-edge revealed distinct peaks, providing information about the coordination environment of diatomic CuAu in the sample. The Cu K-edge FT-EXAFS spectrum exhibited peaks at 1.49 Å and 2.53 Å, corresponding to CuAu and CuO coordination; while the Au L-edge spectrum exhibited peaks at 1.52 and 2.49 Å, attributed to AuO and AuCu/AuAu coordination, respectively (Figure 9f,g). The coordination environments of Et7Cu5Au1TiO2 were further confirmed by the WT-EXAFS colored maps, as they revealed the existence of maxima intensities related to CuAu, CuO, AuO, and AuCu/AuAu coordination structures. Besides, the XPS results exhibited that the Au 4f peaks of Et7Cu5Au1TiO2 are moved to lower binding energies, this is attributed to the migration of photogenerated electrons from TiO2 to the diatomic CuAu, which is advantageous for the CC coupling reactions. These results reveal that diatomic CuAu is the main catalytic center for PRCO2 and CC coupling processes. Nevertheless, charge kinetics was explored using the PL and TRPL spectroscopy. The PL emission peak of Et7Cu5Au1TiO2 at 425 nm exhibited the lowest intensity compared to that of pure TiO2 or Cu5Au1TiO2, revealing the key role of diatomic CuAu in reducing the electron–hole recombination in Et7Cu5Au1TiO2. Also, the shorter calculated τ3 lifetime of Et7Cu5Au1TiO2 in the TRPL measurements compared with those of the other catalysts suggested that the photogenerated electrons preferred to transfer from TiO2 to the diatomic CuAu rather than recombining in the Cu and diatomic CuAu recombination centers. Nevertheless, Et7Cu5Au1TiO2 exhibited exceptional performance for CO2 reduction to C2 products with light irradiation (320 nm < λ < 780 nm). It achieved a magnificent ethylene evolution rate of 71.6 μmol g−1 h−1 with a selectivity of 68.3%, outperforming the other as-prepared photocatalysts. The addition of Cu5Au1 to bare TiO2, along with optimizing the etching time, contributed to the obvious improvement in ethylene generation and selectivity. And the optimal performance was achieved by Et7Cu5Au1TiO2 (Figure 9h–i). Interstingly, the generation of ethane was observed in the reaction, with Et7Cu5Au1TiO2 exhibiting the highest ethane evolution rate (8.5 μmol g−1 h−1) with a selectivity of 9.4%. These photocatalytic performances highlight the key role of incorporating diatomic CuAu over TiO2 for achieving higher performance in ethylene production. Moreover, Figure 9j exhibited the free energy diagram for PRCO2 to ethylene over Et7Cu5Au1TiO2 alongside the structure of the formed intermediates. The above results revealed that the diatomic centers regulated the electronic/optical properties of the support, which can reduce the overall energy barriers of the CO2 reduction reaction and raise the sluggish CC coupling rates to acquire higher production rates and selectivity for ethylene/ethane.
The aforementioned references revealed the significance of anchoring Cu,[56, 57, 59, 63, 64, 67] Au,[68] Ag,[58] Mo,[71] Ni,[72] P/Cu,[65] and CuAu[61] AACs over a range of support photocatalysts to raise the PRCO2. This enhancement results in higher production rates and selectivity of valuable ethylene/ethane. The integrated AAcs can regulate the coordination environments and microstructures of the photocatalysts, creating novel active centers at the atomic level with unique electronic properties. These characteristics can help overcome the complex reaction pathways involved in the photosynthesis of ethylene/ethane. Through regulating the electronic densities distributed over the active centers and their coordination structures, we can accumulate photogenerated charge carriers to obtain highly active AACs. These AACs can reduce the thermodynamic energy barriers of CO2 adsorption/activation, resulting in desired intermediates for subsequent reaction pathways. Moreover, these AACs can boost the slow kinetics of CC coupling and competitive hydrogenation steps, resulting in higher evolution rates and selectivities of the targeted renewable hydrocarbons. However, the utilization of AACs anchored photocatalysts for the photosynthesis of ethylene/ethane is not extensively reported. Further insightful mechanistic research is urgently required to fill the current gap of very low production rates and selectivities of ethylene/ethane in realistic conditions. Besides, the exploration of various AACs anchored photocatalysts with remarkable performances will greatly facilitate industrial-scale applications, and meet the high demand in this area.
4 Conclusion and Outlook
In this review, we critically introduce the latest atomic-scale engineering strategy for preparing composite photocatalysts with superior performances for CO2 reduction to ethylene/ethane. The possible reaction pathways and challenges for photosynthesis of ethylene/ethane have been summarized, including the thermodynamic feasibility of CO2 adsorption/activation, the associated energy barriers, the multielectron transfer requirement, and the challenges associated with sluggish CC bonding and competing hydrogenation processes. An insightful review of the intrinsic properties of various AACs, including Cu, Au, Ag, Mo, Ni, Cu/P, and CuAu anchored photocatalysts, is provided. This review highlights their remarkable production rates and selectivities in the generation of ethylene/ethane. Particularly, Cu AACs anchored photocatalysts are the most explored AACs photocatalysts for ethylene/ethane generation. These are attributed to their unique electronic properties that reduce the overall energy barriers in PRCO2 and boost the CC coupling reactions. Besides, Cu is also an earth-abundant and nontoxic metal, greatly contributing to the cost-effectiveness and sustainability impacts of employing the Cu AACs anchored photocatalysts. Furthermore, this review also critically summarized and analyzed the synergetic effect from anchoring dual AACs over support photocatalysts and the subsequent photocatalytic activity enhancement. Various preparation routes (e.g., impregnation, up-bottom ion-cutting, atomic layer deposition, calcination, photoreduction, hydrothermal, and two-step thermal polymerization) have been comprehensively evaluated for the integration of AACs over a wide range of photocatalysts supports, such as metal oxides (e.g., TiO2, W18O49, and CeO2TiO2), 2D materials (e.g., CN, PCN, and RP), COFs (e.g., TpBpy COF), and bimetallic sulfide (e.g., CIS) to prepare robust AACs anchored photocatalyst with optimum scattering for the immobilized AACs. The electronic configuration, coordination structure, morphology, charge kinetics, optical properties, and reaction mechanism of the reported AACs anchored photocatalysts have been thoroughly explored utilizing state-of-art in situ/ex situ characterization techniques. The optimized AACs anchored photocatalysts exhibited remarkable evolution rates, selectivities, and robustness for the photosynthesis of ethylene/ethane. The robust photocatalytic performance of the AACs anchored photocatalysts highlighted the significance of this emerging class of photocatalysts, offering novel concepts and valuable insights in the area of heterogeneous photocatalysis. Moreover, recent advancements in the preparation of AACs anchored photocatalysts have opened up opportunities for their application in other photocatalytic reactions. Although significant achievement have been made recently in the preparation of AAC anchored photocatalysts with remarkable catalytic performances, there are still obvious restrictions and challenges in their application for the photosynthesis of ethylene/ethane as below: 1) Emerging AACs with unique electronic properties/chemical compositions, such as Ru, Fe, Ni, Co, Pt, and Ir, can be explored for their potential to raise the PRCO2 to ethylene/ethane. Additionally, the utilization of various diatomic AACs requires further investigation owing to their synergistic effect and great potential to significantly raise the photocatalytic performance. The sluggish CC coupling process could also be obviously accelerated through regulating the coordination structure of anchored AACs on the photocatalyst supports. Furthermore, although most references focused on metal oxides and 2D materials, it is urgently required to explore the emerging photocatalyst supports, such as metal organic framework (MOF), covalent organic framework (COF), and transition metal chalcogenides (TMC), to achieve higher generation and selectivity for ethylene/ethane. 2) The atomic structure, coordination environment, and charge kinetics of anchored AACs play a key role in the conversion of CO2 to ethylene/ethane. Therefore, it is urgently required to gain a more insightful understanding of the catalytic centers and reaction pathways in realistic condition for achieving higher catalytic performances. These insights can further boost the exploration of emerging AACs anchored photocatalysts with regulated properties to raise generation and selectivity for photosynthesis of ethylene/ethane. To fill the current knowledge gap in this area, it is of great importance to employ a variety of state-of-art in situ characterization techniques, such as in situ X-ray photoelectron spectroscopy (XPS), in situ X-ray absorption spectroscopy (XAS), in situ transmission electron microscopy (TEM), in situ transient absorption spectroscopy (TAS), in situ transient-state photoluminescence (TSPL), in situ infrared (IR) spectroscopy and in situ electron paramagnetic resonance (EPR). 3) The reported-generation amounts for the photosynthesis of ethylene/ethane by the AACs anchored photocatalysts are currently in the micromole range, despite using triethanolamine (TEOA) as a sacrificial agent. These performances are still unsatisfactory for large scale applications owing to the high energy barriers for CO2 adsorption/activation, complex reaction pathways, and slow kinetics of the CC coupling process. To address these challenges, there is an urgent requirement to explore emerging AACs. They are expected to obviously raise the transfer of photogenerated electrons to the active centers, regulate the coordination structure of the active centers to increase CO2 adsorption/activation, and promote the CC coupling reactions to generate higher yields of ethylene/ethane. More insightful research is also required to raise the cost-effectiveness of AACs anchored photocatalysts. This involves exploring the utilization of more earth-abundant metals/non-metals as AACs and testing their corresponding performances. Besides, it is highly important to explore the design of the photocatalytic reactors, which can obviously raise the production rate to the industrial level, for example, flow reactors and stainless-steel reactors with solar concentrator. Additionally, investigating the critical role of reaction conditions, such as temperature, pressure, and pH, in raising the selectivity can provide more insights regarding promoting the activity for such products. Furthermore, it is of great significance to utilize alternative feedstocks (e.g., waste plastic and biomass) as electron donors to not only accelerate reaction kinetics but also generate more valuable chemicals. This will raise a economic and sustainable impact of the process.
Acknowledgements
E.M.H. and A.T.-K. contributed equally to this work. The authors gratefully acknowledge the financial support from the Australian Research Council (ARC) through the Discovery Project programs (FT230100192). E.M.H. acknowledges the financial support from The University of Adelaide Research Scholarship.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Elhussein M. Hashem: Writing—original draft (equal). Amin Talebian-Kiakalaieh: Writing—original draft (equal). Meijun Guo: Writing—review and editing (supporting). Tian Chen: Writing—review and editing (supporting). Jaenudin Ridwan: Writing—review and editing (supporting). Philip Kwong: Writing—review and editing (supporting). Jingrun Ran: Supervision (lead). Elhussein M. Hashem and Amin Talebian-Kiakalaieh contributed equally to this work.
Biographies

Elhussein M. Hashem is currently pursuing his Ph.D. degree in the School of Chemical Engineering at the University of Adelaide as a part of Prof. Shi-Zhang Qiao's research group under the supervision of Dr. Jingrun Ran. His research is primarily focused on the development and rational design of advanced atomic-level photocatalysts that can efficiently produce solar fuels and value-added chemicals across a wide range of applications.

Amin Talebian-Kiakalaieh received his bachelor's degree in petroleum engineering in 2007. Then he changed his major to Chemical Engineering and received his master's degree in chemical engineering from Universiti Teknologi Malaysia (UTM) in 2012. He joined Prof. Shi-Zhang Qiao group in 2021 as a Ph.D. candidate under the supervision of Dr. Jingrun Ran at the University of Adelaide. Currently, he is working on synthesizing advanced photocatalysts for renewable energy applications.

Jingrun Ran received his Ph.D. degree in Chemical Engineering from the University of Adelaide under the supervision of Professor Shi-Zhang Qiao. Currently he is appointed as a senior lecturer in the School of Chemical Engineering at the University of Adelaide and working in Prof. Qiao's group. In 2020-2024, he has been recognized as a Clarivate Highly Cited Researcher. In 2023, he was awarded the ARC Future Fellowship. His current research is focused on the atomic-scale design/synthesis of photocatalysts for producing energy fuels and value-added chemicals using renewable solar energy.
Open Research
Data Availability Statement
Research data are not shared.




